Introduction
Although the majority of breast cancers are
currently diagnosed by needle biopsy, there are valid exceptions
for which this may not be possible or necessary, and thus require
surgical excision (1). In addition,
surgical excision is the first biopsy choice for breast cancer in
certain centers, due to cultural reasons. Approximately half of
breast cancer patients in China are diagnosed by excision biopsy
(2). following diagnosis, a
proportion of patients opt directly for surgical excision. Surgical
biopsy of suspicious breast lesions enables histological diagnosis
and facilitates appropriate treatment planning. However, the use of
excision biopsy raises several issues (3,4), among
which is the residual disease that may be associated with a higher
incidence of recurrence. Particularly for patients who are
candidates for breast conservation, the presence of residual
disease may require multiple re-excisions (5).
The incidence of residual disease following initial
excisional biopsy of breast cancer is variable, ranging from 45 to
70% in the literature (6,7). Accurate assessment of residual tumor
following bioptic lumpectomy is crucial for subsequent treatment
planning. Patients with no residual disease following bioptic
lumpectomy may only require excising and pathologically evaluating
the cavity margins instead of aggressive re-excision
mastectomy.
Mammography (MMG) and ultrasound (US) are currently
the most commonly used imaging modalities for the diagnosis of
primary breast cancer, with a fairly high accuracy (8–13).
However, it may be more difficult to detect residual disease
following lumpectomy with these modalities. The most significant
concerns are associated with the fact that the process of wound
healing following surgical excision may lead to changes in breast
architecture (14), which may affect
the diagnostic accuracy of the imaging modalities (15) and the identification of residual
tumor. In the present study, we aimed to evaluate the accuracy of
MMG and breast US in detecting residual tumor following bioptic
lumpectomy in breast cancer patients.
Patients and methods
Ethics statement
Patients with primary breast cancer who were
initially diagnosed by surgical excision followed by re-excision
breast-conserving surgery or mastectomy were recruited to
participate in this study, which was approved by the Institutional
Review Board of Fujian Provincial Tumor Hospital. Written informed
consent was obtained from all the patients prior to their
participation in this study.
Study population
All the primary breast cancer cases from the
surgical pathology files of the Fujian Provincial Tumor Hospital
over a 10-year period (2003–2012) were reviewed. The study
population came from two different sources: A proportion of the
patients were diagnosed at our center, whereas others were referred
to our institution for definitive surgery after undergoing excision
biopsy performed at an external institution. All the patients had
primary breast cancer diagnosed by excision biopsy.
Diagnostic imaging equipment
MMG was performed using two digital full-field
instruments, namely Senographe 2000D (GE Healthcare, Munich,
Germany) and Selenia (Hologic GmbH, Frankfurt am Main,
Germany).
Sonography was performed using a linear transducer
with a 50-mm width and a frequency of 12 MHz, using Philips models
iU22 and HD11 (Philips GmbH Healthcare, Hamburg, Germany).
Real-time spatial compound imaging (CT) in combination with
adaptive image processing (XRES) was a method to analyze
sonographic criteria for the differentiation of benign and
malignant breast lesions.
MMG and breast US were performed to evaluate
residual tumor prior to reoperation (mastectomy or
breast-conserving surgery) as part of routine patient care at the
discretion of the breast surgeon. Magnetic resonance imaging (MRI)
is not routinely used as part of patient care at our center;
therefore, it was not included in this study. MMG evaluation
included standard craniocaudal and mediolateral oblique views. All
the mammograms were reviewed independently by two radiologists
experienced in the interpretation of breast MMG based on the
morphological pattern and density on the MMG images. Similarly,
another two radiologists experienced in the interpretation of
breast US, independently reviewed all US images in terms of echoic
structures and acoustic shadowing. The radiologists were blinded to
any clinical or histopathological information of the patients.
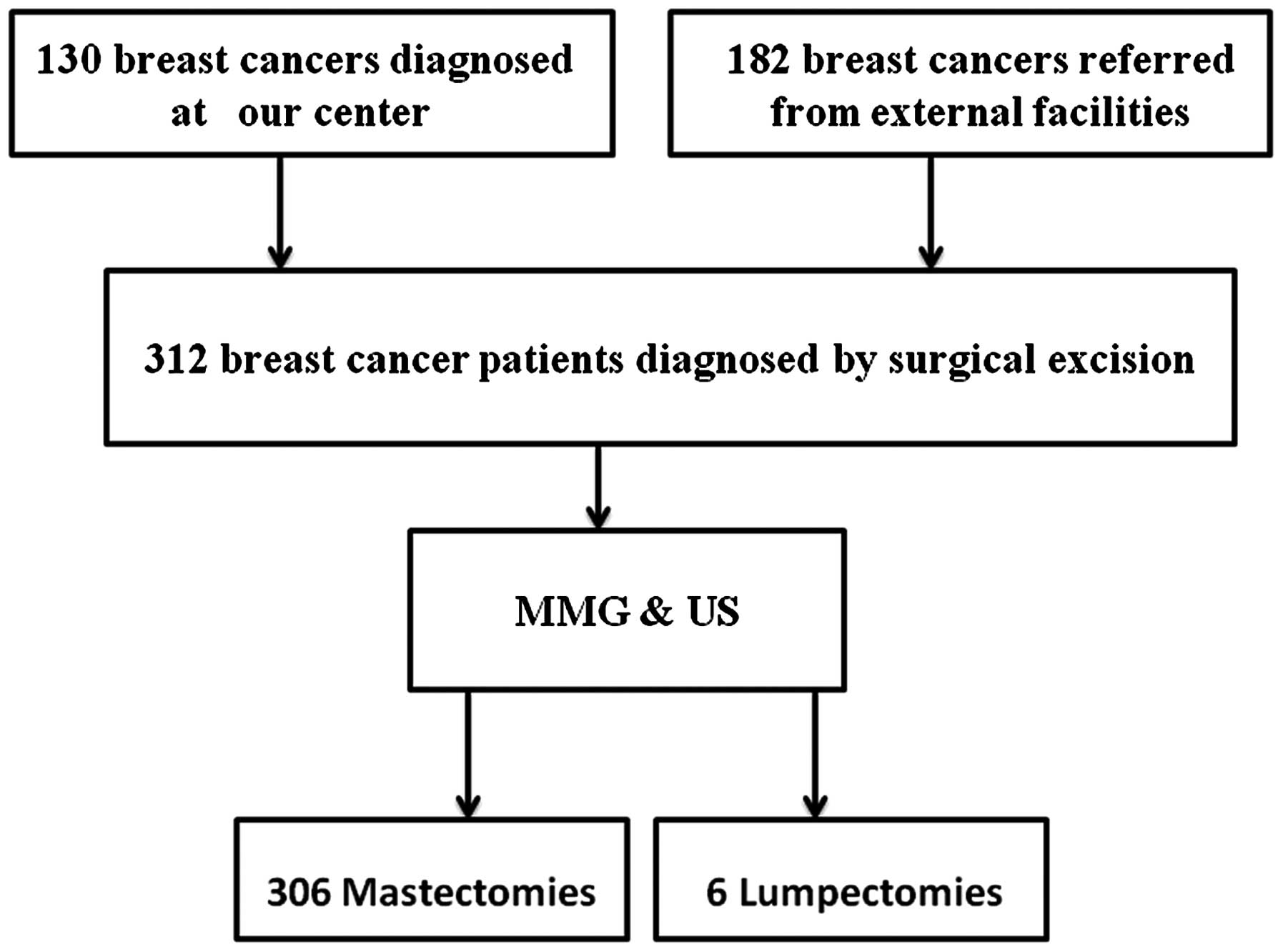
Following diagnostic imaging, all the patients underwent
reoperation (mastectomy or breast-conserving surgery) (Fig. 1). The re-excision specimens were sent
to the histopathology laboratory for histological examination by
two histopathologists specialized in breast pathology. residual
disease was defined as microscopically confirmed invasive or in
situ carcinoma identified within the mastectomy or re-excision
specimens.
Statistical analysis
The diagnostic performance of MMG and breast US was
evaluated by calculating the accuracy, sensitivity and negative
predictive values. Categorical variables were compared between the
two groups using the chi-square test. IBM SPSS software, version 20
(IBM Corp, Armonk, NY, USA) was used for the statistical analyses.
P-values <0.05 were considered to indicate statistically
significant differences.
Results
Patient characteristics
A total of 320 patients were considered as eligible
for this study, 8 of whom did not participate due to scheduling
constraints. Of the 312 eligible patients, 130 (41.7%) were
diagnosed in our center and were clinically and radiologically
considered to have benign disease; however, the subsequent surgical
excision and histological examination revealed a positive result.
The remaining 182 patients (58.3%) were referred from other
institutions following diagnosis; radiological information was not
available for these patients. The median patient age was 49 years
(range, 27–85 years). All the patients underwent MMG and breast US
prior to reoperation. The re-excision procedure was mastectomy in
306 cases and lumpectomy in the remaining 6 cases, based on the
surgeon's discretion and the patient's preference, irrespective of
imaging findings. Due to inherent cultural barriers and cancer
fatalism in Chinese women, the majority of the patients opted for
mastectomy upon breast cancer diagnosis.
Residual disease was confirmed in 118 of 306
patients by final pathology in re-excision mastectomy specimens.
There was no residual disease in the 6 re-excision lumpectomy
specimens. residual invasive ductal carcinoma (IDC) or ductal
carcinoma in situ (DCIS) alone were found in 15.2 and 58.5%
of the cases, respectively, whereas 17.8% of the patients were
allocated to the IDC+DCIS group. Other types of tumors in this
study included residual invasive lobular carcinoma and mucinous
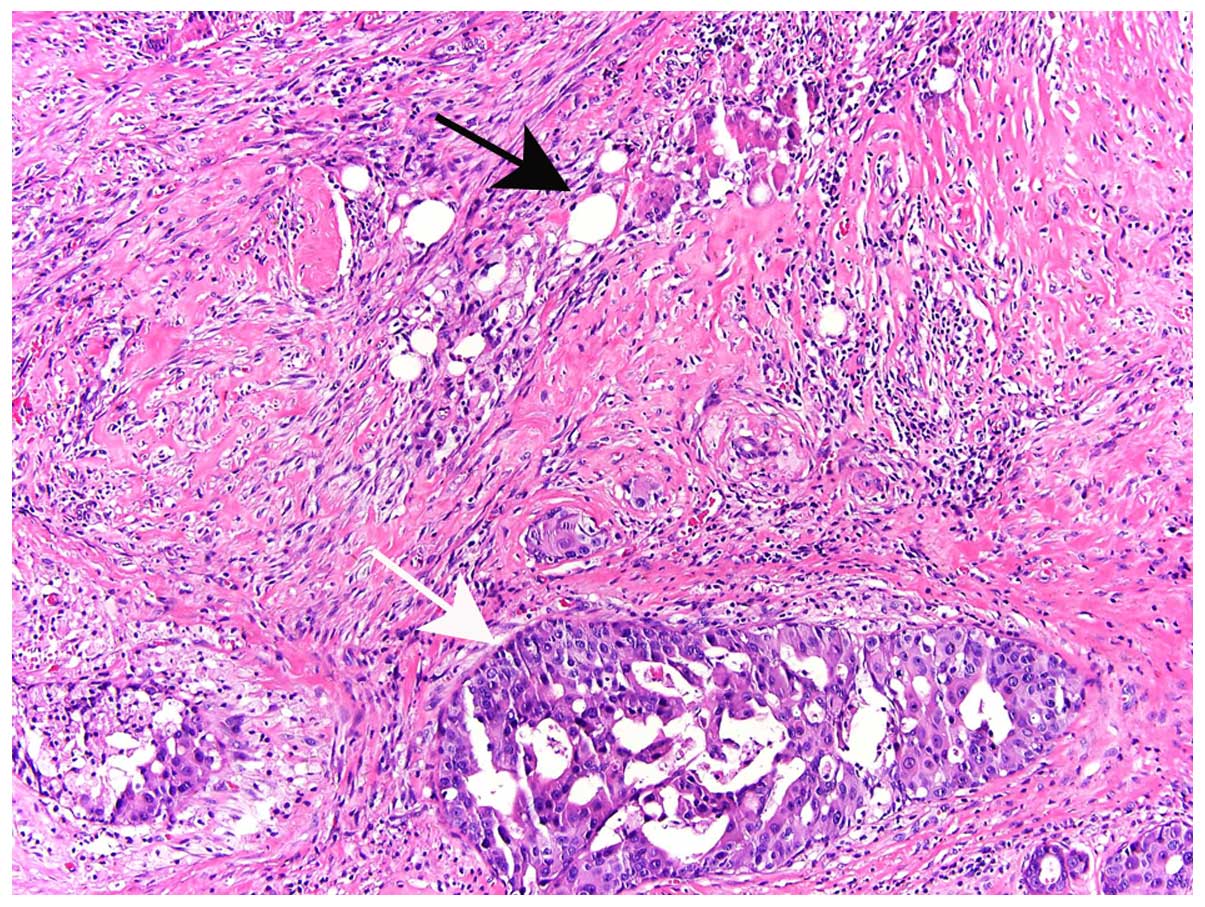
carcinoma, occurred in 8.5% of the cases (Table I). Residual disease was not identified
in 194 of the 312 cases, despite extensive sectioning of the
re-excision specimens (16). Residual
tumor was usually present in adjacent tissues, rather than within
the excision cavity (Fig. 2).
 | Table I.Final pathological diagnosis in cases
with residual disease on re-excision specimens. |
Table I.
Final pathological diagnosis in cases
with residual disease on re-excision specimens.
| Residual disease
cases | Mixed IDC+DCIS | DCIS | IDC | Othersa |
|---|
| 118 | 21 (17.8%) | 69 (58.5%) | 18 (15.2%) | 10 (8.5%) |
Identifying residual disease with
imaging modalities
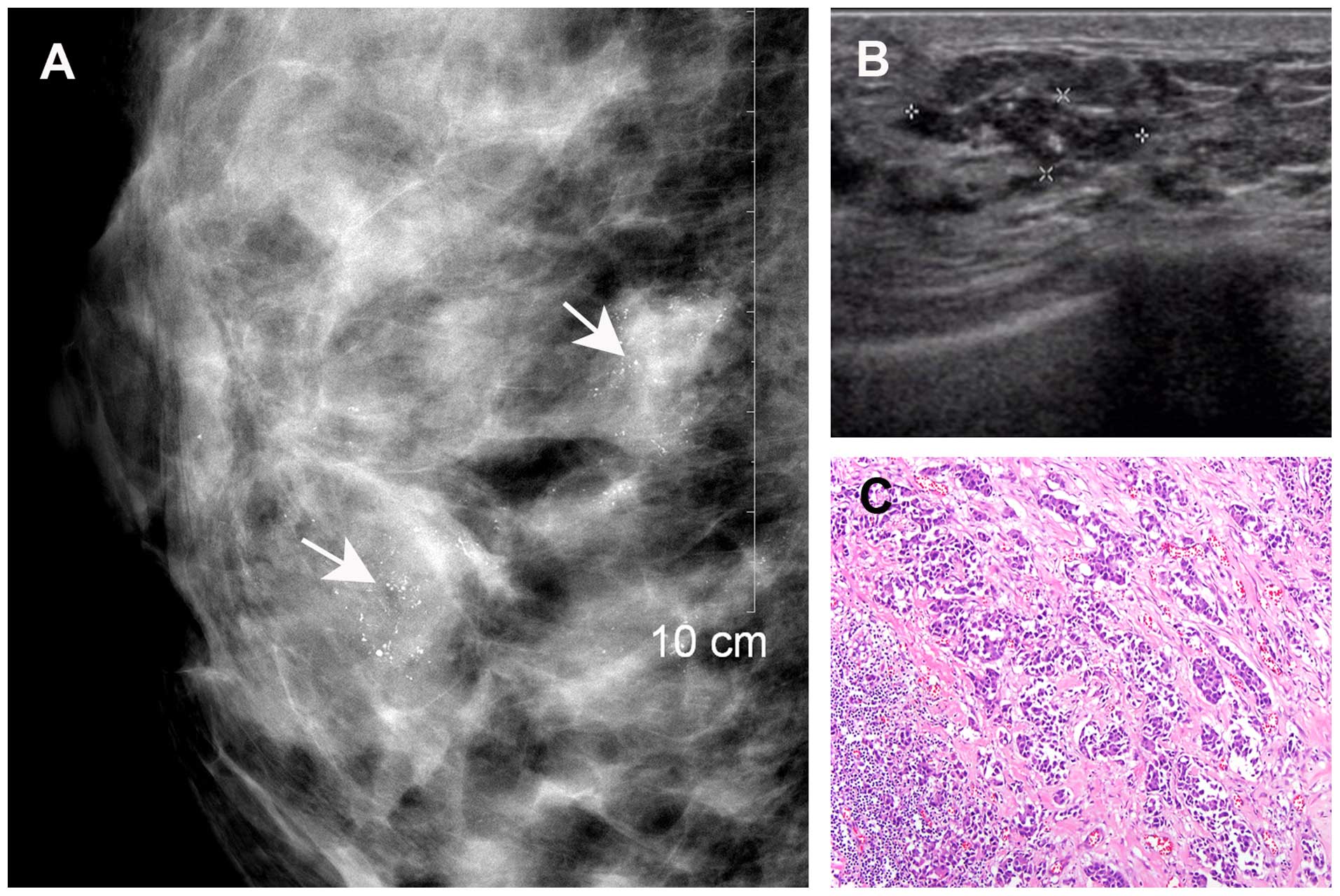
Both MMG and breast US detected residual disease in
20 of the 118 cases that were were correlated with the pathological
findings (Fig. 3). However, 28 cases
with pathologically confirmed residual disease were not detected by
either MMG or US. Of the 86 cases with residual disease identified
by MMG, 77 were correlated with histopathological findings. The
remaining 9 cases did not have residual disease on final pathology.
US detected residual disease in 35 cases, of which 32 were
confirmed by pathology, whereas the remaining 3 cases turned out to
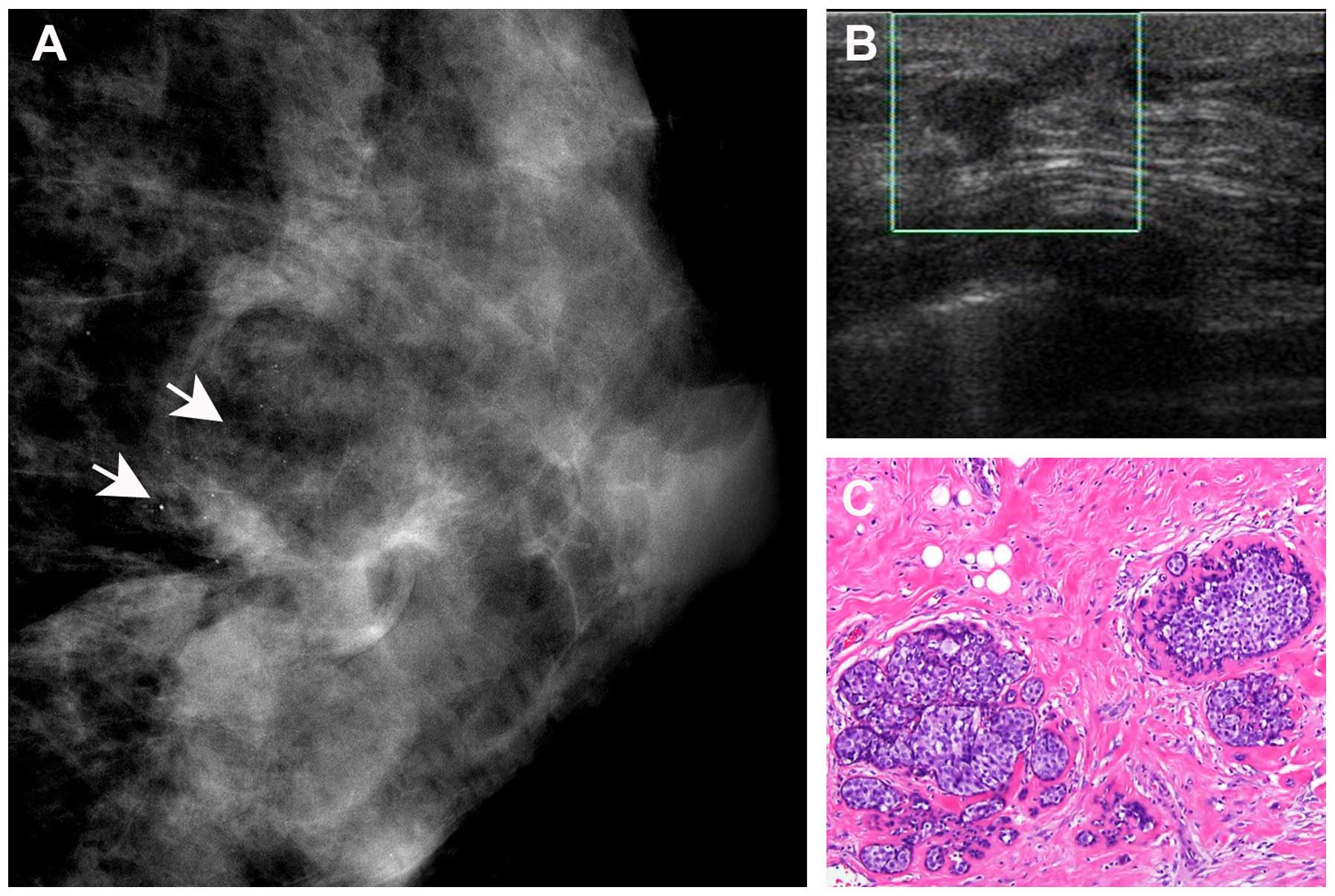
be negative for residual disease on final pathology. A total of 57
cases with residual disease identified on MMG were not detected by
US (Fig. 4). However, 12 cases of
residual disease detected by US were not identified on MMG. As
regards the 89 cases with residual DCIS, 65 were accurately
detected by MMG, whereas only 23 were accurately detected by
US.
Sensitivity and accuracy of MMG and
US
We next evaluated the sensitivity and accuracy of
the two methods for the detection of residual disease in
re-excision specimens. the overall performance of each of the
imaging modalities is summarized in Table IV. MMG was more sensitive, detecting
77/118 (65.3%) of all residual disease cases. This was
significantly superior to US, which detected 32/118 (27.1%)
residual disease cases (P﹤0.001) (Tables
II–IV). as regards the 89 cases
with residual DCIS, the sensitivity of MMG was also significantly
superior to that of US [65/69 (94.2%) vs. 23/69 (33.3%) cases of
residual disease detected, respectively] (Table V). These data clearly demonstrate that
MMG is a reliable method for identifying residual disease,
particularly DCIS.
 | Table IV.Results of data synthesis. |
Table IV.
Results of data synthesis.
|
Characteristics | MMG (%) | US (%) |
P-valuea |
|---|
| Accuracy | 83.9 | 71.5 |
<0.001 |
| Sensitivity | 65.3 | 27.1 |
<0.001 |
| Specificity | 95.4 | 98.5 |
0.07 |
| NPV | 81.9 | 68.9 |
0.001 |
| PPV | 89.5 | 91.4 | 0.5 |
 | Table II.Diagnostic accuracy of MMG in
detecting residual disease. |
Table II.
Diagnostic accuracy of MMG in
detecting residual disease.
|
| residual disease |
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| MMG results | Present | Absent | Total | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|
| Present | 77 |
9 | 86 | 65.3% | 95.4% | 89.5% | 81.9% | 83.9% |
| Absent | 41 | 185 | 226 | (77/118) | (185/194) | (77/86) | (185/226) | (262/312) |
| Total | 118 | 194 | 312 |
|
|
|
|
|
 | Table V.Sensitivity and accuracy of MMG and
US in the detection of residual ductal carcinoma in
situ. |
Table V.
Sensitivity and accuracy of MMG and
US in the detection of residual ductal carcinoma in
situ.
| Variables | MMG (%) | US (%) |
P-valuea |
|---|
| Sensitivity | 94.2 (65/69) | 33.3 (23/69) | <0.001 |
| Accuracy |
95.8
(113/118) | 58.5
(69/118) | <0.001 |
Discussion
Surgical biopsy is widely used in China, due to
inherent cultural barriers and cancer fatalism in Chinese women.
Accurate prediction of residual disease following surgical excision
is crucial for treatment selection. The present study compared MMG
and US in detecting residual disease following bioptic lumpectomy
in Chinese women. Our results demonstrated that the sensitivity,
accuracy and negative predictive value were significantly higher
with MMG compared with US. Further analyses suggested that
sensitivity and accuracy were also higher with MMG regarding the
detection of residual DCIS following bioptic lumpectomy. Therefore,
our data suggest that MMG was more accurate compared with breast US
in identifying residual tumor following bioptic lumpectomy.
Currently, MMG and US are the most commonly used
imaging modalities for primary breast cancer diagnosis, although it
remains unclear which modality is superior for accurate diagnosis
of breast abnormalities. Berg et al (8), Bosch et al (9) and Madjar et al (12) confirmed the sonographic superiority to
MMG in detecting primary tumors. However, studies comparatively
analyzing the diagnostic accuracy of MMG and sonography
demonstrated that the two modalities perform equally well in
detecting primary breast cancer (11,17), or
that MMG is superior to breast US in the accurate diagnosis of
primary breast cancer (2,18). Although it has been reported that US
was more accurate compared with MMG in predicting residual tumor
size following neoadjuvant chemotherapy (19), there is no available literature
regarding the optimal method for accurate assessment of residual
disease following bioptic lumpectomy. Identifying residual tumor
following lumpectomy may be even more challenging. In this
prospective study, we observed that a greater proportion of
residual tumors following bioptic lumpectomy were detected using
MMG rather than US, whereas MMG was more accurate compared with
breast US in identifying residual tumor following bioptic
lumpectomy.
The presence of DCIS was a significant predictor of
an increased likelihood of residual disease at re-excision
following breast-conserving therapy (20–25). In
our series, residual DCIS accounted for 58.5% (69/118) of the
cases, indicating that DCIS is also a risk factor for residual
disease following bioptic lumpectomy. of the 69 residual DCISs, 65
(94.2%) were visible on MMG as abnormal lesions, with or without
microcalcifications. Compared with MMG, only 23 residual disease
cases with DCIS were detected by US, which indicates that MMG may
be more effective compared with US in the detection of residual
DCIS (26,27).
In this study, ~23.7% of the residual tumors could
not be detected by either MMG or US. Since MRI has been proven to
have a better sensitivity for evaluating the extent of breast
cancer and detecting additional breast lesions compared with
conventional visualizing methods (28), MRI is very useful for evaluating
residual disease that cannot be detected using US or MMG. further
investigations should focus on evaluating the accuracy of MRI in
detecting residual disease following bioptic lumpectomy.
The superiority of MMG regarding residual disease
detection following bioptic lumpectomy has not been documented. MMG
in our study cohort was associated with a high detection rate of
residual disease following bioptic lumpectomy. Thus, the use of
MMG-guided stereotactic biopsy may enable complete removal of
malignant foci with clear excision margins, resulting in a reduced
rate of second operations. Moreover, the use of preoperative MMG
may enable accurate evaluation of residual disease size following
bioptic lumpectomy, allowing for selecting eligible candidates for
breast-conserving surgery. Finally, our results further confirmed a
previous study reporting that MMG is useful in early detection of
breast cancer (29).
The present study had certain limitations. First, we
only enrolled breast cancer patients diagnosed by initial surgical
excision followed by mastectomy or lumpectomy, without considering
the time interval between surgical excision and re-excision;
however, the incidence of residual disease in breast cancer may be
affected by the time interval between lumpectomy and subsequent
re-excision (14). Second, a
proportion of the patients were referred from other facilities, and
information on primary tumors, such as palpability, size,
localization and biology, were not available; however, tumor size
and biology are significant predictive factors for residual disease
(30).
Our prospective analysis of the ability of MMG and
breast US to identify residual disease following bioptic
lumpectomy, compared with surgical pathology evaluation of the
residual tumor, demonstrated that 65.3% of residual tumors were
accurately detected using MMG, compared with 27.1% using breast US.
The diagnostic accuracy of MMG was associated with the presence of
residual DCIS. Our results underscore the significance of MMG in
treatment selection and residual disease localization following
bioptic lumpectomy.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81272574), the Natural
Science Foundation of Fujian Province (nos. 2014J01300 and
2014J05086), the Medical Innovation Program of Fujian Province (no.
2012-CXB-7), the Program from Education Bureau of Fujian Province
(no. JB13127), and the Scientific Research Foundation for High
Level Talents in Nanfang Hospital of Southern Medical
University.
References
|
1
|
Corben AD, Edelweiss M and Brogi E:
Challenges in the interpretation of breast core biopsies. Breast J.
16(Suppl 1): S5–S9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osanai T, Gomi N, Wakita T, Yamashita T,
Ichikawa W, Nihei Z and Sugihara K: US-guided core needle biopsy
for breast cancer: Preliminary report. Jpn J Clin Oncol. 30:65–67.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fishman JE, Milikowski C, Ramsinghani R,
Velasquez MV and Aviram G: US-guided core-needle biopsy of the
breast: How many specimens are necessary? Radiology. 226:779–782.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Menes TS, Tartter PI, Bleiweiss I, Godbold
JH, Estabrook A and Smith SR: The consequence of multiple
re-excisions to obtain clear lumpectomy margins in breast cancer
patients. Ann Surg Oncol. 12:881–885. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jardines L, Fowble B, Schultz D, Mackie J,
Buzby G, Torosian M, Daly J, Weiss M, Orel S and Rosato E: Factors
associated with a positive reexcision after excisional biopsy for
invasive breast cancer. Surgery. 118:803–809. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gwin JL, Eisenberg BL, Hoffman JP, Ottery
FD, Boraas M and Solin LJ: Incidence of gross and microscopic
carcinoma in specimens from patients with breast cancer after
re-excision lumpectomy. Ann Surg. 218:729–734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berg WA, Gutierrez L, NessAiver MS, Carter
WB, Bhargavan M, Lewis RS and Ioffe OB: Diagnostic accuracy of
mammography, clinical examination, US and MR imaging in
preoperative assessment of breast cancer. Radiology. 233:830–849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bosch AM, Kessels AG, Beets GL, Rupa JD,
Koster D, van Engelshoven JM and von Meyenfeldt MF: Preoperative
estimation of the pathological breast tumour size by physical
examination, mammography and US: US: A prospective study on 105
invasive tumours. Eur J Radiol. 48:285–292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hieken TJ, Harrison J, Herreros J and
Velasco JM: Correlating sonography, mammography and pathology in
the assessment of breast cancer size. Am J Surg. 182:351–354. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kald BA, Boiesen P, Ronnow K, Jonsson PE
and Bisgaard T: Preoperative assessment of small tumours in women
with breast cancer. Scand J Surg. 94:15–20. 2005.PubMed/NCBI
|
|
12
|
Madjar H, Ladner HA, Sauerbrei W,
Oberstein A, Prömpeler H and Pfleiderer A: Preoperative staging of
breast cancer by palpation, mammography and high-resolution US. US
Obstet Gynecol. 3:185–190. 1993.
|
|
13
|
Yang WT, Lam WW, Cheung H, Suen M, King WW
and Metreweli C: Sonographic, magnetic resonance imaging and
mammographic assessments of preoperative size of breast cancer. J
US Med. 16:791–797. 1997.
|
|
14
|
Wiley EL, Diaz LK, Badve S and Morrow M:
Effect of time interval on residual disease in breast cancer. Am J
Surg Pathol. 27:194–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wiratkapun C, Wibulpholprasert B,
Wongwaisayawan S and Pulpinyo K: Nondiagnostic core needle biopsy
of the breast under imaging guidance: Result of rebiopsy. J Med
Assoc Thai. 88:350–357. 2005.PubMed/NCBI
|
|
16
|
Wiley E and Keh P: Diagnostic
discrepancies in breast specimens subjected to gross reexamination.
Am J Surg Pathol. 23:876–879. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang JT, Chang LM, Song X, Zhao LX, Li JT,
Zhang WG, Ji YB, Cai LN, Di W and Yang XY: Comparison of primary
breast cancer size by mammography and sonography. Asian Pac J
Cancer Prev. 15:9759–9761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Golshan M, Fung BB, Wiley E, Wolfman J,
Rademaker A and Morrow M: Prediction of breast cancer size by US,
mammography and core biopsy. Breast. 13:265–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keune JD, Jeffe DB, Schootman M, Hoffman
A, Gillanders WE and Aft RL: Accuracy of ultrasonography and
mammography in predicting pathologic response after neoadjuvant
chemotherapy for breast cancer. Am J Surg. 199:477–484. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boyages J, Recht A, Connolly J, Schnitt
SJ, Gelman R, Kooy H, Love S, Osteen RT, Cady B, Silver B, et al:
Early breast cancer: Predictors of breast recurrence for patients
treated with conservative surgery and radiation therapy. Radiother
Oncol. 19:29–41. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan KC, Knox WF, Sinha G, Gandhi A, Barr
L, Baildam AD and Bundred NJ: Extent of excision margin width
required in breast conserving surgery for ductal carcinoma in situ.
Cancer. 91:9–16. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holland R, Connolly JL, Gelman R, Mravunac
M, Hendriks JH, Verbeek AL, Schnitt SJ, Silver B, Boyages J and
Harris JR: The presence of an extensive intraductal component
following a limited excision correlates with prominent residual
disease in the remainder of the breast. J Clin Oncol. 8:113–118.
1990.PubMed/NCBI
|
|
23
|
Aziz D, Rawlinson E, Narod SA, Sun P,
Lickley HL, McCready DR and Holloway CM: The role of reexcision for
positive margins in optimizing local disease control after
breast-conserving surgery for cancer. Breast J. 12:331–337. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schnitt SJ, Connolly JL, Khettry U,
Mazoujian G, Brenner M, Silver B, Recht A, Beadle G and Harris JR:
Pathologic findings on re-excision of the primary site in breast
cancer patient considered for treatment by primary radiation
therapy. Cancer. 59:675–681. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Solin LJ, Fourquet A, Vicini FA, Haffty B,
Taylor M, McCormick B, McNeese M, Pierce LJ, Landmann C, Olivotto
IA, et al: Mammographically detected ductal carcinoma in situ of
the breast treated with breast-conserving surgery and definitive
breast irradiation: Long-term outcome and prognostic significance
of patient age and margin status. Int J Radiat Oncol Biol Phys.
50:991–1002. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cilotti A, Bagnolesi P, Moretti M,
Gibilisco G, Bulleri A, Macaluso AM and Bartolozzi C: Comparison of
the diagnostic performance of high-frequency US as a first- or
second-line diagnostic tool in non-palpable lesions of the breast.
Eur Radiol. 7:1240–1244. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jackson VP: The current role of
ultrasonography in breast imaging. Radiol Clin North Am.
33:1161–1170. 1995.PubMed/NCBI
|
|
28
|
Wasif N, Garreau J, Terando A, Kirsch D,
Mund DF and Giuliano AE: MRI versus ultrasonography and mammography
for preoperative assessment of breast cancer. Am Surg. 75:970–975.
2009.PubMed/NCBI
|
|
29
|
Ernster VL and Barclay J: Increases in
ductal carcinoma in situ (DCIS) of the breast in relation to
mammography: A dilemma. J Natl Cancer Inst Monogr. 22:151–156.
1997.PubMed/NCBI
|
|
30
|
Miller AR, Brandao G, Prihoda TJ, Hill C,
Cruz AB Jr and Yeh IT: Positive margins following surgical
resection of breast carcinoma: Analysis of pathologic correlates. J
Surg Oncol. 86:134–140. 2004. View Article : Google Scholar : PubMed/NCBI
|