Introduction
Numerous studies and reviews on circulating tumor
cells (CTCs) have been found to be critically useful for clinical
application in the diagnosis, therapy and research of cancer
(1–14). CTCs are known to exist in the
peripheral blood at an extremely low concentration and are
difficult to isolate from the blood. Subsequently, a number of
efforts have been made to develop devices and apparatus that are
able to isolate CTCs. Our previous study designed and fabricated a
novel CTC isolation device, a polymeric microfluidic device termed
the ‘CTC-chip’, which is now commercially available (15). Although most microfluidic CTC
isolation devices, including the conventional CTC-chip, are made of
silicon or polydimethylsiloxane (16–19), these
materials are not necessarily suitable for clinical applications in
terms of cost, producibility and their material properties. The
current polymeric devices are comprised of light-curable resins,
which were formulated for easy production and clinical usability,
and our previous study demonstrated that the device overcame
practical usage issues faced by conventional chips. The device
captures cancer cells by targeting the epithelial cell adhesion
molecule (EpCAM) expressed on the surface of cancer cells using an
immobilized anti-EpCAM antibody. As EpCAM expression is considered
heterogeneous among CTCs from within a single patient and varies
based on cancer type, it is important to estimate the influence of
EpCAM expression on the efficiency of capture by the device. The
present study evaluated the efficiency of capture by use of
esophageal and breast cancer cell lines. The esophageal cancer cell
lines were chosen as they exhibit different EpCAM expression levels
measured by flow cytometry for the evaluation. As for the breast
cancer cell lines, attention was focused not only on EpCAM
expression but also on subtypes of breast cancer and used cell
lines representing estrogen receptor positive/progesterone receptor
positive (ER+/PR+), human epidermal growth
factor receptor 2 (HER2+) and triple-negative breast
cancers.
Materials and methods
Cancer cell lines and preparation of
cell suspensions
Esophageal cancer cell lines KYSE150, KYSE220 and
KYSE510 were kindly provided by Dr Yutaka Shimada (one of the
authors of this manuscript), and breast cancer cell lines MCF7,
SKBR3 and MDA-MB-231 were obtained from the American Type Culture
Collection (Manassas, VA, USA). EpCAM expression levels were
measured in the esophageal cancer lines using a BD FACSCanto flow
cytometer (Becton-Dickinson, San Jose, CA, USA), mouse anti-human
EpCAM antibody (cat. no. sc-59906; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and PE anti-mouse immunoglobulin G (IgG)
antibody (cat. no. IM0551; Beckman Coulter, Brea, CA, USA).
For capture efficiency measurement, cancer cells
were fluorescently labeled with CellTrace (Life Technologies,
DriveRockville, MD, USA) and were either spiked into
phosphate-buffered saline (PBS) containing 1% bovine serum albumin
or whole blood, which had been drawn from a healthy donor
(following approval by the Ethics Comittee of the University of
Toyama and after obtaining written informed concent) and stored in
a vacuum blood collection tube containing
ethylenediaminetetraacetic acid. The cell suspensions were prepared
at concentrations between 100 and 400 cells/ml.
Immunostaining of MDA-MB-231 and KYSE510 was
performed using mouse anti-EpCAM and Cy3 goat anti-mouse IgG
antibodies (cat. no. CLCC35010; Cedarlane, Hornby, ON, Canada).
Cells cultured in 96-well microplates were fixed in 4%
paraformaldehyde for 30 min and washed with PBS. Mouse anti-EpCAM
antibody at a concentration of 20 µg/ml was applied to the cells
for 2 h at room temperature. Cells were washed with PBS and stained
with Cy3 goat anti-mouse IgG antibody at a concentration of 4
µg/ml. Following a final wash with PBS, fluorescent microscopic
images were captured using a digital camera.
Preparation of the polymeric
CTC-chip
Production of the polymeric CTC-chip and antibody
coating of the chip surface were carried out as described
previously (15). The microstructure
of the chip consisted chiefly of an array of two different types of
microposts, modified from the previous design to prevent clogging
by whole blood. The gap between microposts was enlarged to 200 µm
in the area around the chip inlet. Goat anti-mouse IgG antibody
(cat. no. 1032-01; Southern Biotech, Birmingham, AL, USA) and mouse
anti-human EpCAM antibody were used for chip coating.
Evaluation of cell capture efficiency
by the polymeric CTC-chip
The polymeric CTC-chip was set in a holder and the
efficiency of capture was evaluated using the method described
previously with the cancer cell suspensions. Efficiency of capture
was calculated by measuring the number of cells remaining on the
chip following sample passage compared to the number of cells that
passed through the chip inlet.
Results
EpCAM expression levels
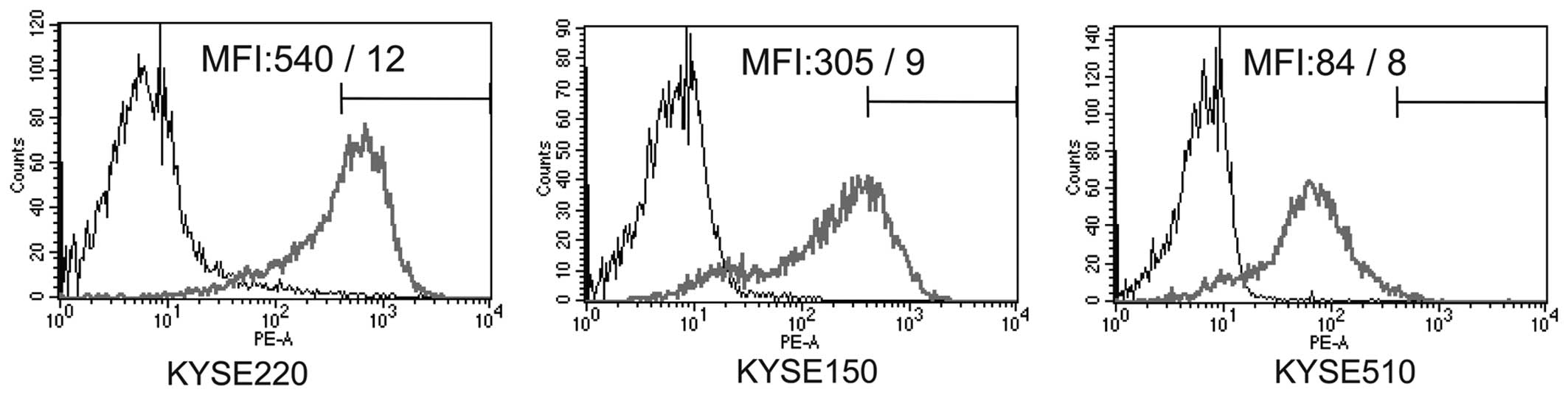
EpCAM expression levels in the esophageal cancer
cell lines were measured by flow cytometry and represented as
relative mean fluorescent intensity (MFI), which changed between
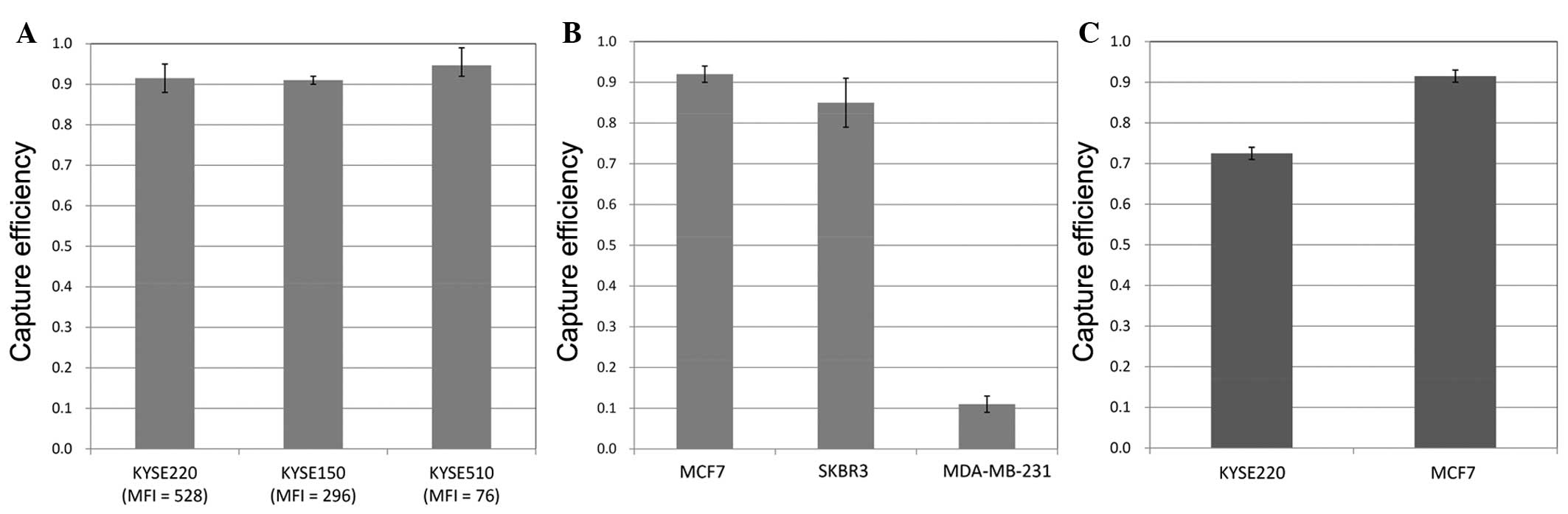
528 and 76 for the different cell lines (Fig. 1). Capture efficiencies of the
esophageal cancer cell lines in PBS were ~0.9 irrespective of EpCAM
expression levels (Fig. 2A). In the
breast cancer cell lines, MCF7 and SKBR3 in PBS were efficiently
captured, however a low value of ~0.1 was obtained for MDA-MB-231
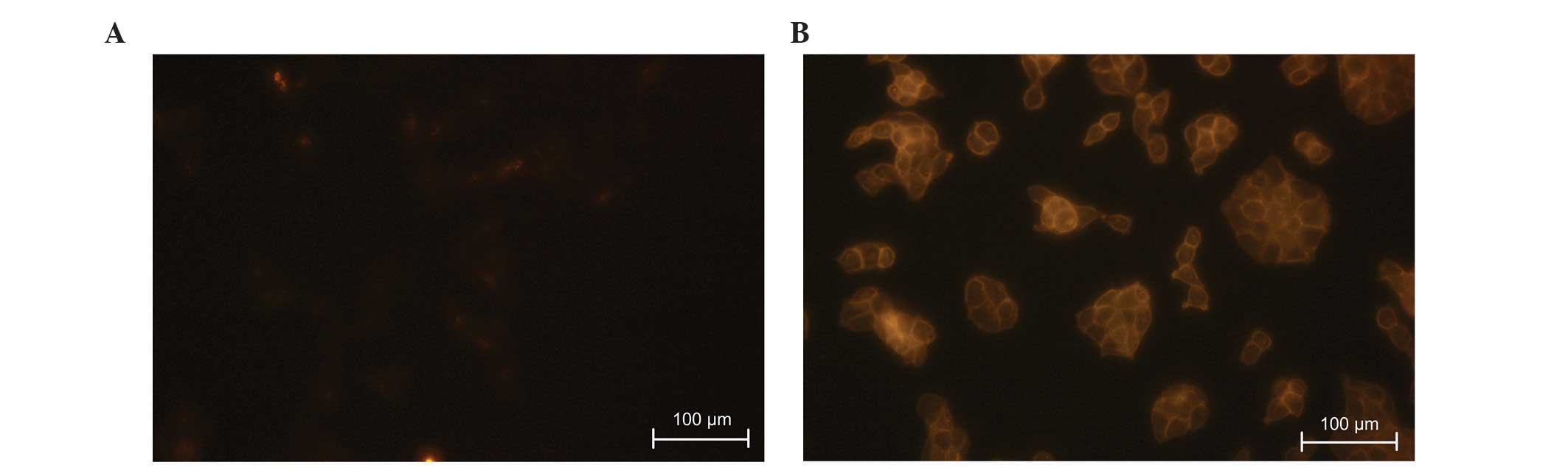
(Fig. 2B). Fluorescent images of
immunostained MDA-MB-231 and KYSE510 were captured and compared to
roughly estimate EpCAM expression level of MDA-MB-231 (Fig. 3).
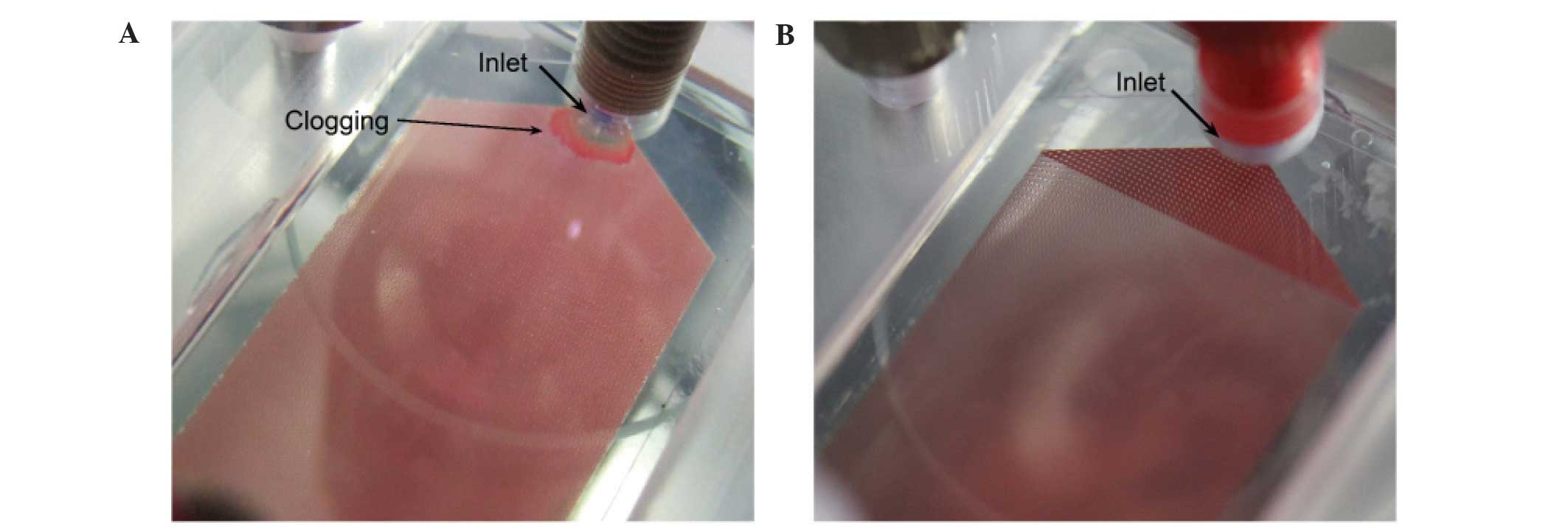
There was no clogging of the modified chip when
using whole blood (Fig. 4) and the
efficiency of cell capture was successfully evaluated. Capture
efficiencies for KYSE220 and MCF7 in whole blood were >0.7, but
were of either equal or lesser efficiency in comparison to PBS
(Fig. 2C).
Discussion
In the present study, the polymeric CTC-chip
efficiently captured esophageal and breast cancer cells except for
MDA-MB-231 in PBS. All the esophageal cancer cells used exhibited
an MFI between 528 and 76, indicating EpCAM expression, with the
lowest EpCAM-expressing cell line, KYSE510, still efficiently
captured as shown by the bright image of the stained cells in
Fig. 3B. The breast cancer lines MCF7
and SKBR3 have been previously shown to express sufficient EpCAM to
allow efficient capture by other microfluidic devices (19–21), which
was in accordance with the present results. Capture efficiency of
SKBR3 by conventional CTC-chip was lower than the polymeric chip.
Capture efficiency of MDA-MB-231 was extremely low and appeared
reasonable due to marginal EpCAM expression, as shown in Fig. 3A. Previous studies have confirmed
MDA-MB-231 to have extremely low EpCAM expression (22–25).
However, the capture efficiency obtained for MDA-MBA-231 suggests
that the extremely low level of EpCAM still had a role in capture
due to obtaining a capture efficiency of only 0.02 when coating the
polymeric CTC-chip with only anti-mouse IgG antibody. Low capture
efficiencies of MDA-MB-231 and other cancer cell lines exhibiting
downregulation of EpCAM, often caused by epithelial-mesenchymal
transition (EMT), have also been observed in PBS or whole blood
using other microfluidic devices or methods utilizing
antibody-based capture of EpCAM (20,21,23).
Recently, other microfluidic devices have targeted cell surface
antigens, such as prostate-specific membrane antigen (PSMA), HER2
and epidermal growth factor receptor (EGFR) by incorporating
antibodies against these antigens, and have successfully isolated
CTCs from the blood of cancer patients (20,26,27).
MDA-MB-231 cells have been reported to be captured efficiently by a
microfluidic device using anti-EGFR antibody, and this modification
may be effective in the polymeric CTC-chip and easy to apply.
Various antibodies are able to be simply attached to the chip
surface by initial anti-IgG antibody bonding. However, these cell
surface antigens are only able to target a narrow range of cancer
type, such as PSMA to prostate cancer, and are known to change
expression by EMT; therefore, universal targets for efficient
capture of a wide range of CTCs are still required. Much
challenging work is still required to find ideal markers for the
identification of cancer cells, which may subsequently be used as
targets for capture (6).
Although whole blood samples caused the polymeric
CTC-chip to clog in our previous study, whole blood was able to
pass through the modified chip to successfully evaluate the
efficiency of cell capture. This change was due to enlargement of
the gap between microposts in the modified chip. Shear stress at
the interface between the microposts and whole blood sample
decreased by gap enlargement and local instances of blood
coagulation appeared to be reduced, resulting in reduction of
clogging.
As determined in the present study, capture
efficiency may be generally lower in the whole blood compared to
PBS. However, further studies must be carried out to clarify this
issue, due to efficiency being influenced by numerous factors, such
as viscosity, adsorption of cells and proteins, leading to
complications.
Acknowledgements
The present study was supported by the Grant-in-Aid
for Scientific Research (no. 25350582).
References
|
1
|
Sheng W, Ogunwobi OO, Chen T, Zhang J,
George TJ, Liu C and Fan ZH: Capture, release and culture of
circulating tumor cells from pancreatic cancer patients using an
enhanced mixing chip. Lab Chip. 14:89–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babayan A, Hannemann J, Spötter J, Müller
V, Pantel K and Joosse SA: Heterogeneity of estrogen receptor
expression in circulating tumor cells from metastatic breast cancer
patients. PLoS One. 8:e750382013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Liu Q, Wang T, Bian L, Zhang S, Hu
H, Li S, Hu Z, Wu S, Liu B, et al: Circulating tumor cells in
HER2-positive metastatic breast cancer patients: A valuable
prognostic and predictive biomarker. BMC Cancer. 13:2022013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onstenk W, Gratama JW, Foekens JA and
Sleijfer S: Towards a personalized breast cancer treatment approach
guided by circulating tumor cell (CTC) characteristics. Cancer
Treat Rev. 39:691–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torino F, Bonmassar E, Bonmassar L, De
Vecchis L, Barnabei A, Zuppi C, Capoluongo E and Aquino A:
Circulating tumor cells in colorectal cancer patients. Cancer Treat
Rev. 39:759–772. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokobori T, Iinuma H, Shimamura T, Imoto
S, Sugimachi K, Ishii H, Iwatsuki M, Ota D, Ohkuma M, Iwaya T, et
al: Plastin3 is a novel marker for circulating tumor cells
undergoing the epithelial-mesenchymal transition and is associated
with colorectal cancer prognosis. Cancer Res. 73:2059–2069. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Franken B, de Groot MR, Mastboom WJB,
Vermes I, van der Palen J, Tibbe AGJ and Terstappen LWMM:
Circulating tumor cells, disease recurrence and survival in newly
diagnosed breast cancer. Breast Cancer Res. 14:R1332012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou JM, Krebs MG, Lancashire L, Sloane R,
Backen A, Swain RK, Priest LJC, Greystoke A, Zhou C, Morris K, et
al: Clinical significance and molecular characteristics of
circulating tumor cells and circulating tumor microemboli in
patients with small-cell lung cancer. J Clin Oncol. 30:525–532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsusaka S, Suenaga M, Mishima Y,
Kuniyoshi R, Takagi K, Terui Y, Mizunuma N and Hatake K:
Circulating tumor cells as a surrogate marker for determining
response to chemotherapy in Japanese patients with metastatic
colorectal cancer. Cancer Sci. 102:1188–1192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park Y, Kitahara T, Urita T, Yoshida Y and
Kato R: Expected clinical applications of circulating tumor cells
in breast cancer. World J Clin Oncol. 2:303–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stott SL, Lee RJ, Nagrath S, Yu M,
Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z,
et al: Isolation and characterization of circulating tumor cells
from patients with localized and metastatic prostate cancer. Sci
Transl Med. 2:25ra232010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okegawa T, Nutahara K and Higashihara E:
Association of circulating tumor cells with tumor-related
methylated DNA in patients with hormone-refractory prostate cancer.
Int J Urol. 17:466–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okegawa T, Nutahara K and Higashihara E:
Prognostic significance of circulating tumor cells in patients with
hormone refractory prostate cancer. J Urol. 181:1091–1097. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wülfing P, Borchard J, Buerger H, Heidl S,
Zänker KS, Kiesel L and Brandt B: HER2-positive circulating tumor
cells indicate poor clinical outcome in stage I to III breast
cancer patients. Clin Cancer Res. 12:1715–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohnaga T, Shimada Y, Moriyama M, Kishi H,
Obata T, Takata K, Okumura T, Nagata T, Muraguchi A and Tsukada K:
Polymeric microfluidic devices exhibiting sufficient capture of
cancer cell line for isolation of circulating tumor cells. Biomed
Microdevices. 15:611–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dickson Nora M, Tsinberg P, Tang Z,
Bischoff FZ, Wilson T and Leonard EF: Efficient capture of
circulating tumor cells with a novel immunocytochemical
microfluidic device. Biomicrofluidics. 5:34119–3411915. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stott SL, Hsu CH, Tsukrov DI, Yu M,
Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK,
et al: Isolation of circulating tumor cells using a
microvortex-generating herringbone-chip. Proc Natl Acad Sci USA.
107:18392–18397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gleghorn JP, Pratt ED, Denning D, Liu H,
Bander NH, Tagawa ST, Nanus DM, Giannakakou PA and Kirby BJ:
Capture of circulating tumor cells from whole blood of prostate
cancer patients using geometrically enhanced differential
immunocapture (GEDI) and a prostate-specific antibody. Lab Chip.
10:27–29. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, et al: Isolation of rare circulating tumour cells in cancer
patients by microchip technology. Nature. 450:1235–1239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Punnoose EA, Atwal SK, Spoerke JM, Savage
H, Pandita A, Yeh RF, Pirzkall A, Fine BM, Amler LC, Chen DS, et
al: Molecular biomarker analyses using circulating tumor cells.
PLoS One. 5:e125172010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martowicz A, Spizzo G, Gastl G and
Untergasser G: Phenotype-dependent effects of EpCAM expression on
growth and invasion of human breast cancer cell lines. BMC Cancer.
12:5012012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pecot CV, Bischoff FZ, Mayer JA, Wong KL,
Pham T, Bottsford-Miller J, Stone RL, Lin YG, Jaladurgam P, Roh JW,
et al: A novel platform for detection of CK+ and CK- CTCs. Cancer
Discov. 1:580–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sieuwerts AM, Kraan J, Bolt J, van der
Spoel P, Elstrodt F, Schutte M, Martens JWM, Gratama JW, Sleijfer S
and Foekens JA: Anti-epithelial cell adhesion molecule antibodies
and the detection of circulating normal-like breast tumor cells. J
Natl Cancer Inst. 101:61–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prang N, Preithner S, Brischwein K, Göster
P, Wöppel A, Müller J, Steiger C, Peters M, Baeuerle PA and da
Silva AJ: Cellular and complement-dependent cytotoxicity of
Ep-CAM-specific monoclonal antibody MT201 against breast cancer
cell lines. Br J Cancer. 92:342–349. 2005.PubMed/NCBI
|
|
26
|
Galletti G, Sung MS, Vahdat LT, Shah MA,
Santana SM, Altavilla G, Kirby BJ and Giannakakou P: Isolation of
breast cancer and gastric cancer circulating tumor cells by use of
an anti HER2-based microfluidic device. Lab Chip. 14:147–156. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kirby BJ, Jodari M, Loftus MS, Gakhar G,
Pratt ED, Chanel-Vos C, Gleghorn JP, Santana SM, Liu H, Smith JP,
et al: Functional characterization of circulating tumor cells with
a prostate-cancer-specific microfluidic device. PLoS One.
7:e359762012. View Article : Google Scholar : PubMed/NCBI
|