Introduction
Polymorphous low-grade adenocarcinoma (PLGA) is an
uncommon malignant salivary gland tumor exhibiting cytologic
uniformity, morphologic diversity, infiltrative growth pattern and
low metastatic potential (1,2). The various appellations include terminal
duct carcinoma and lobular carcinoma (1). PLGA originated from the reserve cells of
the salivary gland and was introduced by the World Health
Organization (WHO) as a new entity in 1990 (2). PLGA most commonly affects the minor
salivary glands, primarily in the oral cavity, particularly the
palate and buccal mucosa (1,2). Complete surgical excision with close
evaluation of surgical margin remains the cornerstone of surgical
management of PLGA. PLGA has a local recurrent rate of 9–17% and a
regional metastatic rate of 9–15% (3). PLGA arising from the larynx is extremely
rare and, to the best of our knowledge, only two cases have been
previously reported (4,5) and primary epiglottic PLGA has never been
reported. The present study reported a rare case of epiglottic PLGA
in a 65-year-old male.
Case report
Clinical summary
A 65-year-old Thai male patient living in Thailand
was admitted to the faculty of Medicine, Ramathibodi Hospital
(Bangkok, Thailand), due to intermittent sore throat and painful
dysphagia for a duration of 3 months. The patient had an 8 year
history of hypertension and dyslipidemia. The patient did not drink
alcohol or smoke, and had no history of tuberculosis and cancer
among the members of the families. Physical examination was
significant for a solitary firm mass involving the left
aryepiglottic fold. Laryngopharyngeal endoscopy revealed a bulging
of the left epiglottis and left aryepiglottic fold by a submucosal
mass measuring 2.1×2.3×1.2 cm. No abnormalities were observed in
the nasopharynx, oropharynx and hypopharynx. No impairment of the
laryngeal motion was observed and the cervical lymph node could not
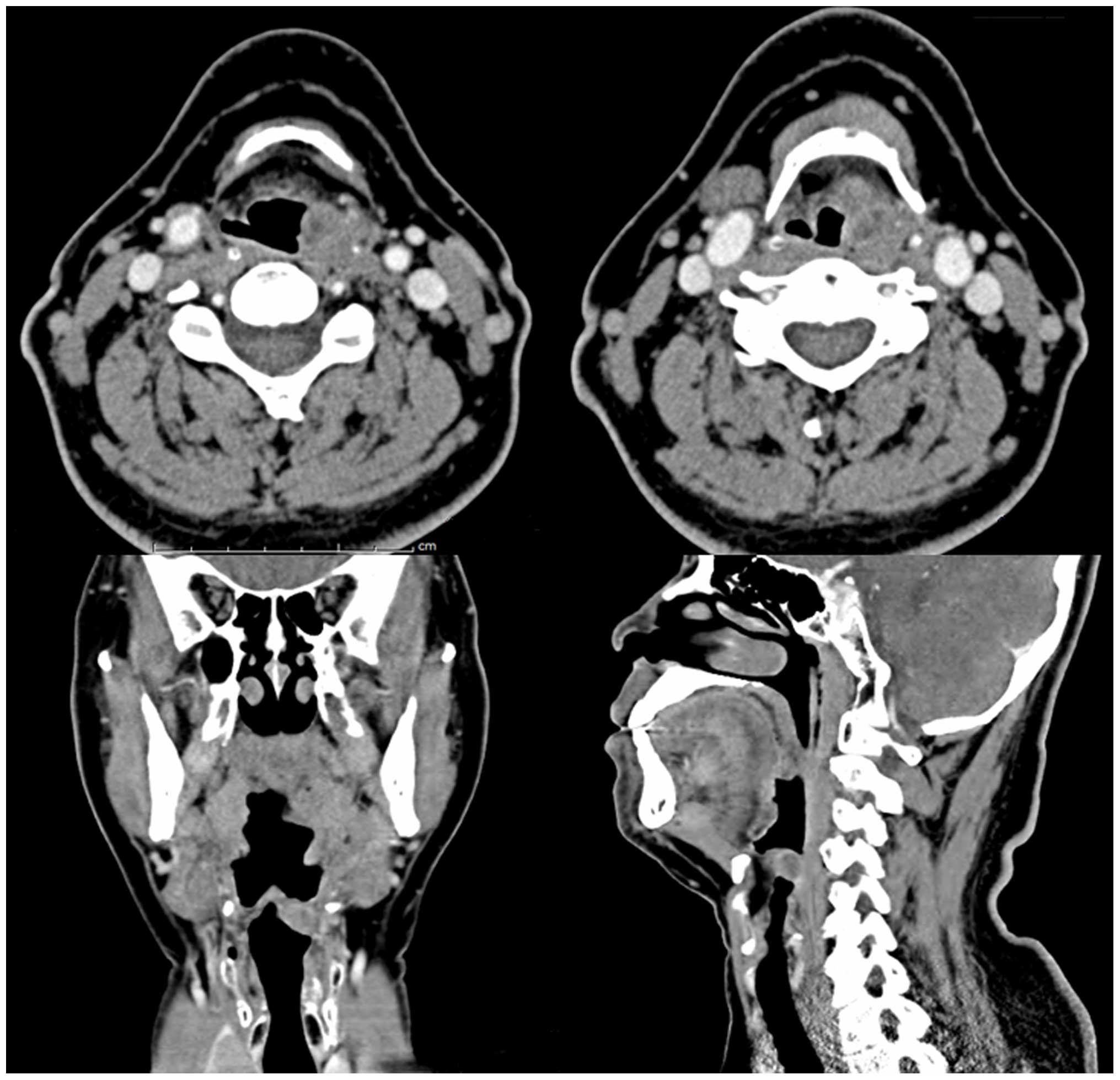
be palpated. Computed tomography (CT) revealed an infiltrative
heterogeneous enhancing mass, which invaded the left-sided
epiglottis and left aryepiglottic fold (Fig. 1). The patient underwent tumor removal
with frozen section for evaluating the surgical margin. Subsequent
supraglottic laryngectomy was performed. The definite diagnosis was
T2N0M0 stage 2 PLGA. The patient recovered uneventfully and no
additional therapy was administered. An improvement in the sore
throat was also observed. At the 7 year follow-up, the patient
remains well and exhibits no evidence of recurrence and systemic
metastasis.
Pathological findings
The gross pathological specimen consisted of a
supraglottic laryngectomy specimen showing a single polypoid
submucosal lesion involving the left aryepiglottic fold with intact
overlying laryngeal epiglottic mucosa. The lesion was an
unencapsulated firm gray-tan-white mass measuring 2.2×2.3×1.2 cm.
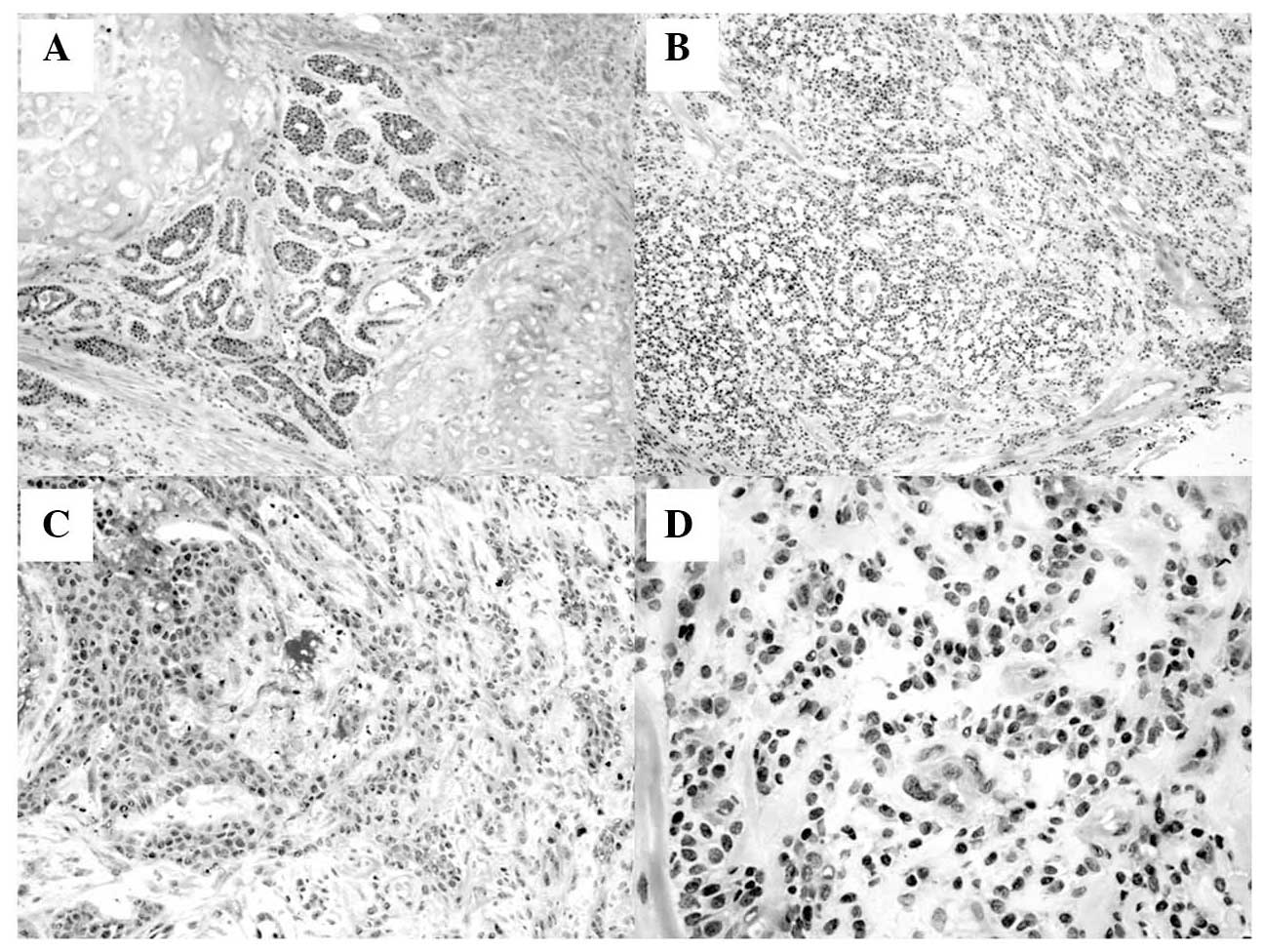
The sections of the left epiglottic mass revealed bland uniformity
of cell type with variation in architectural patterns consisting of
tubular, cribriform, solid and fascicular formations. The
peripheral section of the tumor revealed invasive features into the
epiglottic cartilage (Fig. 2). The
neoplastic cells were small and uniform with bland, round to oval,
basophilic nuclei, inconspicuous nucleoli and a moderate quantity
of eosinophilic cytoplasm. Mitotic activity was inconspicuous. The
stroma between the nests of tumor cells revealed hyalinized
fibroconnective tissue without myoepithelial cell proliferation.
Neither lymphovascular nor perineural invasion was detected.
Immunohistochemical staining revealed that tumor cells were
positive with epithelial cell markers, including cytokeratin (both
AE1/AE3 and cytokeratin 7) and epithelial membrane antigen (EMA),
confirming the epithelial origin of the tumor. Tumor cells were
also immunoreactive with antibodies against vimentin and CD117.
Tumor cells were negative immunohistochemically for gross cystic
disease fluid protein 15 (GCDFP15), estrogen receptor, progesterone
receptor, androgen receptor, HER-2/neu (CD340), prostatic specific
antigen (PSA), thyroid transcription factor 1 (TTF1), PAX8, napsin
A, CD43 and smooth muscle actin, excluding metastatic mammary,
prostatic, thyroid and pulmonary adenocarcinoma, as well as adenoid
cystic carcinoma and salivary duct carcinoma. The proliferative
index of the tumor cells, demonstrated by Ki67, ranged between 2
and 8% throughout the tumor, with a mean index of 5%.
Discussion
PLGA is an uncommon, low-grade malignant salivary
gland neoplasm characterized by cytological uniformity, morphologic
diversity, infiltrative growth pattern and low metastatic potential
(1,2).
PLGAs originate from the reserve cells exhibiting both luminal and
myoepithelial differentiation of the ductal cells of the salivary
glands (6). PLGA shows a female
predilection of ~2:1 (1,3). The average age at presentation occurs
principally during the fifth to seventh decade (1,3). The
patients have an average age of 59 years with a range between 16
and 94 years (1). The tumors are
usually found in the junction between the soft and hard palates,
although cases with involvement of the major salivary glands and
the minor salivary glands have been reported (3). The minor salivary glands are scattered
throughout the upper respiratory tract, including laryngeal
aryepiglottic fold, which may be a potential site for PLGA. PLGAs
usually remain asymptomatic until they produce a mass effect
causing a sore throat and airway obstruction. The routine initial
laboratory investigations are non-contributory. The imaging
procedures, including CT and magnetic resonance imaging may allow
early recognition of PLGAs. Diagnosis is often delayed as a result
of protean non-specific and diverse clinical manifestations at
presentation, with resultant poor outcome resulting from advance
local invasion and regional metastasis. The common metastatic sites
include the lymph nodes and lungs (1,3,7). The tumor size range varies between 0.4
and 6 cm with an average size of 2.2 cm (8). The microscopic findings demonstrated
uniform tumor cells exhibiting small to medium sized, oval pale
nuclei and occasional nucleoli (1,3,8). Mitoses were uncommon. The morphological
configurations included lobular, papillary, cribriform and
trabeculae (1–3). Whorls or targetoid arrangements around
the nerve and blood vessel have been previously described (3,8). The
authors' case was characterized by a variety of histopathological
features and uniform cytological appearance, without definite
perineural infiltration. Previous immunohistochemical studies have
reported that PLGA stained positive for cytokeratin, variably
positive for the CD117 and EMA, and negative for PSA, androgen and
estrogen receptors (1,8,9). The
immunohistochemical result in the present patient was compatible
with PLGA, which has been immunohistochemically reported in the
literature (1,8,9).
The differential diagnoses of a primary epiglottic
tumor include squamous cell carcinoma (SCC), pleomorphic adenoma,
adenoid cystic carcinoma or metastatic tumor. SCC originates from
the surface mucosa of the larynx and can directly invade the
adjacent structure. The intact mucosa on gross examination in
combination with histopathology and the positive results of
cytokeratin 7 immunohistochemistry may be helpful in excluding SCC
in this patient. Adenoid cystic carcinoma, arising on the
aryepiglottic fold, has been reported (10). Adenoid cystic carcinoma may be
confused with PLGA by its architectural patterns (tubular,
cribriform and solid formations), as well as uniform cytological
appearance. However, the fascicular pattern of tumor cells and
negative CD43 and smooth muscle actin immunohistochemical staining
are not described in adenoid cystic carcinoma (11–13).
Pleomorphic adenoma can arise from minor salivary gland of the
epiglottis (14). However, the
histological features of the epithelial and modified myoepithelial
elements exhibiting mucoid, myxoid or chondroid appearances were
not observed in the present case.
Surgical excision with close evaluation of surgical
margin remains the cornerstone of surgical management of PLGA.
Lymph node dissection is not generally performed and should usually
only be considered if evidence of cervical lymph node involvement
is document. PLGAs generally behave in an indolent manner and
generally do not recur following complete surgical excision. The
overall survival rate of the patients with conventional PLGA is
excellent. However, dedifferentiation of PLGA has been reported and
carries a less favorable outcome and a poor prognosis (1,15).
Postoperative radiotherapy and/or chemotherapy is generally
avoided, except in dedifferentiated PLGA cases due to the risk of
aggressive dedifferentiation.
Perineural invasion is frequently noted with PLGA,
generally characterized by indolent course and has considerable
implications for prognosis and treatment. Therefore, the early and
accurate detection of perineural invasion may enhance surgical
planning and appropriate use of adjuvant radiotherapy.
To the best of our knowledge, this is the first
reported case of the epiglottic PLGA in a 65 year-old male,
presenting with intermittent sore throat and painful dysphagia, and
receiving curatively treatment by a supraglottic laryngectomy. The
post-operative course was uneventful with a 7 year disease free
survival. In conclusion, PLGA must be considered in the
differential diagnosis of laryngeal tumor. The application of
immunohistochemical investigation correlating with the clinical,
radiological, endoscopic and histopathological findings may assist
in making the diagnosis, and lead to the appropriate treatment.
References
|
1
|
Lhuna MA and Wenig BM: World Health
Organization Classification of Tumours of the Salivary glands:
Polymorphous low-grade adenocarcinoma. Pathology and Genetics of
Head and Neck Tumours. Barnes L, Eveson JW, Reichart P and
Sidransky D: IARC Press. (Lyon). 223–224. 2005.
|
|
2
|
Seifert G: Histological typing of salivary
gland tumours (2nd). Springer-Verlag Berlin Heidelberg. New York:
22–23. 1991.
|
|
3
|
Ellis GL and Auclair PL: Polymorphous
low-grade adenocarcinoma. Tumors of the salivary glands (3rd).
Armed Forces Institute of Pathology. (Washington, DC). 216–228.
1996.
|
|
4
|
Takagi S, Tsunetomi K, Tsuda K, Mizokami
H, Ootani S and Shin T: A Case of Polymorphous Low-Grade
Adenocarcinoma (PLGA) of the Larynx. Larynx Japan. 10:136–139.
1998. View Article : Google Scholar
|
|
5
|
Jafarian AH, Khazaeni K, Rahpeyma A and
Khajehahmadi S: Polymorphous low-grade adenocarcinoma of the
larynx: A rare case report. Arch Iran Med. 16:560–562.
2013.PubMed/NCBI
|
|
6
|
Edwards PC, Bhuiya T and Kelsch RD:
Assessment of p63 expression in the salivary gland neoplasms
adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma and
basal cell and canalicular adenomas. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 97:613–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olusanya AA, Akadiri OA, Akinmoladun VI
and Adeyemi BF: Polymorphous low grade adenocarcinoma: Literature
review and report of lower lip lesion with suspected lung
metastasis. J Maxillofac Oral Surg. 10:60–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eveson JW and Nagao T: Diseases of the
salivary glands. Surgical pathology of the head and neck. Barnes L:
(3rd). Informa Healthcare. (New York). 557–561. 2008.
|
|
9
|
Mino M, Pilch BZ and Faquin WC: Expression
of KIT (CD117) in neoplasms of the head and neck: An ancillary
marker for adenoid cystic carcinoma. Mod Pathol. 16:1224–1231.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Kerviler E, Bely N, Laccourreye O,
Clément O, Halimi P and Frija G: The aryepiglottic fold as a rare
location of adenoid cystic carcinoma. AJNR Am J Neuroradiol.
16:1375–1377. 1995.PubMed/NCBI
|
|
11
|
Darling MR, Schneider JW and Phillips VM:
Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma:
A review and comparison of immunohistochemical markers. Oral Oncol.
38:641–645. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woo VL, Bhuiya T and Kelsch R: Assessment
of CD43 expression in adenoid cystic carcinomas, polymorphous
low-grade adenocarcinomas and monomorphic adenomas. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 102:495–500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Epivatianos A, Poulopoulos A,
Dimitrakopoulos I, Andreadis D, Nomikos A, Vlahou S, Papazoglou G
and Barbatis C: Application of alpha-smooth muscle actin and c-kit
in the differential diagnosis of adenoid cystic carcinoma from
polymorphous low-grade adenocarcinoma. Oral Oncol. 43:67–76. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baptista PM, Garcia-Tapia R and Vazquez
JJ: Pleomorphic adenoma of the epiglottis. J Otolaryngol.
21:355–357. 1992.PubMed/NCBI
|
|
15
|
Pelkey TJ and Mills SE: Histologic
transformation of polymorphous low-grade adenocarcinoma of salivary
gland. Am J Clin Pathol. 111:785–791. 1999. View Article : Google Scholar : PubMed/NCBI
|