Introduction
The most common sites of metastatic breast cancer
are the brain, lungs, liver, soft tissue and bone (1). Although less frequent than solid organ
and bone metastasis, malignant effusion, including pleural and
pericardial effusion, and meningeal infiltrates are somewhat common
in breast cancer. Approximately 7–11% of patients with breast
cancer develop malignant pleural effusion during the course of the
disease (2), and in 43% of these
patients, pleural effusion is the first symptom of metastatic
disease (3). The prevalence of
pericardial involvement varies from 4% in general autopsies to
15–30% in autopsies of cancer patients (4). Only 12–25% of patients who have
metastasis to the pericardium develop pericardial effusion, of whom
only a small percentage develop tamponade (5). Therefore, there are few reported cases
of cardiac metastases from breast cancer. Pericardial effusion with
cardiac tamponade as the initial manifestation of breast cancer is
quite rare. The estimated incidence of leptomeningeal metastasis in
clinical and autopsy series of patients with breast cancer is 2–5
and 3–6%, respectively (6). The
incidence of metastasis to the pleura, pericardium or meninges is
lower compared with those to other sites in breast cancer, whereas
simultaneous involvement of all three sites in one patient, without
metastases to other sites, is extremely rare. To the best of our
knowledge, no similar cases have been reported in the literature to
date.
It has been reported that ~3–6% of new breast cancer
cases have already metastasized to distant sites at the time of
diagnosis (7), with the median
survival ranging between 18 and 39 months. Several studies have
reported a range of prognostic factors affecting survival, such as
age at diagnosis, hormone receptor status, human epidermal growth
factor receptor 2 status and site of metastasis (8). Negative hormone receptor expression,
cardiac tamponade and pleural effusion as the initial
manifestation, and meningeal metastases, are poor prognostic
factors.
Case report
A 44-year-old woman presented to the West China
Hospital (Chengdu, China) in October, 2013 complaining of pain in
the right breast for 2 months, a mass in the right breast,
progressive dyspnea and productive cough accompanied by general
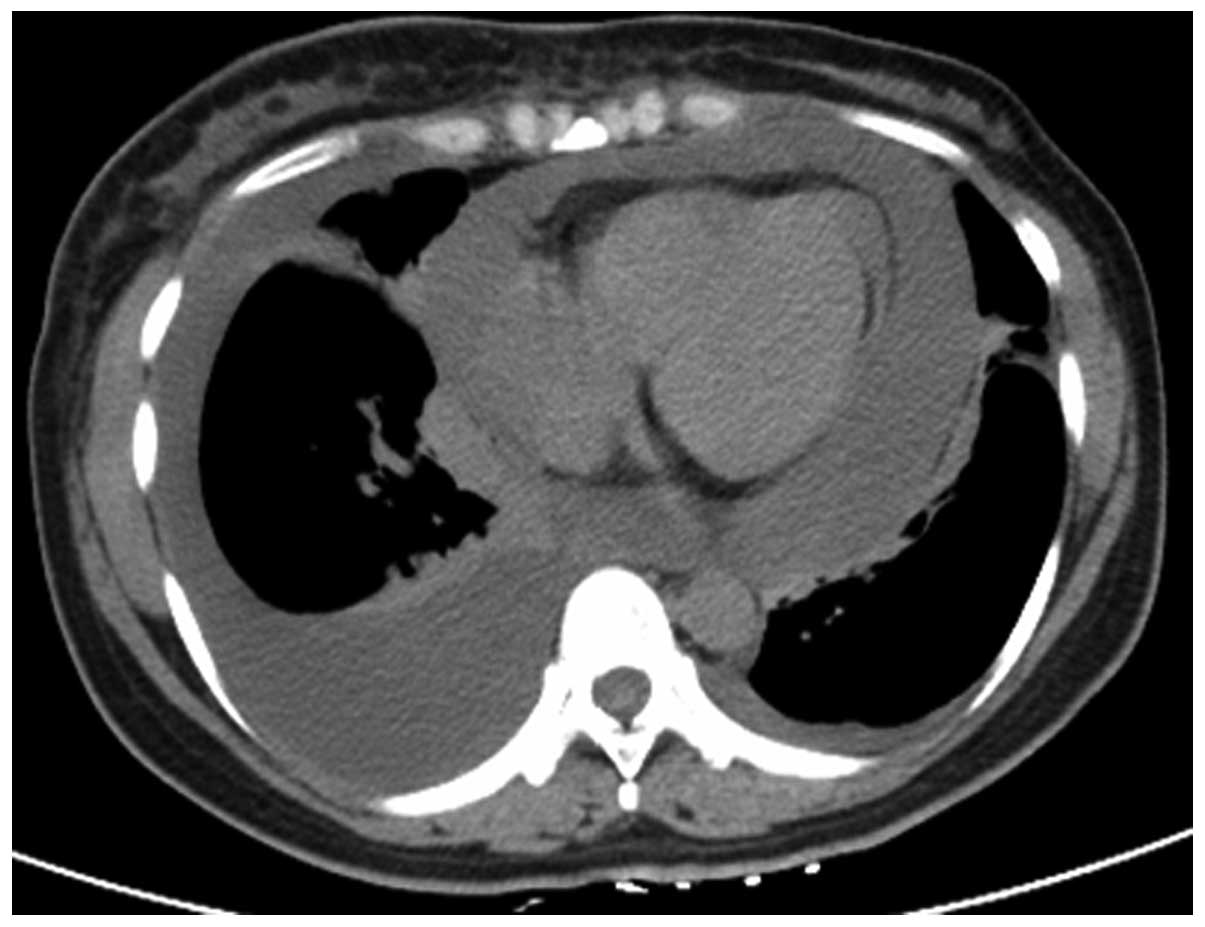
malaise for 1 month. Prior to admission, a plain chest computed
tomography (CT) scan revealed bilateral pleural effusions and a
large amount of pericardial effusion with characteristics of
tamponade (Fig. 1). The cytological
examination of the pleural and pericardial effusions demonstrated
atypical cells suspicious of breast ductal carcinoma metastasis.
Thoracentesis and pericardiocentesis were performed and drainage
tubes were placed. The dyspnea and cough were significantly
alleviated following drainage of the fluid and the subsequent
intrathoracic and intraperitoneal administration of cisplatin. A
color Doppler ultrasound revealed a firm mass (33×19×32 mm) in the
right breast and several enlarged lymph nodes in the right axilla.
The biopsy and subsequent histological examination of the mass
revealed an infiltrative carcinoma (estrogen receptor, 0%;
progesterone receptor, 0%; Ki-67, 20%; and human epidermal growth
factor receptor 2, 1+). Further examinations, including abdominal
ultrasound, cranial CT scan and bone scan, detected no other
metastasis. Upon admission, re-examination with color Doppler
ultrasound revealed that the breast mass had increased to 52×23×46
mm in size over a few days. On the basis of these results, the
patient was diagnosed with invasive ductal breast carcinoma
(cT3N2M1) of the right breast, with malignant pleural and
pericardial effusion.
The patient was then treated with docetaxel (75
mg/m2, day 1) and gemcitabine (1,000 mg/m2,
days 1 and 8) every 3 weeks, for 2 cycles, achieving stable
disease. Subsequently, the chemotherapy was changed to carboplatin
(400 mg, day 1) and vinorelbine (40 mg/m2, days 1 and 8)
every 3 weeks, achieving partial response after the second cycle.
Prior to receiving the third cycle, the patient complained of
headache, dizziness and tinnitus, but no disorder of consciousness.
An enhanced magnetic resonance imaging (MRI) scan of the head
revealed absence of metastases; palliative therapy did not mitigate
the symptoms. Several days later, the patient presented with
exacerbation of the headache, diplopia, nausea, vomiting,
convulsions, palpitations and dyspnea. On physical examination, the
patient was dyspnoeic, with diminished breath sounds on the right
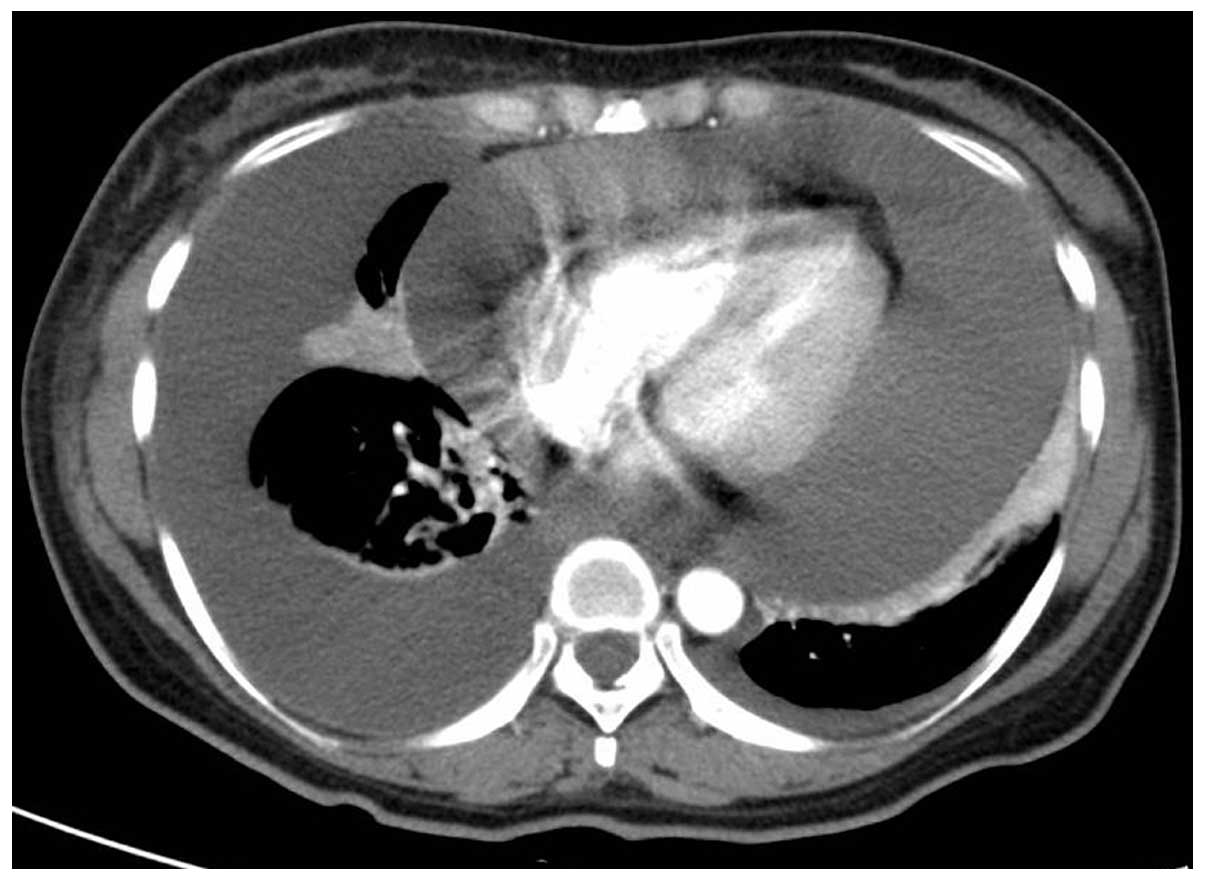
pulmonary field and muffled cardiac tones. A chest-abdominal CT
scan was performed, which revealed a large amount of pericardial
effusion and a moderate amount of right pleural effusion (Fig. 2). Lumbar puncture was performed and
revealed elevated opening pressure, protein, and lymphocytes, and
reduced glucose, but without evidence of malignant cells in the
cerebrospinal fluid (CSF). Thoracentesis and pericardiocentesis
were performed and drainage tubes were again placed. In addition,
treatments for reducing the intracranial pressure and antiepileptic
agents were also administered. With these measures, the symptoms
were controlled. However, symptoms of meningeal metastasis
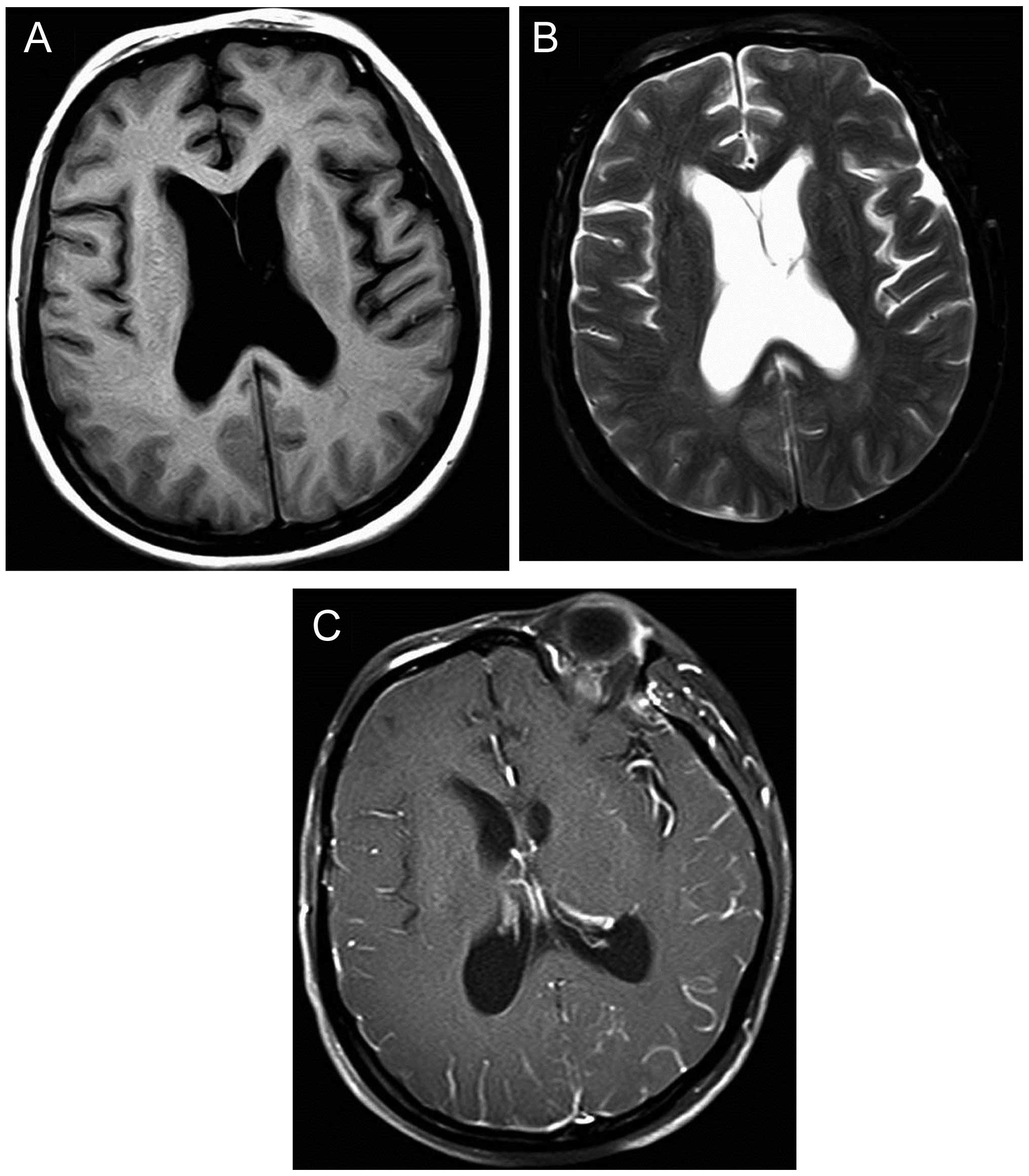
reappeared only 10 days later. The head MRI scan this time revealed
narrowed bilateral frontal lobe grooves, noticeable hydrocephalus
and enlarged ventricles; the enhanced scan revealed mild
enhancement of the gyrus, suggesting meningeal metastasis (Fig. 3). Therefore, whole-brain radiotherapy
(WBRT) with a dose of 40 Gy in 20 fractions was administered, with
supportive treatment with mannitol, glycerin fructose, sodium
valproate, levetiracetam and nutritional support. The patient's
consciousness improved with these treatments. However, the
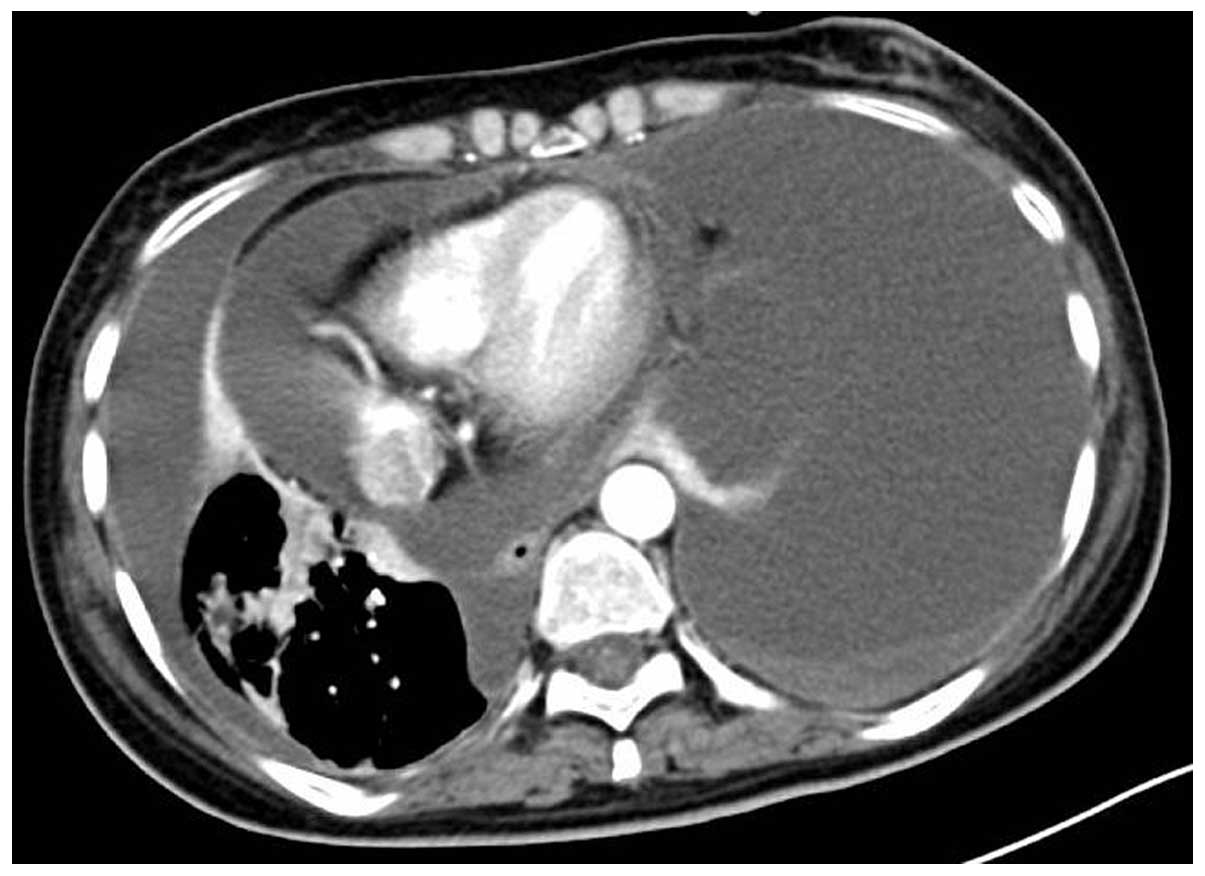
patient's heart rate increased from 90–100 to 120–130 beats per
minute, accompanied by dyspnea; re-examination with chest CT scan
revealed a large amount of pericardial and pleural effusion
(Fig. 4). Pericardial cavity drainage
and intracavitary cisplatin was administered 3 times.
Unfortunately, the general condition of the patient
deteriorated with rapid progression of the pericardial effusion and
the patient succumbed to pericardial tamponade on May 3, 2014.
Discussion
It is well known that breast cancer spreads to
different distant organs, preferentially to the bones, lungs,
liver, soft tissues and brain, whereas metastasis to the pleura,
pericardium or meninges is less common. Absence of metastasis to
organs other than the pleura, pericardium and meninges during the
entire course of the disease is extremely rare and, to the best of
our knowledge, this is the first report of such a case. Of note,
there was no solid evidence for meningeal metastases in the present
report, as no neoplastic cells were found in the CSF examination
and no obvious metastatic sites were identified on MRI. However, a
negative MRI following negative CSF cytology cannot exclude
meningeal metastases. It was previously reported that the first
lumbar puncture exhibits low sensitivity, identifying neoplastic
cells only in 45% of patients with meningeal metastases, whereas
≤40% of patients with clinically suspected meningeal metastases
proven at the time of autopsy have negative CSF cytology; in
addition, the false-negative rate of MRI is ~30% (9). Thus, we may infer the presence of
meningeal metastases in this patient based on indirect evidence:
First, the patient displayed typical clinical characteristics,
including headache, giddiness, tinnitus, diplopia, nausea,
vomiting, convulsions and disorders of consciousness; second,
although no neoplastic cells were found on CSF examination and no
obvious metastatic sites were identified on MRI, there was elevated
intracranial pressure, protein and lymphocytes, and decreased
glucose on CSF examination, as well as noticeable hydrocephalus and
enlarged ventricles on MRI. Finally, the patient's mental status
improved and the clinical symptoms were alleviated following WBRT.
The molecular basis of the metastatic process in breast cancer,
particularly the mechanism underlying organ-specific metastasis, is
poorly understood. Recent research data suggested that primary
tumor gene signatures predict tumor metastatic potential and
organ-specific tropism (10). The
mechanism underlying breast cancer metastasis in this patient may
be different from other common types of cancer, and it requires
extensive investigation. However, the patient's family members
refused the autopsy; thus, no further specimens were obtained to
investigate the potential mechanisms.
Although metastatic breast cancer remains an
incurable disease, survival has improved in recent years, due to a
wide range of new therapeutic agents and improved supportive care.
Although rarely, long-term survival, occasionally for >20 years,
has been reported in 1–3% of metastatic breast cancer cases
(11,12). Several reports suggested that women
with de novo disease exhibited better survival compared with
patients with relapsed disease (13,14), with
the median survival of de novo stage IV disease ranging
between 18 and 39 months. In our case, however, the patient only
survived for 7 months after diagnosis of breast cancer. We consider
the reasons for the patient's short survival to be the multiple
metastatic sites, triple-negative phenotype and initial
presentation with malignant pleural effusion and pericardial
tamponade caused by pericardial effusion. The majority of deaths in
metastatic breast cancer are not due to the primary tumor, but
rather the result of metastasis to other organs (15). Visceral and central nervous system
metastases are predictive of a poor prognosis and shorter survival
compared with bone and soft tissue metastases (8,16,17).
In the present case, the patient initially presented
with malignant pleural and pericardial effusion, which were
considered a grave prognostic sign. In previous studies, ~86% of
patients succumbed within the first year and aproximately one-third
within the first month. A median survival of 5–16 months has been
reported in cases with malignant pleural effusion, and of 8–26
months in cases with malignant pericardial effusion (18–20). The
prognosis of patients presenting with cardiac tamponade is dismal,
with survival ranging from a few days to 14 months and a median
survival of 5.5 months (21).
Additionally, our patient developed meningeal metastases after 4.5
months. Prospective studies have reported a median overall survival
of 9–30.3 weeks in patients with breast cancer following the
diagnosis of meningeal metastases (22); in the present case, the patient
survived for 2.5 months after the diagnosis of meningeal
metastases. Finally, the prognosis of the triple-negative breast
cancer patients is worse compared with that of other breast cancer
phenotypes, as these tumors are more aggressive, with a higher
incidence of distant metastases, particularly to visceral organs
and the brain (23–25), and survival is shorter. Some studies
demonstrated that the triple-negative breast cancer phenotype is an
unfavorable characteristic in patients with malignant pleural
effusion (26) and meningeal
metastases (27), adversely affecting
prognosis and reducing life expectancy (17,24,26,28).
Breast cancer patients with malignant effusion and
meningeal metastases are practically incurable; therefore,
relieving the symptoms and improving the quality of life is very
important. In our case, systemic chemotherapy was administered when
the patient was in good overall condition, with thoracentesis and
pericardiocentesis, as well as intracavitary instillation of
cisplatin to control the malignant effusion. As regards meningeal
metastases, WBRT was applied. However, although the disease was
initially controlled, it progressed within a short time period, and
the patient succumbed 7 months later.
In conclusion, metastasis to the pleura, pericardium
and meninges, without metastases to other organs during the entire
course of the disease, is a rare metastatic pattern of breast
cancer. Treatment is usually aimed at relieving the symptoms and
improving the quality of life, and the prognosis is typically poor.
Clinicians should be aware of these rare metastatic patterns of
breast cancer.
References
|
1
|
Koo JS, Jung W and Jeong J: Metastatic
breast cancer shows different immunohistochemical phenotype
according to metastatic site. Tumori. 96:424–432. 2010.PubMed/NCBI
|
|
2
|
Apffelstaedt JP, Van Zyl JA and Muller AG:
Breast cancer complicated by pleural effusion: Patient
characteristics and results of surgical management. J Surg Oncol.
58:173–175. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Evans TRJ, Stein RC, Pepper JR, Gazet JC,
Ford HT and Coombes RC: A randomized prospective trial of surgical
against medical tetracycline pleurodesis in the management of
malignant pleural effusions secondary to breast-cancer. Eur J
Cancer. 29A:316–319. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Posner MR, Cohen GI and Skarin AT:
Pericardial disease in patients with cancer. The differentiation of
malignant from idiopathic and radiation-induced pericarditis. Am J
Med. 71:407–413. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levitan Z, Kaplan AL and Gordon AN:
Survival after malignant pericardial effusion and cardiac tamponade
in advanced ovarian cancer. South Med J. 83:241–242. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shetty J, Prasad K, Rao M and Nayak A:
Isolated leptomeningeal metastases originating from infiltrating
ductal carcinoma of breast - A case report. IJRRMS. 2:2012.
|
|
7
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Largillier R, Ferrero JM, Doyen J,
Barriere J, Namer M, Mari V, Courdi A, Hannoun-Levi JM, Ettore F
and Birtwisle-Peyrottes I: Prognostic factors in 1,038 women with
metastatic breast cancer. Ann Oncol. 19:2012–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruna J, Simó M and Velasco R:
Leptomeningeal metastases. Curr Treat Options Neurol. 14:402–415.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ugenskiené R and Juozaityté E: The
molecular mechanisms of breast cancer metastasis. Biologija.
58:2012.
|
|
11
|
Hortobagyi GN: Can we cure limited
metastatic breast cancer? J Clin Oncol. 20:620–623. 2002.PubMed/NCBI
|
|
12
|
Greenberg PA, Hortobagyi GN, Smith TL,
Ziegler LD, Frye DK and Buzdar AU: Long-term follow-up of patients
with complete remission following combination chemotherapy for
metastatic breast cancer. J Clin Oncol. 14:2197–2205.
1996.PubMed/NCBI
|
|
13
|
Dawood S, Broglio K, Ensor J, Hortobagyi
GN and Giordano SH: Survival differences among women with de novo
stage IV and relapsed breast cancer. Ann Oncol. 21:2169–2174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Güth U, Magaton I, Huang DJ, Fisher R,
Schötzau A and Vetter M: Primary and secondary distant metastatic
breast cancer: Two sides of the same coin. Breast. 23:26–32. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solomayer EF, Diel IJ, Meyberg GC, Gollan
C and Bastert G: Metastatic breast cancer: Clinical course,
prognosis and therapy related to the first site of metastasis.
Breast Cancer Res Treat. 59:271–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cutler SJ, Ardyce JA and Taylor SG III:
Classification of patients with disseminated cancer of the breast.
Cancer. 24:861–869. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fentiman IS, Millis R, Sexton S and
Hayward JL: Pleural effusion in breast cancer: A review of 105
cases. Cancer. 47:2087–2092. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dieterich M, Goodman SN, Rojas-Corona RR,
Emralino AB, Jimenez-Joseph D and Sherman ME: Multivariate analysis
of prognostic features in malignant pleural effusions from breast
cancer patients. Acta Cytol. 38:945–952. 1994.PubMed/NCBI
|
|
20
|
Hirata T, Yonemori K, Hirakawa A, Shimizu
C, Tamura K, Ando M, Katsumata N, Tanimoto M and Fujiwara Y:
Efficacy of pleurodesis for malignant pleural effusions in breast
cancer patients. Eur Respir J. 38:1425–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stitt VJ Jr: Breast carcinoma with
pericardial metastasis. J Natl Med Assoc. 79:195–197.
1987.PubMed/NCBI
|
|
22
|
Scott BJ and Kesari S: Leptomeningeal
metastases in breast cancer. Am J Cancer Res. 3:117–126.
2013.PubMed/NCBI
|
|
23
|
Rodríguez-Pinilla SM, Sarrió D, Honrado E,
Hardisson D, Calero F, Benitez J and Palacios J: Prognostic
significance of basal-like phenotype and fascin expression in
node-negative invasive breast carcinomas. Clin Cancer Res.
12:1533–1539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang BW, Decker RH, Haffty BG and Knisely
JPS: Incidence of brain metastases in early-stage triple negative
breast cancer patients. Int J Radiat Oncol Biol Phys. 69(Suppl):
S210–S211. 2007. View Article : Google Scholar
|
|
25
|
Lin Y, Yin W, Yan T, Zhou L, Di G, Wu J,
Shen Z, Shao Z and Lu J: Site-specific relapse pattern of the
triple negative tumors in Chinese breast cancer patients. BMC
Cancer. 9:3422009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SS, Ahn JH, Kim MK, Sym SJ, Gong G,
Ahn SD, Kim SB and Kim WK: Brain metastases in breast cancer:
Prognostic factors and management. Breast Cancer Res Treat.
111:523–530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Santos GT, Prolla JC, Camillo ND, Zavalhia
LS, Ranzi AD and Bica CG: Clinical and pathological factors
influencing the survival of breast cancer patients with malignant
pleural effusion. J Bras Pneumol. 38:487–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang EL and Lo S: Diagnosis and
management of central nervous system metastases from breast cancer.
Oncologist. 8:398–410. 2003. View Article : Google Scholar : PubMed/NCBI
|