Introduction
Primary malignant tumors of the hypopharynx are
predominantly squamous cell carcinomas (SqCCs), as primary
small-cell carcinoma (SmCC) of the hypopharynx is rare. Combined
primary SmCC and SqCC of the hypopharynx, referred to as composite
tumor of the hypopharynx, is even more rare, with only 3 cases
previously reported (1–3). Therefore, there is little information
available on the optimal management of these patients. We herein
report a the case of a patient with primary combined SmCC with an
SqCC element and investigate the expression of specific proteins
for molecular-targeted therapy.
Case report
A 74-year-old man, with a ~50-year history of
excessive alcohol consumption and smoking, presented with a 3-month
history of throat pain and hoarseness. There was no history of
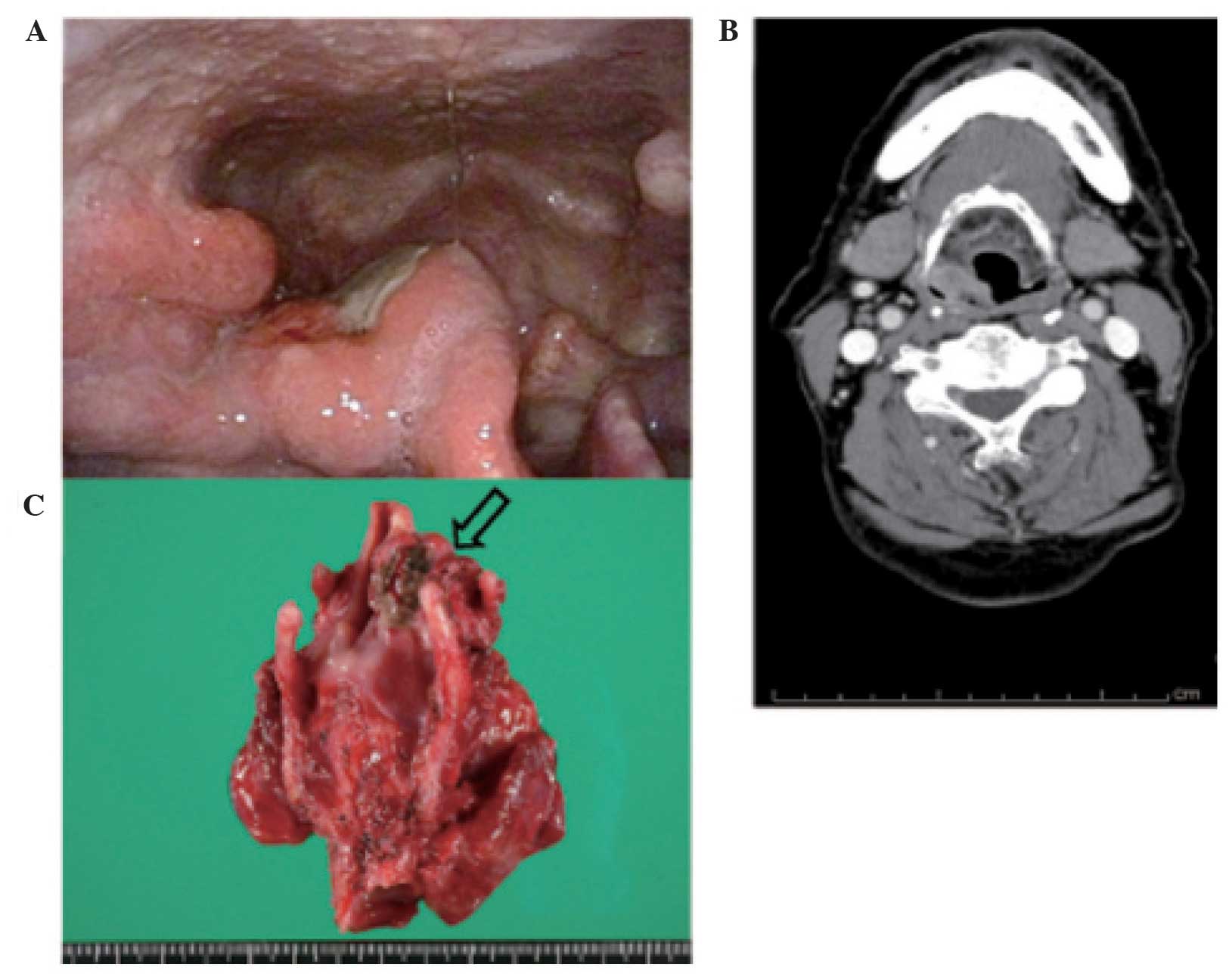
weight loss, dysphagia, or dyspnea. On hypopharyngoscopy, a tumor
was identified in the right anterior wall of the piriform sinus
(Fig. 1A). A pathologist analyzed the
biopsy sample and diagnosed the lesion as SqCC, as no SmCC
component was identified in the biopsy specimen. The right vocal
cord was not fixed. A contrast computed tomography (CT) scan of the
neck revealed a heterogeneously enhanced tumor sized 25×17×38 mm
extending through the right piriform sinus (Fig. 1B). Moreover, fluorodeoxyglucose
positron emission tomography (FDG-PET) revealed high-level
accumulation in the primary tumor, with a maximum standardized
uptake value of 14.6. There was no evidence of cervical lymph node
metastasis, primary lung tumor, or distant metastasis. The patient
was diagnosed with hypopharyngeal cancer classified as T2N0M0,
according to the 2009 Union for International Cancer Control
staging system (4). We performed a
tracheostomy, total laryngectomy, right hemithyroidectomy and
bilateral lateral neck dissection (level II–IV). The macroscopic
surgical specimen is presented in Fig.
1C. The specimen consisted of the resected larynx and right
piriform sinus tumor. A 25×20-mm deeply ulcerated tumor was found
in the right piriform sinus. Microscopically, two components were
identified in the lesion, namely a poorly differentiated SqCC and
an SmCC containing chromatin-rich nuclei with scanty cytoplasm
(Fig. 2A). Pathologically, multiple
metastatic cervical lymph nodes were identifed bilaterally (right
side, levels II and III; and left side, level III). Although the
surgical margin was narrow, it was negative for cancer cells.
Concomitant chemoradiotherapy (cisplatin + etoposide
with 45 Gy) was administered postoperatively. However, after the
first course, adjuvant chemotherapy was discontinued due to
prolonged bone marrow suppression, although the therapy was
effective. However, despite treatment, distant metastases in the
thoracic vertebrae (T5, 9 and 10) were confirmed by CT 6 months
later. The patient's condition started to deteriorate and he
succumbed to the disease 7 months after the initial treatment.
Pathology
On gross examination of the laryngectomy specimen, a
mass involving the right piriform sinus was palpable. The tumor
extended superiorly to involve the right supraglottis and exhibited
variable gross morphology, ranging from ulcerated, to nodular, to
plaque-like growth. The tumor infiltrated 1.3 cm in depth, but did
not involve the thyroid cartilage, vocal cord, or trachea.
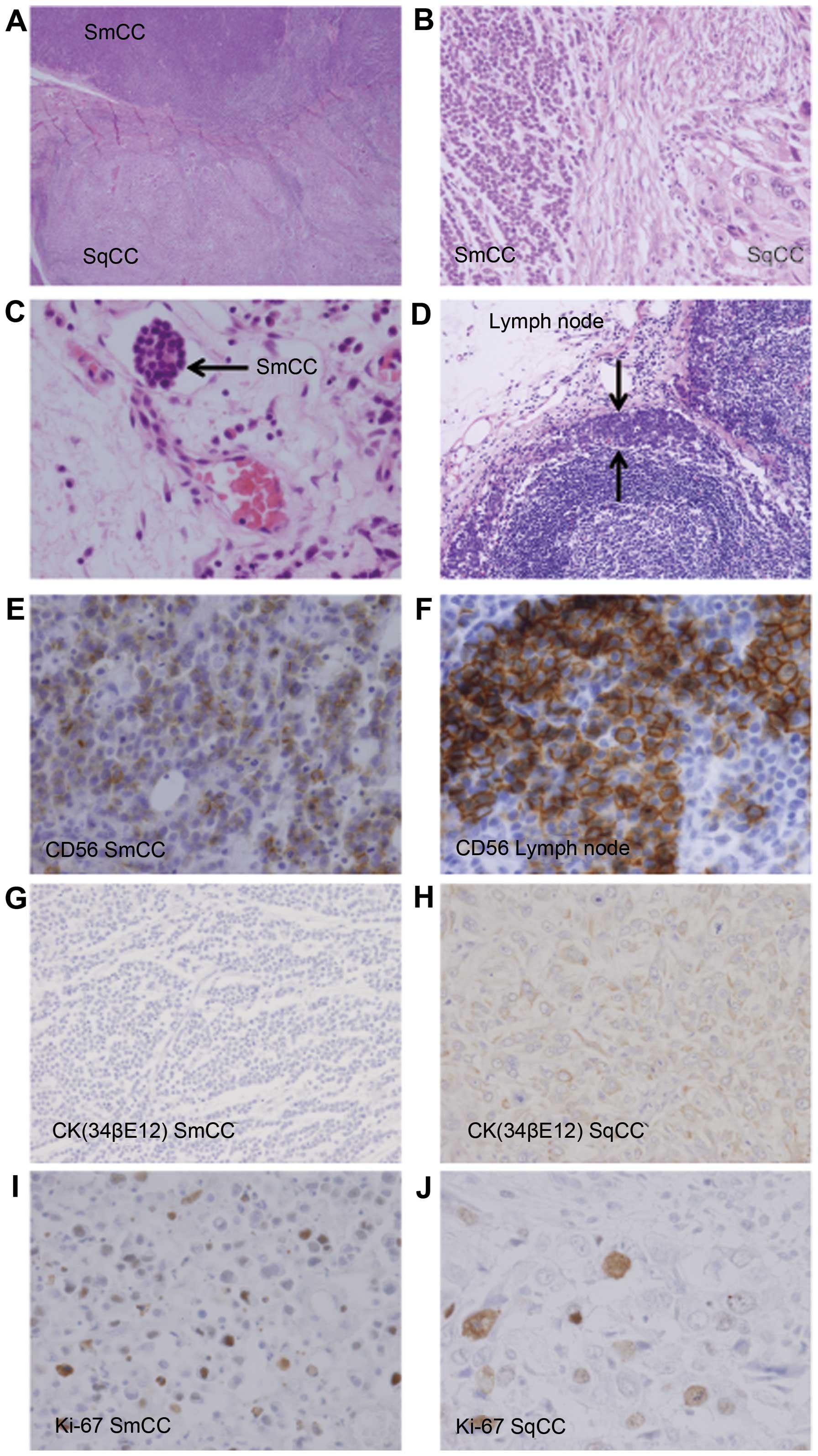
Microscopic sections from the larynx revealed a combined tumor,
composed predominantly of SmCC localized in the upper side and SqCC
localized in the lower side (Figs. 1
and 2A). We observed 2 components in
the lesion: A poorly differentiated SqCC and a small-sized SmCC
with finely granular, hyperchromatic nuclei, inconspicuous nucleoli
and scanty cytoplasm. There was a transitional area between the
SmCC and SqCC (Fig. 2A and B). The
metastatic potential of the lesion was further supported by the
SmCC component found to infiltrate lymphatic vessels (Fig. 2C). The metastatic lymph nodes
primarily involved the SmCC component (Fig. 2D).
Immunohistochemically, the SmCC strongly expressed
CD56 (Fig. 2E) and cytokeratin (CK)
AE1/AE3. Diffuse CD56 positivity in the SmCC component in the
cervical lymph nodes supported the histological diagnosis (Fig. 2F). The SmCC component was also
negative for CK 34βE12 (Fig. 2G),
p63, synaptophysin, chromogranin A and CD45. The SqCC component was
negative for neuroendocrine markers, including CD56, synaptophysin
and chromogranin A, although it was positive for CK 34βE12
(Fig. 2H). Immunoreactivity to Ki-67
was seen in the nuclei of tumor cells. In the SmCC component, the
Ki-67 labeling index was 50.2%, while that in the SqCC component
was 47.5% (Fig. 2I and J).
To predict the prognosis of this patient, we
evaluated the expression of epidermal growth factor receptor
(EGFR). Furthermore, to evaluate the possibility of using
molecular-targeted therapy for combined SmCC and SqCC, the
expression of proteins such as platelet-derived growth factor
receptor α (PDGFRα), vascular endothelial growth factor receptor 2
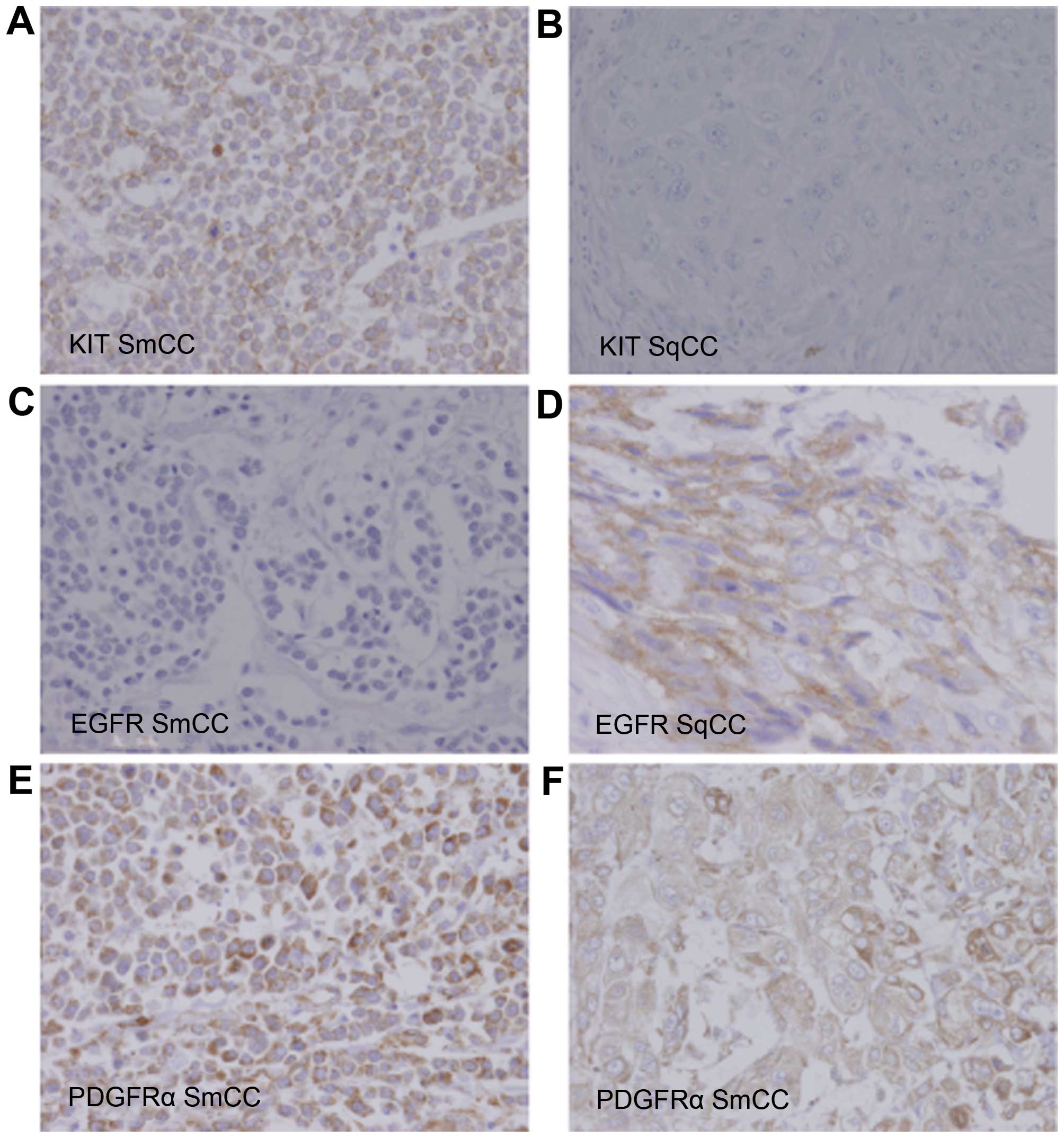
(VEGFR2) and KIT was examined. On immunohistochemical analysis, the
SmCC element was strongly positive for KIT and PDGFRα; however,
EGFR and VEGFR2 were not expressed (Fig.
3A, C and E). The SqCC element was mildly positive for PDGFRα
and EGFR; however, KIT and VEGFR2 were not expressed (Fig. 3B, D and F).
DNA extraction, genetic analysis and
human papillomavirus (HPV) polymerase chain reaction
DNA was isolated from the SmCC element in specimens
obtained during surgery. Genomic DNA was extracted from frozen
tumor specimens using the QIAamp DNA Mini Kit (Qiagen, Hilden,
Germany), according to the manufacturer's protocol.
A molecular genetic analysis of KIT (exons 9,
11, 13 and 17) and PDGFRα (exons 12, 14 and 18) was
performed using the polymerase chain reaction (PCR) direct
sequencing method, as previously reported (5–8). PCR
products were extracted and subjected to a computed automatic DNA
sequencing (ABI PRISM 3100 Genetic Analyzer; Applied Biosystems,
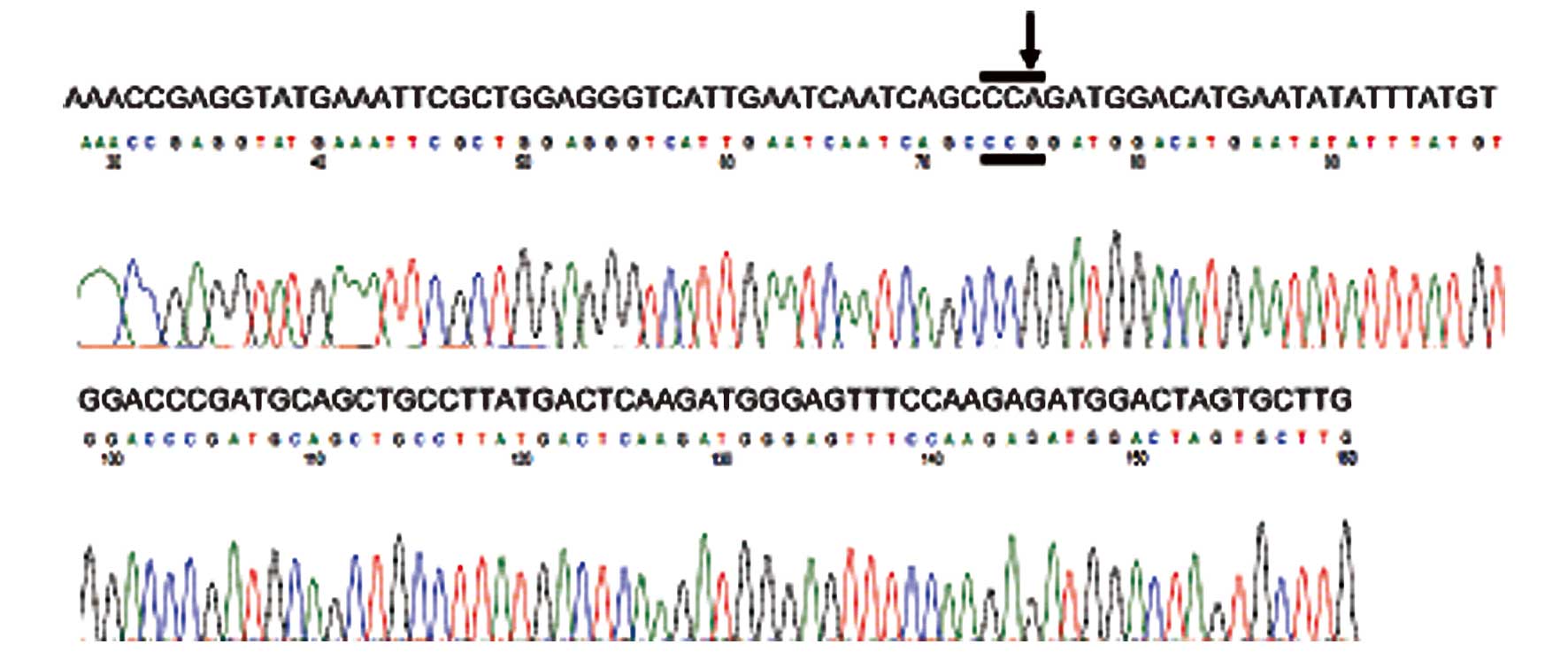
Carlsbad, CA, USA). This case exhibited a silent mutation in exon
12 of PDGFRα (Fig. 4); we
observed an A→G change in codon 567 (CCA→CCG). Analysis of exon 12
of PDGFRα from other normal tonsillar tissues revealed a
similar A→G change at the 1849 nucleotide position. Comparison of
this sequence variation with the single-nucleotide polymorphism
(SNP) database revealed the presence of a known SNP at codon 567 of
PDGFRα (9).
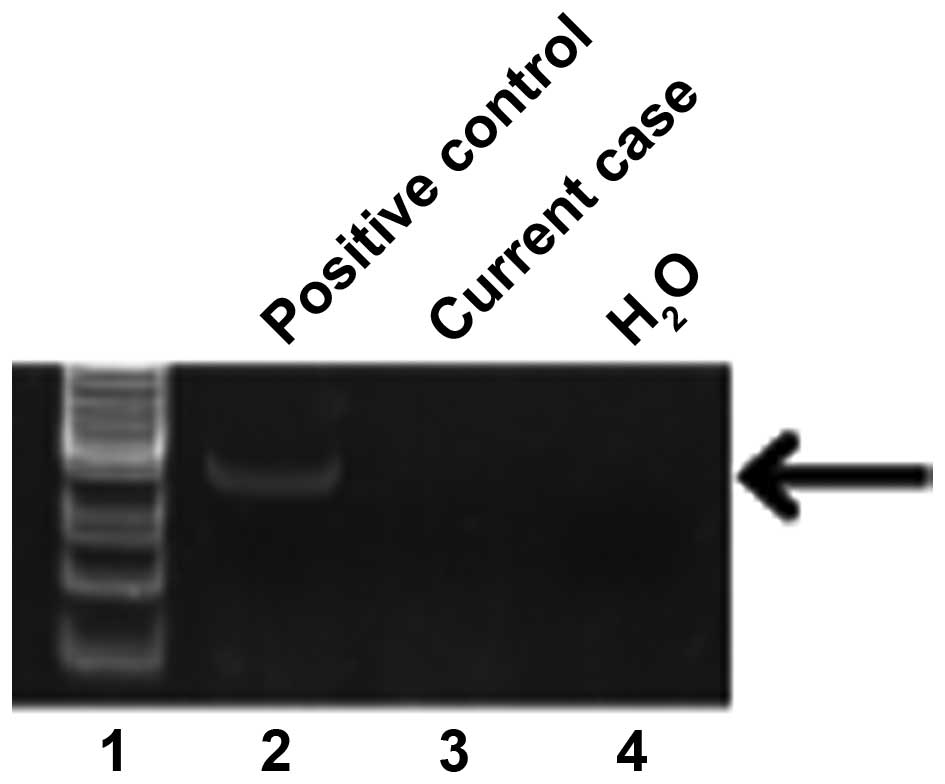
The HPV status was determined using the HPV Typing
Set (Takara Bio., Tokyo, Japan), a PCR primer set specifically
designed to identify HPV genotypes 16, 18, 31, 33, 35, 52 and 58 in
genomic DNA. The PCR HPV Typing Set method was used according to
the manufacturer's protocol. The PCR products were separated by
electrophoresis through a 9% polyacrylamide gel and stained with
ethidium bromide. Our case was negative and not considered as a
high-risk HPV status (Fig. 5).
Discussion
In the head and neck, SmCC most commonly arises in
the larynx, but has also been reported in the sinonasal tract and
salivary glands (10,11). Combined SmCC of the larynx has only
been reported in 17 cases in the literature to date. The majority
of these patients were men and in the 6th-7th decade of life.
Moreover, the majority of the patients succumbed to the disease
within 2 years of diagnosis, even with adjuvant radiation and
chemotherapy (12–14). Despite the rarity of these tumors, the
clinical behavior of combined SmCC of the larynx appears to be
similar to that of the hypopharynx. Metastasis from a primary lung
SmCC must be carefully distinguished from a primary hypopharyngeal
or laryngeal SmCC by imaging studies of the lung (15). In this case, FDG-PET revealed
high-level accumulation in the hypopharynx, without evidence of a
lung tumor or distant metastasis. A chest contrast CT scan
confirmed the absence of a primary lung tumor.
According to the World Health Organization
classification of head and neck tumors in 2005, neuroendocrine
carcinomas may be classified as typical carcinoid, atypical
carcinoid, and SmCC (16).
Neuroendocrine types of SmCC associated with an SqCC component are
referred to as combined carcinomas. They are unusual, representing
<10% of all neuroendocrine types (17). Neuroendocrine neoplasms of the
hypopharynx and larynx constitute a morphologicaly heterogeneous
group of tumors, with considerable differences in clinical behavior
and very different treatment strategies (12). Combined SmCC of the hypopharynx is
extremely rare and is often difficult to pathologically diagnose
prior to surgical treatment. Moreover, the prognosis is very poor
due to early metastasis, without established treatment regimen
(3). Of the 4 reported cases of
combined SmCC of the hypopharynx, including our case, 3 succumbed
to the disease within 1 year (Table
I). Multi-modality therapy should be performed for combined
SmCC of the head and neck region. Therefore, new therapeutic
strategies are required to improve the survival rate. The present
case reports new findings: Positive expression of KIT and PDGFRα in
the SmCC element and positive expression of EGFR and PDGFRα in the
SqCC element. Furthermore, to the best of our knowledge, the
present study is the first to report a KIT and PDGFRα
mutation analysis in combined hypopharyngeal SmCC. KIT and
PDGFRα, both mapped to chromosome 4q12, have structural
similarities with other PDGFR family members. A majority of
gastrointestinal stromal tumors (GISTs) display a gain-of-function
mutation in the KIT proto-oncogene that encodes the KIT
protein. The majority of mutations occur in KIT exon 11, but
mutations may also be found in exon 9 and rarely in exons 13 and
17. The PDGFRα gene is also very similar to the KIT
gene, and PDGFRα mutations have been found in exons 12, 14
and 18. Terada et al (18)
reported KIT protein expression in 100% and PDGFRα expression in
65% of small-cell lung carcinoma cases; however, there were no
genetic mutations of KIT and PDGFRα in small-cell
lung carcinoma (18). In a previously
reported case of esophageal combined SmCC, KIT and PDGFRα were
expressed in the SmCC component, but not in the SqCC component
(5). It was found that SmCC of the
lung and extrapulmonary organs expressed KIT and PDGFRα, but there
were no mutations in these genes within the hot spots of GISTs.
These findings are almost completely in accord with those of our
case study.
 | Table I.Summary of cases of combined SmCC in
the hypopharynx. |
Table I.
Summary of cases of combined SmCC in
the hypopharynx.
| Authors | Age (years) | Gender | Smoking history
(years) | Site | Initial therapy | Histological
phenotype | Metastatic
histological phenotype | Recurrence after
initial therapy | Salvage therapy | Follow-up interval
(months) | Prognosis | (Refs.) |
|---|
| Ferlito et
al | 57 | M | 40 | RPs | TLP+RND, RT | + | SmCC (many LNs)
combined (2LNs) | DM (bone) | None | 3.5 | DOD (DM) | (1) |
| Milis et
al | 49 | M | 30 | RPs | TLP+ND+thyroid
lobectomy, RT | + | SqCC (2LNs) SmCC
(1LN) | None | None | 6 | NED | (2) |
| Uwa et al | 73 | M | 50 | LPs | TLP+BilND+hemithyroid
lobectomy, CRT | + | SmCC (11LNs) | N, DM (lung,
liver) | Chemo | 9 | DOD (N,DM) | (3) |
| Present case | 74 | M | 50 | RPs | TLP+BilND+thyroid
lobectomy, CRT | + | SmCC (many LNs)
combined (5LNs) | DM | None | 6 | DOD (DM) (lung,
bone) |
No biomarker has been yet proven to predict response
to targeted therapy in either SmCC or SqCC. Moreover, a predictive
biomarker in one cancer type may not be helpful in another cancer
type, suggesting that different mechanisms may be involved in
combined SmCC. To the best of our knowledge, a molecular-targeted
therapy for treating combined SmCC of the hypopharynx and larynx
has not yet been reported. Our case is the first to report KIT and
PDGFRα expression in combined SmCC in the hypopharynx and larynx;
if similar results are obtained from larger series, these data may
have management and prognostic implications through the possible
use of targeted biological therapy in these tumors. At present,
SmCC treatment remains a significant challenge for the oncologists,
with several targeted agents under evaluation. However, there is no
effective molecular-targeted therapy for SmCC. Evaluating the
expression of the KIT and PDGFRα genes within
hypopharyngeal SmCC may facilitate the development of novel agents
for the molecular-targeted therapy of this tumor. However, further
investigations are necessary regarding these proteins expression
and clinical application of molecular targeted therapy for
hypophageal combined SmCC.
Acknowledgements
The authors would like to thank Ms. Yuko Mohri for
her excellent technical support.
References
|
1
|
Ferlito A, Recher G and Caruso G: Primary
combined small cell carcinoma of the larynx. Am J Otolaryngol.
6:302–308. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mills SE, Cooper PH, Garland TA and Johns
ME: Small cell undifferentiated carcinoma of the larynx. Report of
two patients and review of 13 additional cases. Cancer. 51:116–120.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uwa N, Terada T, Mohri T, Okazaki K,
Tsukamoto Y, Hirota S and Sakagami M: Combined small cell carcinoma
of the hypopharynx. Auris Nasus Larynx. 40:106–109. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sobin LH, Wittekind C and Gospodarowicz M:
TNM Classification of Malignant Tumors (7th). Wiley-Blackwell. New
York, NY: 2009.
|
|
5
|
Terada T and Maruo H: Esophageal combined
carcinomas: Immunohoistochemical and molecular genetic studies.
World J Gastroenterol. 18:1545–1551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agaimy A, Wünsch PH, Hofstaedter F,
Blaszyk H, Rümmele P, Gaumann A, Dietmaier W and Hartmann A: Minute
gastric sclerosing stromal tumors (GIST tumorlets) are common in
adults and frequently show c-KIT mutations. Am J Surg Pathol.
31:113–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lasota J and Miettinen M: KIT and PDGFRA
mutations in gastrointestinal stromal tumors (GISTs). Semin Diagn
Pathol. 23:91–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kikuchi H, Yamashita K, Kawabata T,
Yamamoto M, Hiramatsu Y, Kondo K, Baba M, Ohta M, Kamiya K and
Tanaka T: Immunohistochemical and genetic features of gastric and
metastatic liver gastrointestinal stromal tumors: Sequential
analyses. Cancer Sci. 97:127–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kartha RV and Sundram UN: Silent mutations
in KIT and PDGFRA and coexpression of receptors with SCF and PDGFA
in Merkel cell carcinoma: Implications for tyrosine kinase-based
tumorigenesis. Mod Pathol. 21:96–104. 2008.PubMed/NCBI
|
|
10
|
Renner G: Small cell carcinoma of the head
and neck: A review. Semin Oncol. 34:3–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mineta H, Miura K, Takebayashi S, Araki K,
Ueda Y, Harada H and Misawa K: Immunohistochemical analysis of
small cell carcinoma of the head and neck: A report of four
patients and a review of sixteen patients in the literature with
ectopic hormone production. Ann Otol Rhinol Laryngol. 110:76–82.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barbeaux A, Duck L, Weynand B, Desuter G,
Hamoir M, Gregoire V, Baurain JF and Machiels JP: Primary combined
squamous and small cell carcinoma of the larynx: Report of two
cases and discussion of treatment modalities. Eur Arch
Otorhinolaryngol. 263:786–790. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aggarwal G, Jackson L and Sharma S:
Primary combined small cell carcinoma of larynx with lateralized
histologic components and corresponding side-specific neck nodal
metastasis: Report of a unique case and review of literature. Int J
Clin Exp Pathol. 4:111–117. 2010.PubMed/NCBI
|
|
14
|
Jaiswal VR and Hoang MP: Primary combined
squamous and small cell carcinoma of the larynx: A case report and
review of the literature. Arch Pathol Lab Med. 128:1279–1282.
2004.PubMed/NCBI
|
|
15
|
Ferlito A, Silver CE, Bradford CR and
Rinaldo A: Neuroendocrine neoplasms of the larynx: An overview.
Head Neck. 31:1634–1646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: Pathology and Genetics. Head and Neck Tumours. World
Health Organisation Classification of Tumours. IARC Press. (Lyon).
2005.
|
|
17
|
Wu BZ, Gao Y and Yi B: Primary
neuroendocrine carcinoma in oral cavity: Two case reports and
review of the literature. J Oral Maxillofac Surg. 72:633–644. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Terada T: An immunohistochemical and
molecular genetic analysis of KIT and PDGFRA in small cell lung
carcinoma in Japanese. Int J Clin Exp Pathol. 5:331–338.
2012.PubMed/NCBI
|