Introduction
Ovarian cancer (OVCA) is the most lethal
gynecological cancer, with an estimated 21,980 new cases and 14,270
deaths in 2014 (1). Unfortunately,
75% of cases are diagnosed at an advanced (III–IV) stage due to the
lack of symptomatic early-stage disease or effective screening
tests. Although ≤70% of these women demonstrate a complete response
to primary treatment with cytoreductive surgery and adjuvant
platinum/taxane-based chemotherapy, the majority will eventually
develop recurrent disease that is associated with poor survival
(2,3).
Such patients frequently receive multiple lines of systemic
cytotoxic therapy that may be associated with significant toxicity
and detriment to the quality of life. The search for additional
tolerable but efficacious drugs against OVCA has progressed
slowly.
Acetaminophen is a commonly used analgesic with
minimal side effects that may be active against OVCA cells
(4). Acetaminophen was first
synthesized in 1877 by von Mehring at Johns Hopkins University, but
it was not extensively used until 1949; it was briefly recalled in
1951 due to reports of blood dyscrasias, but has been marketed
since 1955 as an over-the-counter drug (5). Acetaminophen is currently a commonly
used analgesic and antipyretic. Adverse events associated with
acetaminophen are rare when used at therapeutic dosage; however,
overdose may cause fatal hepatotoxicity. Acetaminophen is generally
categorized with aspirin and aspirin-like drugs, which are known to
act by inhibiting prostaglandin synthesis from arachidonic acid or
other fatty acid precursors (6).
While the exact mechanism of action for acetaminophen remains
controversial, the prevailing hypothesis is that acetaminophen
inhibits the synthesis of prostaglandins by competing at the active
site of the cyclooxygenase enzyme with arachidonic acid (7).
The epidemiological reports on the effect of
acetaminophen on OVCA risk and behavior are conflicting. Moysich
et al (8) reported that
regular acetaminophen use reduced the risk of OVCA [odds ratio (OR)
= 0.56; 95% confidence interval (CI): 0.34–0.86]. Rodriguez-Burford
et al (9) were able to
demonstrate a decrease in viable OVCA cells with acetaminophen
treatment compared with controls. Cramer et al (10) reported a statistically significant
inverse association between the use of paracetamol, which is the
European name for acetaminophen, and OVCA risk. A more recent
Danish case-control study found paracetamol use to be associated
with a reduced OR for developing OVCA (OR=0.82, 95% CI: 0.74–0.92;
P<0.001) (11). Others, however,
have failed to demonstrate such an association (12,13). These
inconsistent findings prompted us to investigate: i) The effect of
acetaminophen on OVCA cell lines; ii) the molecular signaling
pathways associated with acetaminophen response; and iii) in an
effort to evaluate the clinical significance of our findings, the
associations of these pathways with overall patient survival from
OVCA.
Materials and methods
Cell line processing
A total of 16 OVCA cell lines were subjected to
treatment with acetaminophen in increasing concentrations (0–10
mM), and the data were used to determine half maximal inhibitory
concentration (IC50) values. Prior to any treatment, the
16 cell lines also underwent Affymetrix-based analysis of baseline
genome-wide expression. The Pearson's correlation test was used to
identify genes that were associated with acetaminophen sensitivity,
which were then analyzed for representation of molecular pathways.
The genes in the top 2 pathways underwent principal component
analysis (PCA) and were assigned a first principal component (PC1)
score, which functions as a summary measure of pathway expression.
A total of 4 clinico-genomic datasets were then queried using the
PC1 scores to assess the associations between acetaminophen
response pathways and patient survival from OVCA.
Cell culture
The OVCA cell lines OV90, OVCAR3, SK-OV-3 and
TOV112D were obtained from the American Type Culture Collection
(Manassas, VA, USA); A2780S was obtained from the European
Collection of Cell Cultures (Salisbury, UK); FUOV1, IMCC3, IMCC5,
IGR-OV1, MCAS, OVCA420, TOV-21-G, OVCAR2, OVCAR5, PEO1 and PEO4
were kind gifts from Dr Patricia Kruk (Department of Pathology,
College of Medicine, University of South Florida, Tampa, FL, USA)
and Dr Susan Murphy (Department of Obstetrics and
Gynecology/Division of Gynecologic Oncology, Duke University,
Durham, NC, USA). The cell lines were maintained in RPMI-1640
medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; Fisher Scientific, Pittsburgh, PA, USA), 1%
sodium pyruvate, 1% penicillin/streptomycin (Cellgro, Manassas, VA,
USA), and 1% nonessential amino acids (HyClone, Hudson, NH, USA).
The cells were genotyped by short tandem repeat profiling to
confirm the tissue of origin, and mycoplasma testing was performed
every 6 months, in accordance with the manufacturer's protocol
(Lonza, Rockland, ME, USA).
RNA extraction and microarray
expression analysis
OVCA cell RNA was extracted at baseline using the
RNeasy mini-kit following the manufacturer's recommendations
(Qiagen, Valencia, CA, USA). The RNA quality was evaluated using an
Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara,
CA, USA). The targets for Affymetrix microarray analysis were
prepared according to the manufacturer's instructions and
hybridized to customized Human Affymetrix HuRSTA gene chips
(HuRSTA-2a520709), which include 60,607 probe sets and
representation of 19,308 genes (Gene Expression Omnibus accession
no. GSE34615).
CellTiter-Blue cell viability
assays
Drug activity was evaluated using a high-throughput
CellTiter-Blue cell viability assay. Cells (2.5×103 per
well) were plated in 384-well plates using complete media with 10%
FBS and allowed to adhere overnight. After cell adherence,
increasing concentrations of acetaminophen were added to
appropriate wells using an automated pipetting station. Four
replicate wells were used for each drug concentration and for
vehicle controls. Drug dilutions initially consisted of serial
dilutions from a maximum concentration of 100 mM. The cells were
incubated with the drug for 72 h, and 5 µl of CellTiter-Blue
reagent (Promega Corp., Madison, WI, USA) were added to each well.
Fluorescence was read at 579-nm excitation/584-nm emission using a
Synergy 4 microplate reader (Bio-Tek Instruments, Inc., Winooski,
VT, USA). IC50 values were determined using a sigmoidal
equilibrium model fit (XLfit 5.2; ID Business Solutions Ltd.,
Alameda, CA, USA). The IC50 was defined as the
concentration of drug required for a 50% reduction in cell
growth/viability.
Statistical analysis
Expression data from the OVCA cell lines were
subjected to background correction and normalization using the
Robust Multichip Average algorithm in the Affymetrix Expression
Console (http://www.affymetrix.com) and
expressed as log2 values. Individual gene expression and
IC50 results were evaluated using Pearson's correlation
test. Probe sets demonstrating P<0.01 were considered to be
significantly correlated with the IC50 results and were
analyzed using GeneGo MetaCore™ for pathway analysis (http://www.genego.com/metacore.php). Pathways
demonstrating P<0.05 (with a 30% false discovery rate) were
considered significant, based on the GeneGo MetaCore™ statistical
test for significance.
Clinical significance of pathways
Pathways found to be associated with acetaminophen
sensitivity were tested for associations with overall survival
using PCA modeling, as previously described (14,15).
Survival associations were evaluated by the long-rank test using
median PCA value as a cut-off in 4 publically available
clinico-genomic datasets from 820 women with OVCA for whom gene
expression and overall survival data were available, including i)
the Australian dataset (n=218, GSE9891) (16), ii) the MD Anderson dataset (n=53,
GSE18520), iii) the Cancer Genome Atlas (n=492), and iv) the Total
Cancer Care (TCC®) dataset (n=57).
Results
Genes and pathways associated with
acetaminophen sensitivity in OVCA cell lines
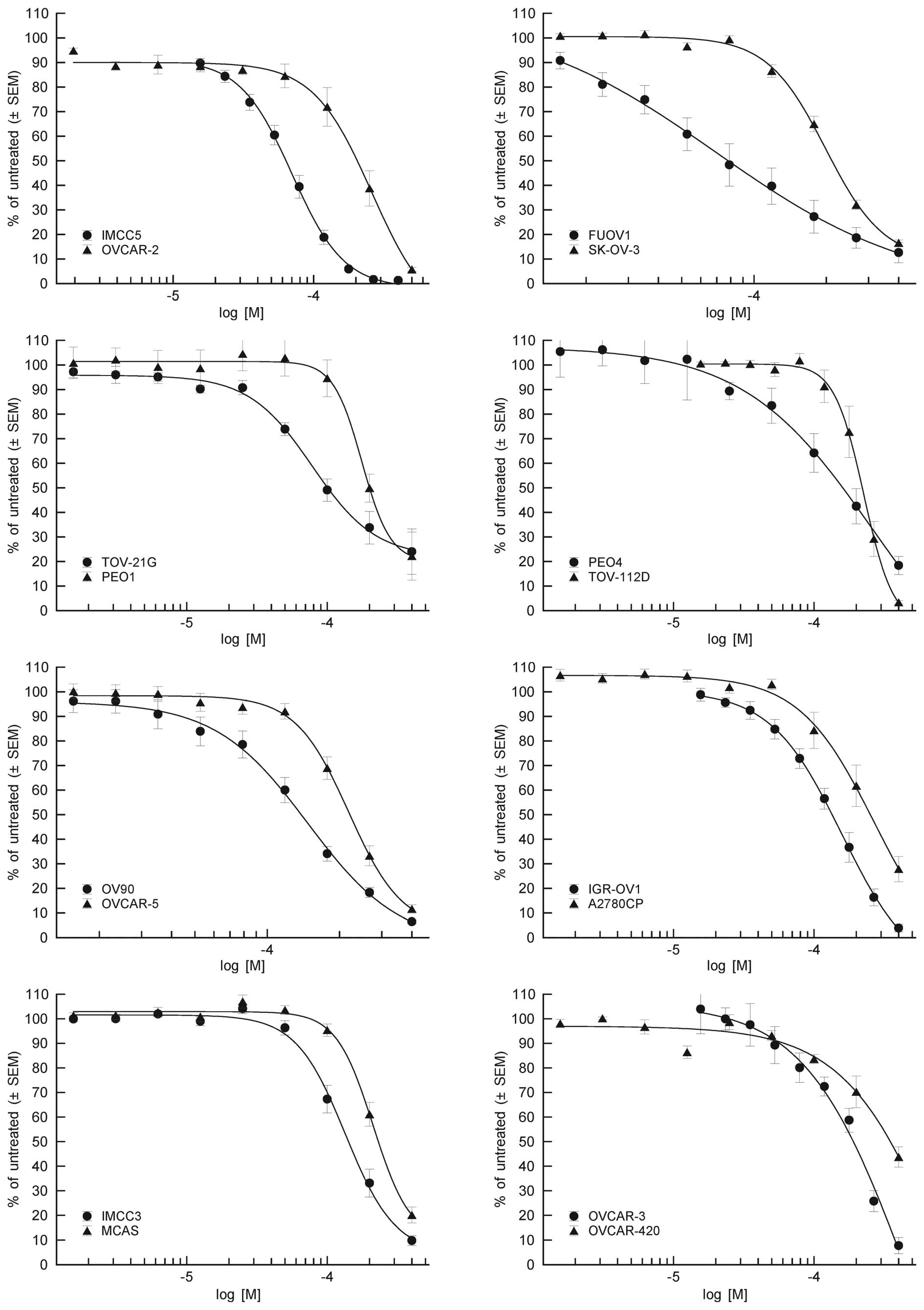
Acetaminophen exhibited antiproliferative activity
against all tested OVCA cell lines, with IC50 values
ranging from 63.2 to 403 µM (Fig. 1).
The Pearson's correlation test using acetaminophen IC50
and OVCA cell line genome-wide expression data revealed the
expression of 727 unique genes (P<0.01) representing 13
molecular signaling pathways (P<0.01) identified by GeneGo
MetaCore™ analysis (Table I).
 | Table I.Biological pathways associated with
acetaminophen sensitivity in 16 OVCA cell lines. |
Table I.
Biological pathways associated with
acetaminophen sensitivity in 16 OVCA cell lines.
| Pathway name | P-value | FDR | Pathway objects |
|---|
| Protein folding and
maturation/angiotensin system maturation/human version | 1.13E-05 | 6.17E-03 | 8/43 |
| Cytoskeleton
remodeling/neurofilaments | 3.76E-04 | 6.86E-02 | 5/25 |
| Signal
transduction/JNK pathway | 6.55E-04 | 8.96E-02 | 6/42 |
| Immune response/MIF -
the neuroendocrine-macrophage connector | 1.07E-03 | 1.18E-01 | 6/46 |
| Androstenedione and
testosterone biosynthesis and metabolism p.2 | 1.86E-03 | 1.29E-01 | 5/35 |
| Cytoskeleton
remodeling/keratin filaments | 2.12E-03 | 1.29E-01 | 5/36 |
| Effect of low doses
of arsenite on glucose-stimulated insulin secretion in pancreatic
cells | 2.12E-03 | 1.29E-01 | 5/36 |
|
Development/angiotensin signaling via
β-arrestin | 3.53E-03 | 1.86E-01 | 4/25 |
| Androstenedione and
testosterone biosynthesis and metabolism p.3 | 3.80E-03 | 1.86E-01 | 5/41 |
| Neurophysiological
process/dopamine D2 receptor transactivation of PDGFR in CNS | 4.09E-03 | 1.86E-01 | 4/26 |
| Cell adhesion/gap
junctions | 6.91E-03 | 2.70E-01 | 4/30 |
| Regulation of lipid
metabolism/FXR-dependent negative feedback regulation of bile acid
concentration | 7.78E-03 | 2.84E-01 | 4/31 |
| Cell cycle/role of
Nek in cell cycle regulation | 8.72E-03 | 2.98E-01 | 4/32 |
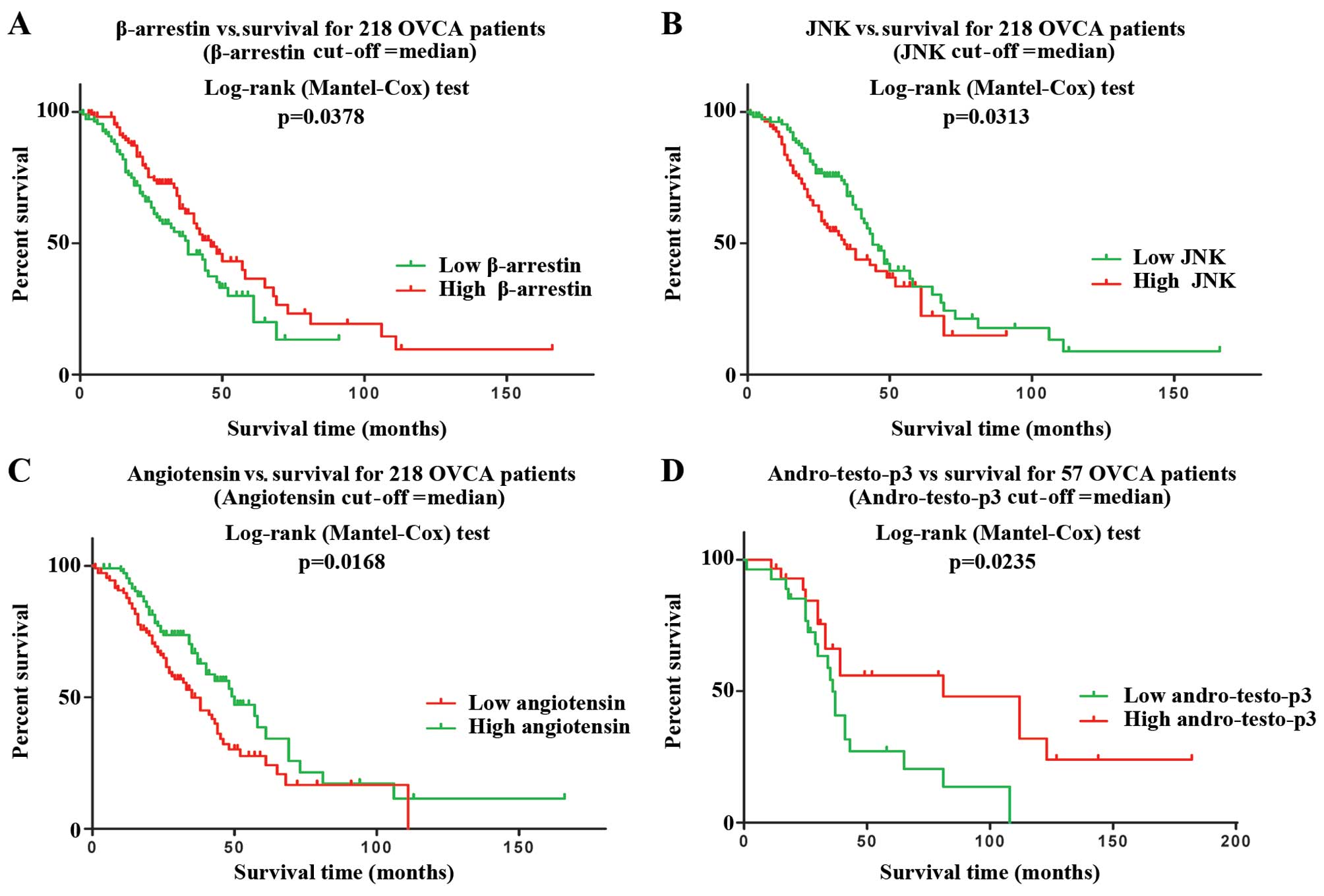
Pathways associated with acetaminophen
sensitivity affect overall survival
PCA modeling of the 13 pathways associated with
in vitro acetaminophen sensitivity in 4 publically available
clinico-genomic datasets identified 4 pathways with expression that
may affect overall survival from OVCA (P<0.05):
Development/angiotensin signaling via β-arrestin (P=0.04,
Australian dataset) (Fig. 2A),
protein folding and maturation/angiotensin system maturation
(P=0.02, Australian dataset) (Fig.
2B), signal transduction/c-Jun N-terminal kinase (JNK) pathway
(P=0.03, Australian dataset) (Fig.
2C), and androstenedione and testosterone biosynthesis and
metabolism (P=0.02, TCC dataset) (Fig.
2D).
Discussion
There are conflicting epidemiological and in
vitro data on the effects of acetaminophen on OVCA. In this
study, we demonstrated that acetaminophen exerts a measurable
effect on OVCA cells and identified possible molecular determinants
of OVCA sensitivity to acetaminophen. In addition, we demonstrated
that overall patient survival is associated with the expression of
4 acetaminophen response molecular signaling pathways. This finding
suggests a clinical relevance of the identified molecular signaling
pathways in OVCA.
Acetaminophen hepatotoxicity is known to be mediated
through the JNK pathway (17).
Antineoplastic properties have been demonstrated in vitro,
where acetaminophen has been shown to enhance the apoptotic effect
of cisplatin and paclitaxel in SKOV3 human ovarian carcinoma cells
through modulation of intracellular glutathione concentration
(4). The epidemiological data vary
widely regarding the protective effect of regular acetaminophen use
against OVCA development (8–13).
Our study identified an antiproliferative effect of
acetaminophen and 4 clinically relevant molecular signaling
pathways that are associated with acetaminophen sensitivity in
vitro. The Signal transduction/JNK, development/angiotensin
signaling via β-arrestin, protein folding and
maturation/angiotensin system maturation, and androstenedione and
testosterone biosynthesis and metabolism pathways were
significantly associated with in vitro sensitivity of OVCA
cell lines to acetaminophen, and clinically relevant given their
association with overall survival.
JNKs are serine/threonine kinases that belong to the
mitogen-activated protein kinase (MAPK) family (18). Phosphorylation of JNK by MAPK kinase
4/7 activates JNK, which may then regulate a number of metabolic
and survival pathways, as well as mediate cell death (18,19).
Sustained JNK activation imparts an increase in apoptosis and
necrotic cell death pathways, while transient JNK activation
protects against cell death (20–22).
However, the presence of JNK is required for development of gastric
cancer, suggesting its role in carcinogenesis (23). The JNK pathway has previously been
implicated in acetaminophen-induced hepatotoxicity (17). JNK expression has been shown in a
substantial number of OVCA patients and its expression was
correlated to progression-free survival, while inhibition of JNK
resulted in decreased tumor growth in vivo (24). While sustained activation of JNK leads
to an increase in apoptosis of OVCA cells, JNK has been shown to be
important in the mechanism of action of acetaminophen and has also
been shown to affect OVCA survival and response to therapy
(25).
We identified a potential novel implication of the
development/angiotensin signaling via β-arrestin, protein folding
and maturation/angiotensin system maturation, and androstenedione
and testosterone biosynthesis and metabolism pathways. These three
pathways have not been previously implicated in OVCA or the
mechanism of action of acetaminophen; further investigation is
required on the interplay of these biological processes in ovarian
carcinogenesis.
In summary, we demonstrated that acetaminophen
exerts antiproliferative effects on OVCA cells in vitro. By
using an integrated methodology, we identified 4 molecular
signaling pathways that appear to be clinically relevant for OVCA.
One of these pathways (signal transduction/JNK pathway) was
previously found to be involved in the mechanism of action of
acetaminophen and to be predictive of decreased survival from
OVCA.
Acknowledgements
We would like to thank Rasa Hamilton (H. Lee Moffitt
Cancer Center and Research Institute) for editorial assistance. We
would like to thank The Foundation of Women's Cancer and Moffitt's
Total Cancer Care® Protocol (TCC). TCC is enabled, in part, by the
generous support of the DeBartolo Family. Our study also received
valuable assistance from the Translation Research Core and Cancer
Informatics Core at H. Lee Moffitt Cancer Center and Research
Institute; a National Cancer Institute-designated Comprehensive
Cancer Center, supported under NIH grant P30 CA76292.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R: Gynecologic Oncology Group: Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
Gynecologic Oncology Group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu YJ, Neuwelt AJ, Muldoon LL and Neuwelt
EA: Acetaminophen enhances cisplatin- and paclitaxel-mediated
cytotoxicity to SKOV3 human ovarian carcinoma. Anticancer Res.
33:2391–2400. 2013.PubMed/NCBI
|
|
5
|
Spooner JB and Harvey JG: The history and
usage of paracetamol. J Int Med Res. 4(Suppl 4): 1–6.
1976.PubMed/NCBI
|
|
6
|
Ameer B and Greenblatt DJ: Acetaminophen.
Ann Int Med. 87:202–209. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Botting RM: Mechanism of action of
acetaminophen: Is there a cyclooxygenase 3? Clin Infect Dis.
31(Suppl 5): S202–S210. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moysich KB, Mettlin C, Piver MS, Natarajan
N, Menezes RJ and Swede H: Regular use of analgesic drugs and
ovarian cancer risk. Cancer Epidemiol Biomarkers Prev. 10:903–906.
2001.PubMed/NCBI
|
|
9
|
Rodriguez-Burford C, Barnes MN,
Oelschlager DK, Myers RB, Talley LI, Partridge EE and Grizzle WE:
Effects of nonsteroidal anti-inflammatory agents (NSAIDs) on
ovarian carcinoma cell lines: Preclinical evaluation of NSAIDs as
chemopreventive agents. Clin Cancer Res. 8:202–209. 2002.PubMed/NCBI
|
|
10
|
Cramer DW, Harlow BL, Titus-Ernstoff L,
Bohlke K, Welch WR and Greenberg ER: Over-the-counter analgesics
and risk of ovarian cancer. Lancet. 351:104–107. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baandrup L, Friis S, Dehlendorff C,
Andersen KK, Olsen JH and Kjaer SK: Prescription use of paracetamol
and risk for ovarian cancer in Denmark. J Natl Cancer Inst.
106:dju1112014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lo-Ciganic WH, Zgibor JC, Bunker CH,
Moysich KB, Edwards RP and Ness RB: Aspirin, nonaspirin
nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of
ovarian cancer. Epidemiology. 23:311–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hannibal CG, Rossing MA, Wicklund KG and
Cushing-Haugen KL: Analgesic drug use and risk of epithelial
ovarian cancer. Am J Epidemiol. 167:1430–1437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marchion DC, Cottrill HM, Xiong Y, Chen N,
Bicaku E, Fulp WJ, Bansal N, Chon HS, Stickles XB, Kamath SG, et
al: BAD phosphorylation determines ovarian cancer chemosensitivity
and patient survival. Clin Cancer Res. 17:6356–6366. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen DT, Nasir A, Culhane A, Venkataramu
C, Fulp W, Rubio R, Wang T, Agrawal D, McCarthy SM, Gruidl M, et
al: Proliferative genes dominate malignancy-risk gene signature in
histologically-normal breast tissue. Breast Cancer Res Treatment.
119:335–346. 2010. View Article : Google Scholar
|
|
16
|
Tothill RW, Tinker AV, George J, Brown R,
Fox SB, Lade S, Johnson DS, Trivett MK, Etemadmoghadam D, Locandro
B, et al: Novel molecular subtypes of serous and endometrioid
ovarian cancer linked to clinical outcome. Clin Cancer Res.
14:5198–5208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Win S, Than TA, Han D, Petrovic LM and
Kaplowitz N: c-Jun N-terminal kinase (JNK)-dependent acute liver
injury from acetaminophen or tumor necrosis factor (TNF) requires
mitochondrial Sab protein expression in mice. J Biol Chem.
286:35071–35078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson GL and Nakamura K: The c-jun
kinase/stress-activated pathway: Regulation, function and role in
human disease. Biochim Biophys Acta. 1773:1341–1348. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weston CR and Davis RJ: The JNK signal
transduction pathway. Curr Opin Cell Biol. 19:142–149. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Czaja MJ: Cell signaling in oxidative
stress-induced liver injury. Semin Liver Dis. 27:378–389. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han D, Ybanez MD, Ahmadi S, Yeh K and
Kaplowitz N: Redox regulation of tumor necrosis factor signaling.
Antioxidants Redox Signal. 11:2245–2263. 2009. View Article : Google Scholar
|
|
22
|
Gunawan BK, Liu ZX, Han D, Hanawa N,
Gaarde WA and Kaplowitz N: c-Jun N-terminal kinase plays a major
role in murine acetaminophen hepatotoxicity. Gastroenterology.
131:165–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shibata W, Maeda S, Hikiba Y, Yanai A,
Sakamoto K, Nakagawa H, Ogura K, Karin M and Omata M: c-Jun
NH2-terminal kinase 1 is a critical regulator for the development
of gastric cancer in mice. Cancer Res. 68:5031–5039. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vivas-Mejia P, Benito JM, Fernandez A, Han
HD, Mangala L, Rodriguez-Aguayo C, Chavez-Reyes A, Lin YG, Carey
MS, Nick AM, et al: c-Jun-NH2-kinase-1 inhibition leads
to antitumor activity in ovarian cancer. Clin Cancer Res.
16:184–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mansouri A, Ridgway LD, Korapati AL, Zhang
Q, Tian L, Wang Y, Siddik ZH, Mills GB and Claret FX: Sustained
activation of JNK/p38 MAPK pathways in response to cisplatin leads
to Fas ligand induction and cell death in ovarian carcinoma cells.
J Biol Chem. 278:19245–19256. 2003. View Article : Google Scholar : PubMed/NCBI
|