Introduction
Multiple myeloma (MM) is a plasma cell tumor, which
has differentiated from B lymphocytes, and the disease is
characterized by the production of monoclonal immunoglobulin (Ig).
Previously, several novel drugs for the treatment of MM have been
developed and the clinical outcome has significantly improved.
Examples of these are bortezomib, a proteasome inhibitor, and the
immunomodulatory drugs (e.g. thalidomide and lenalidomide).
Lenalidomide, a derivative of thalidomide, has been demonstrated to
induce immune responses, including cytokine production, and the
activation of natural killer (NK) cells and T cells, to exert
antitumor effects directly on MM cells by arresting the cell cycle,
and to induce decreases of interleukin (IL)-6 and vascular
endothelial growth factor levels in the microenvironment of the
tumor (1). This has given rise to a
beneficial clinical effect in patients with MM (2). In a clinical trial (MM-009/010), the
time to progression and overall survival were significantly
prolonged in patients treated with lenalidomide in combination with
dexamethasone, compared with those with dexamethasone alone, and
the clinical efficacy was also confirmed (3). In earlier clinical studies, lenalidomide
monotherapy was revealed to be useful as maintenance therapy for
elderly patients (4) or
post-transplant therapy (5,6). Although it is hypothesized that
lenalidomide therapy activates immune cells (7) and leads to good clinical efficacy.
Limited data to show the direct correlation between the increase of
immune cells and the clinical effects of lenalidomide in MM
patients is currently available.
The present study reported a case of refractory MM
with the emergence of large granular lymphocytes (LGLs) during
lenalidomide treatment. Lenalidomide revealed efficacy to suppress
serum M-protein level and tumor size in this case. This suggested a
direct correlation between the proliferation of LGLs and the
therapeutic effect of lenalidomide.
Case report
A 72-year-old Japanese male patient consulted a
doctor for constipation. Laboratory examination revealed a high
serum IgG level (3,305 mg/dl) with IgG κ-type M-protein. However,
aspiration analysis of bone marrow cells revealed no increase of
plasma cells. The patient was diagnosed with monoclonal gammopathy
of undetermined significance and was followed up without therapy.
Three years later, the patient progressed to symptomatic MM.
Subsequently, melphalan + prednisolone (MP) was administered as
first-line chemotherapy. Following MP chemotherapy for ~6 years,
the patient complained of lower back pain and was revealed by CT
examination to exhibit vertebral mass lesions. The patient was
subsequently referred to St. Mary's Hospital (Fukuoka, Japan).
Since the patient was judged to exhibit refractory MM, he
subsequently received radiation therapy on the lumbar spine. Serum
IgG levels were elevated to 3,048 mg/dl, while the proportion of
myeloma cells was 15.2% in the bone marrow. No chromosomal
abnormalities were detected. Durie-Salmon stage classification was
IIA and International Staging System classification was I. As the
disease activity failed to be controlled by MP and radiation
therapy, the patient was enrolled in a clinical trial and received
lenalidomide (15 mg, days 1–21 of a 28-day cycle) + low-dose
dexamethasone (20 mg/week) (Rd) therapy. This clinical study was
designed to investigate the maximum optimal dosage and efficacy of
lenalidomide (UMIN Clinical Trials Registry number, UMIN000010178),
and treatment by the administration of lenalidomide + dexamethasone
was initiated (Fig. 1).
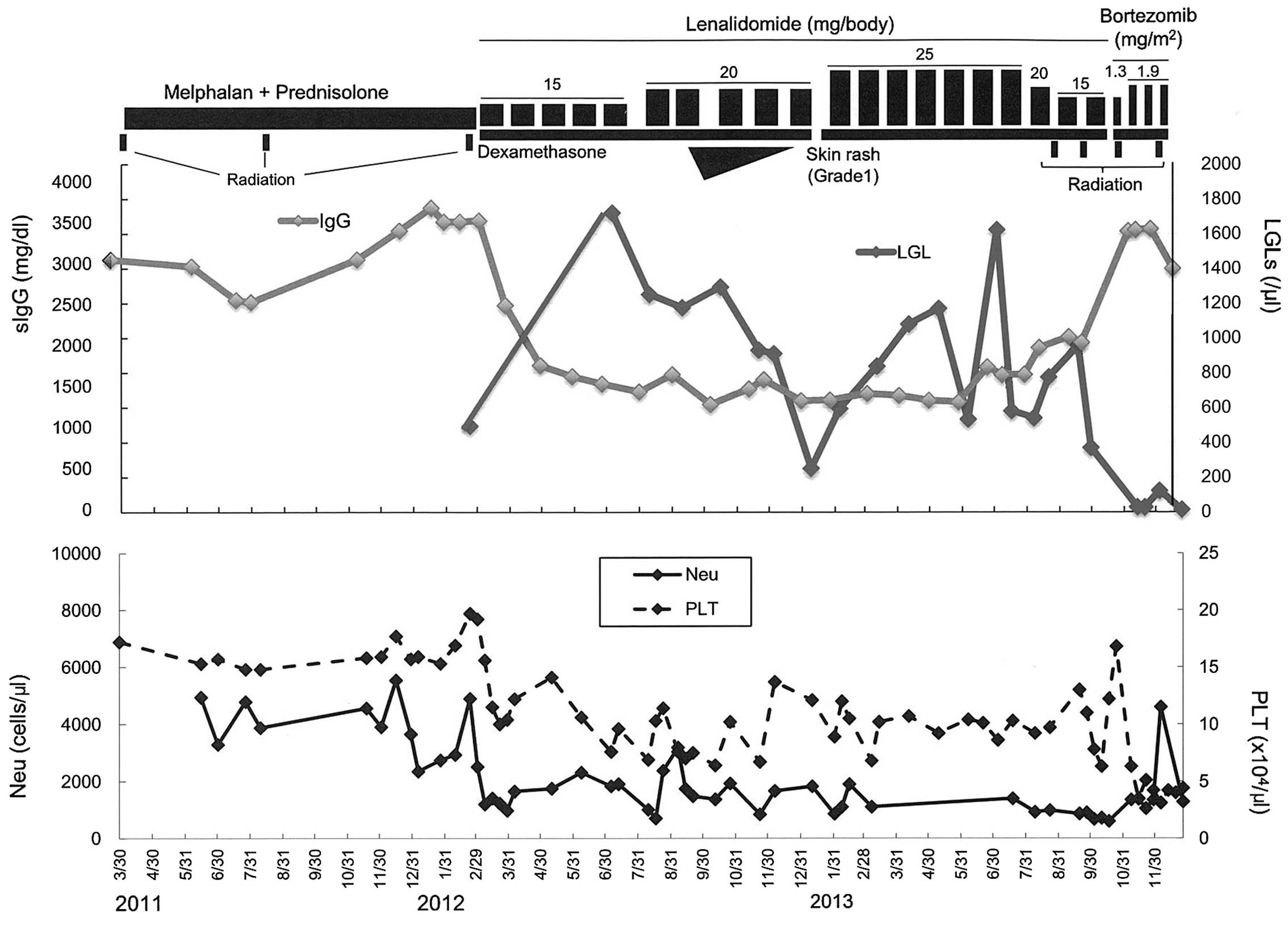 | Figure 1.Clinical course of the present case.
An inverse correlation was observed between the serum IgG and LGL
levels. MP was administered as first-line chemotherapy. The patient
subsequently received radiation therapy on the lumbar spine. As the
disease activity had failed to be controlled by MP and radiation
therapy, the patient was enrolled in a clinical trial and received
Rd therapy. A low level of IgG was sustained during Rd therapy for
~15 months, associated with the increase of LGLs in peripheral
blood. As extramedullary tumor was observed 15 months following the
initiation of Rd therapy, BD therapy was started in addition to
local radiation therapy. Notably, the number of LGLs decreased and
serum IgG level was increased following BD treatment and radiation.
MP, melphalan + prednisolone; Rd, lenalidomide + low-dose
dexamethasone; BD, bortezomib + dexamethasone: LGL, large granular
lymphocyte; sIgG; serum immunoglobulin G; Neu, neutrophil; PLT,
platelet. |
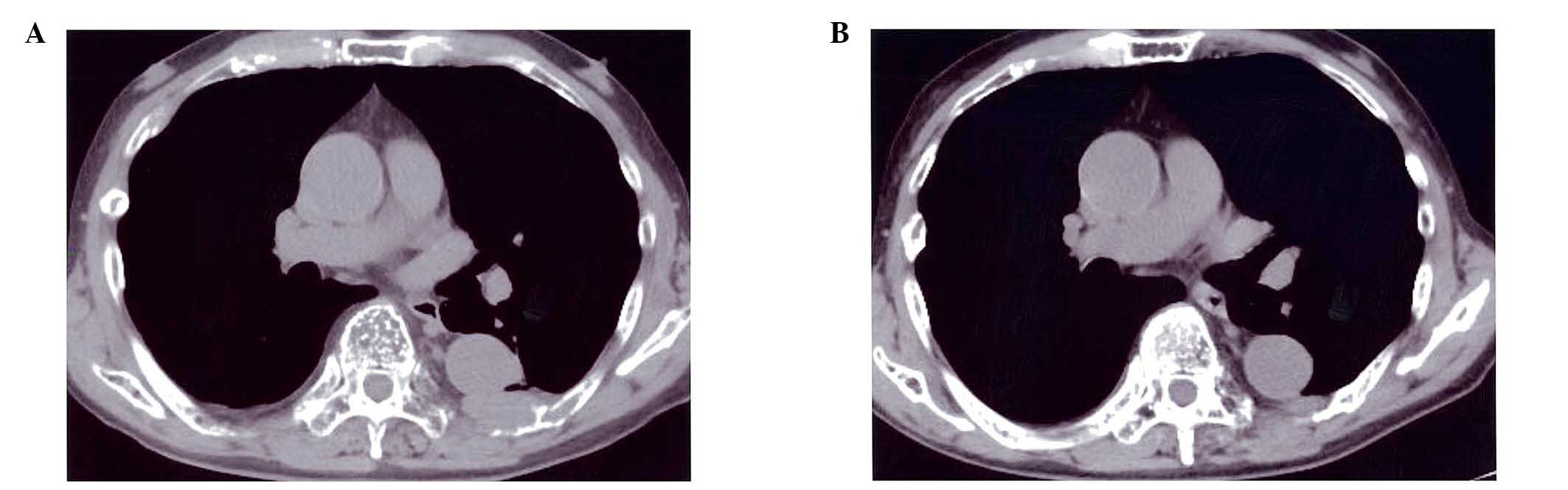
Serum IgG gradually decreased to 1,530 mg/dl and
reduction of the size of the bone tumors was observed (Fig. 2A and B). At this time, LGLs, which
were defined as being more than double the size of red blood cell
with abundant cytoplasm containing azurophilic granules (Fig. 3A), increased to 25–40% of peripheral
blood leukocytes, although the LGLs were present in blood (~8%)
prior to lenalidomide treatment. The patient achieved very good
partial regression following four cycles of Rd therapy. In terms of
the adverse effect of lenalidomide, skin rash (Grade 1) appeared;
however, it subsided spontaneously upon the continuation of
lenalidomide. A low level of IgG was sustained for ~15 months. Flow
cytometry of surface antigens revealed that LGLs exhibited
positivity for cluster of differentiation (CD)2, 7, 8, 16, 56 and
57, and human leukocyte antigen-D related, however, were negative
for CD3, 4, and 5 (Fig. 3B),
suggesting that these LGLs predominantly exhibited an natural
killer (NK) cell phenotype. T-cell receptor β gene rearrangement
was not detected by polymerase chain reaction. A 51Cr
release assay was performed to investigate whether the NK cells
actually possessed activity. The cytotoxic activity was revealed to
be elevated to 60% (normal range, 18–40%).
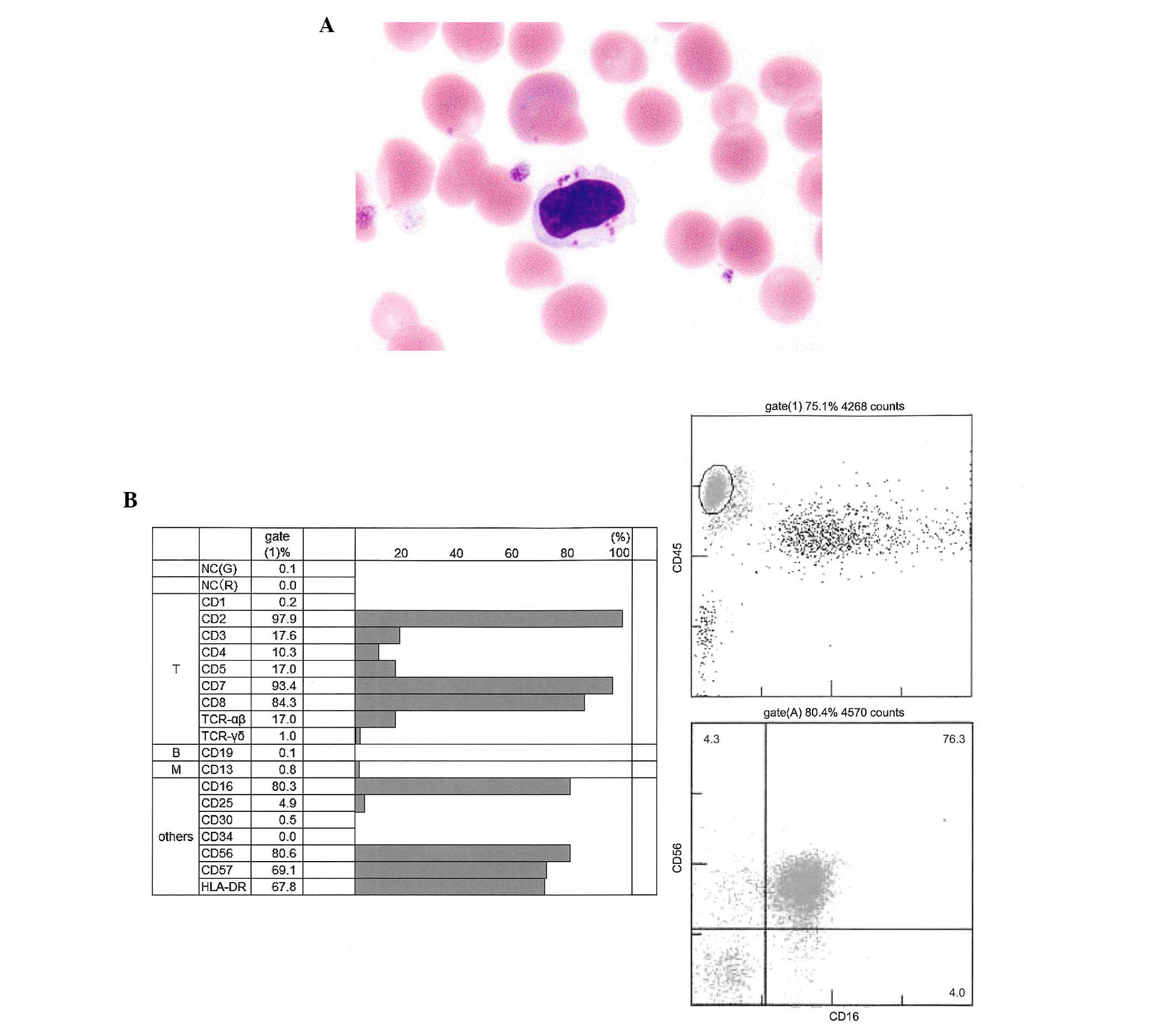 | Figure 3.Morphology and immunophenotype of
LGLs. (A) When the patient achieved VGPR after four cycles of
lenalidomide + low-dose dexamethasone LGLs, which was defineed as
more than double the size of red blood cell and abundant cytoplasm,
containing azurophilic granules, increased to 25–40% of peripheral
blood leukocytes. (May-Giemsa staining; magnification ×400). (B)
Flow cytometry of surface antigens revealed that LGLs were positive
for CD2, 7, 8, 16, 56 and 57 and HLA-DR, however, were negative for
CD3, 4, and 5, suggesting that these LGLs exhibited an NK cell
phenotype. LGL, large granular lymphocyte; CD, cluster of
differentiation; NK, natural killer; VGPR, very good partial
remission; HLA-DR, human leukocyte antigen-D related; TCR, T cell
receptor; NC, negative control. |
Since an extramedullary tumor was observed 15 months
following the initiation of lenalidomide therapy, bortezomib +
dexamethasone (BD) therapy was initiated in addition to local
radiation therapy. Unfortunately, exposure to this treatment was
not effective for this patient. Notably, the number of LGLs
decreased and serum IgG level was elevated following BD treatment
and radiation. As shown in Fig. 1, an
inverse correlation between the serum IgG and LGL levels was
observed.
Discussion
The present case study reported an interesting case
of refractory MM presenting the proliferation of LGLs in peripheral
blood during lenalidomide therapy. These LGLs exhibited an NK cell
phenotype (CD16+CD56+CD57+) and
cytotoxic activity, and may be involved in the elimination of
myeloma cells. In the present case, a long-term decrease in
M-proteins may be achieved by LGL expansion through lenalidomide
treatment.
Induction of LGL cells is well known in chronic
myeloid leukemia patients treated with dasatinib, which acts to
eliminate BCR/ABL-positive cells and is associated with a favorable
prognosis (8).
It has been reported that patients with
relapsed/progressive MM exerted a marked deficit of NK/T cell
numbers and activity, particularly following repeated chemotherapy
and/or autologous peripheral blood stem cell transplantation
following a high-dose melphalan regimen (2). Therefore, it appears to be beneficial
for immunomodulatory therapy to increase the cytotoxic activity and
cytokine production of NK/T cells in chemotherapy-resistant
patients with MM.
The action of immunomodulatory drugs, including
lenalidomide, is considered to be associated with cereblon
(2). However, the detailed mechanisms
remain to be elucidated. Lenalidomide normally induces
immunomodulatory function when used alone. However, when used
together with dexamethasone, the immunomodulatory effect is
suppressed (9). In the current
patient, although peripheral blood levels of IL-2 and interferon
(IFN)-γ were not elevated during lenalidomide treatment, and this
may have been the result of the effect of combination with
dexamethasone.
It was reported that CD57 is a marker of long-lived
‘memory’ NK cells (10). Notably,
although the CD57+ NK cell population produced less
IFN-γ compared with CD57− cells in response to
activation by cytokines, they exhibited higher levels of perforin
and granzymes (10). In addition, it
was also demonstrated that CD57+ NK cells are more
sensitive to CD16 activation (10).
Since lenalidomide can increase CD16 expression in NK cells
(11),
CD16+CD56+CD57+ cells, which were
mentioned in this case report, may have antitumor potency under
lenalidomide treatment. Previously, it was reported that no change
was observed in CD57+ NK cell numbers following three
cycles of Rd treatment (12), whereas
the present study detected LGL expansion during the fifth cycle of
Rd treatment. Therefore, these cells may not proliferate in the
early phase of Rd treatment.
Since anti-myeloma therapy was not effective during
BD treatment and LGL level was decreased, NK cell-mediated
cytotoxic activity was suggested not to be sustained in this
period. The MM cells may result in escape from the immune
surveillance system through alteration in the cellular and/or
extracellular properties during treatment (13). Certain heterogeneous tumor subclones
with low immunogenicity can have a chance to survive and expand
(14). In addition, MM cells are not
fixed on a single evolutionary trajectory and can potentially shift
between stable and evolving genomes during the course of the
disease (15). In addition,
epigenetic silencing of tumor antigen expression may be responsible
for antigen loss and tumor escape (16). In the present case, MM cells may have
escaped from immune surveillance during treatment.
In conclusion, the present case study demonstrated
the proliferation of LGLs during lenalidomide + dexamethasone
therapy, and demonstrated that the expanded LGLs were of the
CD57+ NK lineage. This immune system component may
contribute to disease control.
The accumulation of cases would provide more marked
evidence of the beneficial effects of LGL in lenalidomide therapy.
However, further investigation is required to reveal the precise
mechanism of the correlation between clinical effects and LGLs
during lenalidomide treatment.
References
|
1
|
Quach H, Ritchie D, Stewart AK, Neeson P,
Harrison S, Smyth MJ and Prince HM: Mechanism of action of
immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia.
24:22–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan AC, Neeson P, Leeansyah E, Tainton K,
Quach H, Prince HM, Harrison SJ, Godfrey DI, Ritchie D and Berzins
SP: Natural killer T cell defects in multiple myeloma and the
impact of lenalidomide therapy. Clin Exp Immunol. 175:49–58. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dimopoulos MA, Chen C, Spencer A,
Niesvizky R, Attal M, Stadtmauer EA, Petrucci MT, Yu Z, Olesnyckyj
M, Zeldis JB, et al: Long-term follow-up on overall survival from
the MM-009 and MM-010 phase III trials of lenalidomide plus
dexamethasone in patients with relapsed or refractory multiple
myeloma. Leukemia. 23:2147–2152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palumbo A, Hajek R, Delforge M, Kropff M,
Petrucci MT, Catalano J, Gisslinger H, Wiktor-Jędrzejczak W,
Zodelava M, Weisel K, et al: Continuous lenalidomide treatment for
newly diagnosed multiple myeloma. N Engl J Med. 366:1759–1769.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Attal M, Lauwers-Cances V, Marit G,
Caillot D, Moreau P, Facon T, Stoppa AM, Hulin C, Benboubker L,
Garderet L, et al: Lenalidomide maintenance after stem-cell
transplantation for multiple myeloma. N Engl J Med. 366:1782–1791.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCarthy PL, Owzar K, Hofmeister CC, Hurd
DD, Hassoun H, Richardson PG, Giralt S, Stadtmauer EA, Weisdorf DJ,
Vij R, et al: Lenalidomide after stem-cell transplantation for
multiple myeloma. N Engl J Med. 366:1770–1781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neuber B, Herth I, Tolliver C, Schoenland
S, Hegenbart U, Hose D, Witzens-Harig M, Ho AD, Goldschmidt H,
Klein B and Hundemer M: Lenalidomide enhances antigen-specific
activity and decreases CD45RA expression of T cells from patients
with multiple myeloma. J Immunol. 187:1047–1056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mustjoki S, Ekblom M, Arstila TP, Dybedal
I, Epling-Burnette PK, Guilhot F, Hjorth-Hansen H, Höglund M,
Kovanen P, Laurinolli T, et al: Clonal expansion of T/NK-cells
during tyrosine kinase inhibitor dasatinib therapy. Leukemia.
23:1398–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gandhi AK, Kang J, Capone L, Parton A, Wu
L, Zhang LH, Mendy D, Lopez-Girona A, Tran T, Sapinoso L, et al:
Dexamethasone synergizes with lenalidomide to inhibit multiple
myeloma tumor growth, but reduces lenalidomide-induced
immunomodulation of T and NK cell function. Curr Cancer Drug
Targets. 10:155–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lopez-Vergès S, Milush JM, Schwartz BS,
Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM,
Norris PJ, et al: Expansion of a unique CD57+ NKG2Chi
natural killer cell subset during acute human cytomegalovirus
infection. Proc Natl Acad Sci USA. 108:14725–14732. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Qian Z, Cai Z, Sun L, Wang H,
Bartlett JB, Yi Q and Wang M: Synergistic antitumor effects of
lenalidomide and rituximab on mantle cell lymphoma in vitro and in
vivo. Am J Hematol. 84:553–559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu AK, Gherardin N, Quach H, Harrison SJ,
Prince HM, Ritchie D and Neeson P: CD57+ NK cells are increased in
patients with multiple myeloma and are primed effectors for ADCC,
but not natural cytotoxicity. Blood. 122:19042013.
|
|
13
|
Katira P, Bonnecaze RT and Zaman MH:
Modeling the mechanics of cancer: Effect of changes in cellular and
extra-cellular mechanical properties. Front Oncol. 3:1452013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Magrangeas F, Avet-Loiseau H, Gouraud W,
Lodé L, Decaux O, Godmer P, Garderet L, Voillat L, Facon T, Stoppa
AM, et al: Minor clone provides a reservoir for relapse in multiple
myeloma. Leukemia. 27:473–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keats JJ, Chesi M, Egan JB, Garbitt VM,
Palmer SE, Braggio E, Van Wier S, Blackburn PR, Baker AS,
Dispenzieri A, et al: Clonal competition with alternating dominance
in multiple myeloma. Blood. 120:1067–1076. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DuPage M, Mazumdar C, Schmidt LM, Cheung
AF and Jacks T: Expression of tumour-specific antigens underlies
cancer immunoediting. Nature. 482:405–409. 2012. View Article : Google Scholar : PubMed/NCBI
|