Introduction
Breast cancer, the most commonly diagnosed
malignancy in women, is a heterogeneous disease encompassing
phenotypically distinct tumor subtypes (1,2). It is
estimated that breast cancer is responsible for >1,300,000 cases
and 450,000 deaths annually worldwide (3). In China, newly diagnosed cases and
deaths account for 12.2 and 9.6% of global breast cancer patients,
respectively (4,5). With the advances in treatment and early
detection, breast cancer mortality decreased by 34% from 1990 to
2010 (6). In America, the 5-year
survival rate for breast cancer patients is >75%, while the
5-year survival rate for patients with breast cancer in Shanghai
(the most industrialised city in China) was found to be 78%
compared with 58% reported in Qidong (a rural neighbouring area of
Shanghai) (4). Therefore, there is a
need for simple, reliable and non-invasive prognostic biomarkers to
enable clinicians to perform risk evaluation in breast cancer
patients prior to or during the treatment process.
Tumor markers in breast cancer have been
investigated for nearly 20 years (7,8). Recently,
certain indices from complete blood count (CBC) appear to be useful
for predicting outcomes in patients with breast cancer. The
neutrophil-to-lymphocyte ratio (NLR) has been used not only as a
biomarker of inflammation, but also as a prognostic index for
various common solid tumors, such as gastric cancer, breast
carcinoma, colorectal carcinoma, nasopharyngeal cancer and
malignant melanoma (9). Azab et
al first evaluated NLR in predicting short- and long-term
mortality in breast cancer patients (10) and found that patients in the highest
NLR quartile had higher 1- and 5-year mortality rates compared with
those in the lowest quartile. Several studies reported similar
results (11–16). In addition, low hemoglobin (HGB) level
prior to each cycle of adjuvant polychemotherapy, as a marker of
anemia, was reported to be associated with worse local relapse-free
survival in patients with primary breast cancer (17–21).
Furthermore, mean corpuscular volume (MCV) was also assessed during
capecitabine therapy in metastatic breast cancer (9,22).
However, in the study of Bozkurt et al (23), no significant difference was
identified between tumor response and increase in MCV. Moreover, a
limited number of previous studies were based on Chinese patients
with breast cancer. Thus far, the clinical utility of pretreatment
CBC parameters in breast cancer prognosis requires further
validation.
Consequently, in this retrospective analysis, we
attempted to investigate the prognostic value of red cell indices
(RCIs) and NLR in breast cancer patients with a long follow-up,
taking into account several clinicopathological characteristics,
such as tumor markers, clinical stage and histological grade.
Patients and methods
Patients and clinical data
We performed a retrospective review of patients
undergoing complete resection of breast cancer between 2001 and
2005. A total of 162 breast cancer patients from the Chinese PLA
General Hospital (Beijing, China) were enrolled in this study. The
inclusion criteria were as follows: patients diagnosed with breast
cancer, with data on CBC, including RCIs and leukocyte differential
count, prior to initiating any chemotherapy. The exclusion criteria
were as follows: patients without any blood count data prior to
chemotherapy, presence of active infection, presence of coexisting
hematological malignancies or other hematological disorders,
autoimmune disorders, and patients on recent steroid therapy. These
patients have been followed for up to 9.6 years. The median overall
survival (OS) and disease-free survival (DFS) were 74.6 months
(range, 2.4–115.6 months) and 69.9 months (range, 1.5–115.6
months), respectively. The design of this study was approved by our
Hospital's Ethics Committee and all the patients provided written
informed consent.
Data were collected from patient medical records,
pathology reports, and blood results recorded on admission prior to
the initiation of any treatment, including radiotherapy,
chemotherapy, or surgery. The medical records for each patient were
independently reviewed by two physicians for the baseline
characteristics (Table I). All the
test results were obtained using the same equipment. RCIs from
blood test results were collected, including red blood cell (RBC)
count, HGB, hematocrit (HCT), MCV, mean corpuscular hemoglobin
(MCH) and mean corpuscular hemoglobin concentration (MCHC).
Neutrophil-to-lymphocyte ratio (NLR) was also calculated based on
the CBC. breast cancer patients were divided into two groups (high
vs. low) according to the median value of each index. Accordingly,
the two groups obtained from our data for NLR were NLR <1.81 and
≥1.81. Similarly, the two groups for MCH were MCH <30.6 and
≥30.6. Table I summarizes the patient
characteristics according to the MCH and NLR groups.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
|
| MCH |
| NLR |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristics | Overall | Low | High | P-value | Low | High | P-value |
|---|
| Patients | 162 (100%) | 83 | 79 |
| 80 | 82 |
|
| Age ± SD (years) | 50.8±10.6 | 50.6±10.1 | 50.9±11.1 | 0.847 | 51.9±10.3 | 49.9±10.8 | 0.228 |
| T stage, n |
|
|
| 0.379 |
|
| 0.774 |
| T1 | 53 | 26 | 27 |
| 28 | 25 |
|
| T2 | 89 | 50 | 39 |
| 44 | 45 |
|
| T3 | 17 | 6 | 11 |
| 6 | 9 |
|
| T4 | 3 | 1 | 2 |
| 2 | 1 |
|
| N stage, n (%) |
|
|
| 0.915 |
|
| 0.345 |
| N0 | 73 (45.1) | 36 | 37 |
| 37 | 36 |
|
| N1 | 42 (25.9) | 23 | 19 |
| 23 | 19 |
|
| N2 | 22 (13.6) | 12 | 10 |
| 7 | 15 |
|
| N3 | 25 (15.4) | 12 | 13 |
| 13 | 12 |
|
| Histological grade,
n (%) |
|
|
| 0.216 |
|
| 0.593 |
|
I/II | 120 (74.1) | 65 | 55 |
| 61 | 59 |
|
|
III | 42 (25.9) | 18 | 24 |
| 19 | 23 |
|
| Clinical stage, n
(%) |
|
|
| 0.616 |
|
| 0.241 |
|
I/II | 110 (67.9) | 58 | 52 |
| 58 | 52 |
|
|
III | 52 (32.1) | 25 | 27 |
| 22 | 30 |
|
| ER, n (%) |
|
|
| 0.552 |
|
| 0.156 |
|
Positive | 87 (53.7) | 46 | 41 |
| 38 | 49 |
|
|
Negative | 74 (45.7) | 37 | 37 |
| 42 | 32 |
|
|
Unkonwn | 1 (0.6) | 0 | 1 |
| 0 | 1 |
|
| PR, n (%) |
|
|
| 0.888 |
|
| 0.156 |
|
Positive | 77 (47.6) | 41 | 36 |
| 32 | 45 |
|
|
Negative | 83 (51.2) | 41 | 42 |
| 48 | 35 |
|
|
Unkonwn | 2 (1.2) | 1 | 1 |
| 0 | 2 |
|
| Her2, n (%) |
|
|
| 0.044 |
|
| 0.1406 |
|
Positive | 37 (22.8) | 13 | 24 |
| 22 | 15 |
|
|
Negative | 124 (76.5) | 70 | 54 |
| 58 | 76 |
|
|
Unkonwn | 1 (0.6) | 0 | 1 |
| 0 | 1 |
|
Statistical analysis
Statistical analysis was performed using SPSS
software, version 21.0 (Armonk, NY, USA). Continuous variables are
presented as mean ± standard deviation and categorical variables
are presented as frequencies and percentages. Student's t-test or
Mann-Whitney U test was used to test the differences between the
two groups. For group comparisons, chi-square and Fisher's exact
tests were used for categorical variables. Kaplan-Meier survival
curves were plotted for DFS and OS in the different NLR and MCH
groups. The statistical differences between these curves were
detected using log-rank tests. Clinicopathological parameters are
known to be associated with prognosis, including tumor size (T2-3
vs. T1), histological grade (III vs. I/II), estrogen receptor (ER)
status (negative vs. positive), progesterone receptor (PR) status
(negative vs. positive), human epidermal growth factor receptor 2
(HER2) status (negative vs. positive), and the value of each RCI
separately (high vs. low) were tested with univariate analysis.
Variables that were found to be significant in the univariate
analysis were then entered in a stepwise multivariate Cox
proportional hazard regression model to ascertain the individual
contribution of factors associated with DFS and OS.
Results
Patient characteristics
Table I presents the
general information and characteristics related to the tumors of
the 162 breast cancer patients by MCH and NLR. The mean age of our
population was 50.8 years. According to the American Joint
Committee on Cancer staging guidelines, 110 patients were
classified as stage I/II and 52 as stage III. All the patients were
followed up until death or the last date of this study. The 10-year
DFS and OS of all 162 breast cancer patients were 67 and 77%,
respectively. NLR was not found to be associated with age,
histological grade, tumor size, lymph node status, ER status, PR
status, or HER2 status in the population studied. As regards MCH,
the higher MCH group had significantly higher rates of
HER2-positive tumors (P=0.044; Table
I).
Survival analysis of RCIs and NLR
We evaluated the correlation of RCIs and NLR with
prognosis of breast cancer. Kaplan-Meier survival analysis and
log-rank tests were performed using patients' postoperative
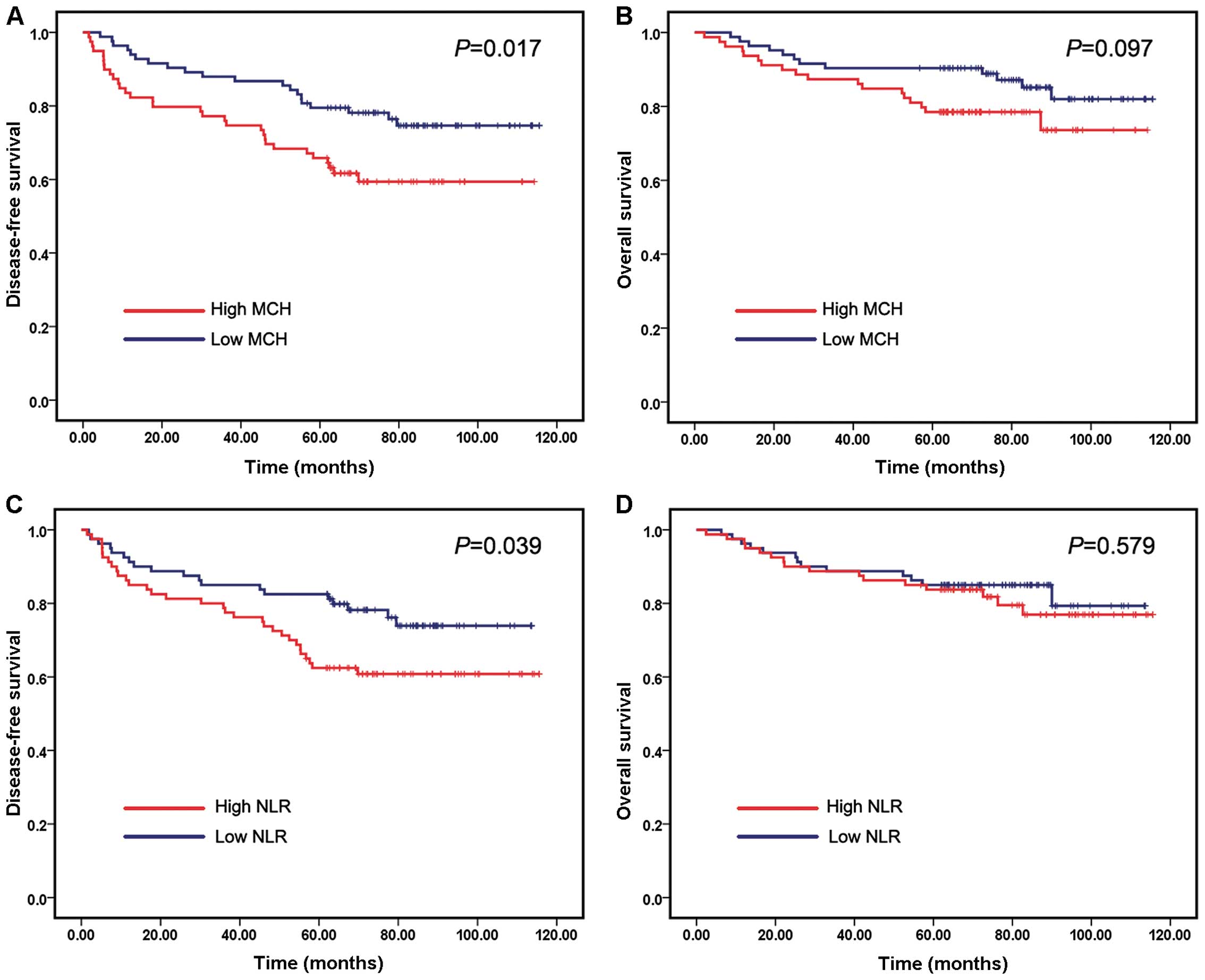
survival. The Kaplan-Meier survival curves demonstrated that
patients with high MCH exhibited worse DFS compared with those with
low MCH (P=0.017) (Fig. 1A), whereas
higher MCH only showed a trend of higher patient mortality
(Fig. 1B). Similarly, patients with
high NLR had shorter DFS times compared with those with low NLR
(P=0.039) (Fig. 1C). However,
patients with low NLR did not exhibit significantly longer OS times
compared with those with high NLR (Fig.
1D). The values of the remaining five RCIs were not correlated
with DFS or OS times.
Predictive value of MCH
The univariate analysis revealed that tumor size,
histological grade, clinical stage and lymph node status were
significantly associated with DFS and OS in our cohort, whereas PR
was significantly associated with OS (Table II). As regards RCIs, the univariate
analysis revealed that only MCH was significantly associated with
DFS, whereas no index was associated with OS (Table II). A Cox proportional hazards model
generated a univariate hazard ratio (HR) of 1.959 [95% confidence
interval (CI): 1.114–3.445, P=0.020] for DFS by comparing high and
low MCH. For OS, the univariate HR of high MCH compared with low
MCH was 1.848 (95% CI: 0.885–3.858, P=0.102). To further
investigate the effect of all the parameters showing significance
in the univariate analysis regarding the prognosis of breast cancer
patients, a multivariate Cox proportional hazards model was yielded
for DFS using the following variables: MCH, tumor size, lymph node
status, clinical stage and histological grade (Table III). Following backward elimination,
the final model consisted of MCH, tumor size and clinical stage.
The multivariate HR of high MCH compared with low MCH was 1.975
(95% CI: 1.118–3.487, P=0.019), which demonstrated that MCH was an
independent factor associated with DFS in these patients (Table IV).
 | Table II.Univariate analysis for disease-free
and overall survival of patients with breast cancer. |
Table II.
Univariate analysis for disease-free
and overall survival of patients with breast cancer.
|
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Factors | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age | 0.987
(0.961–1.014) | 0.341 | 1.003
(0.970–1.038) | 0.848 |
| Tumor size, T2-T4
vs. T1 | 4.203
(1.792–9.857) | 0.001 | 7.243
(1.725–30.412) | 0.007 |
| Lymph node N1–3 vs.
N0 | 3.424
(1.756–6.679) | <0.001 | 5.973
(2.083–17.130) | 0.001 |
| Histology grade,
III vs. I/II | 2.228
(1.302–4.019) | 0.004 | 3.656
(1.777–7.524) | <0.001 |
| Clinical stage, III
vs. I/II | 4.228
(2.402–7.442) | <0.001 | 5.036
(2.352–10.786) | <0.001 |
| ER | 0.696
(0.401–1.206) | 0.196 | 0.602
(0.292–1.241) | 0.169 |
| PR | 0.617
(0.351–1.082) | 0.092 | 0.348
(0.155–0.782) | 0.011 |
| HER2 | 1.607
(0.879–2.940) | 0.124 | 1.760
(0.800–3.873) | 0.160 |
| RBC | 0.640
(0.366–1.119) | 0.117 | 0.862
(0.421–1.766) | 0.684 |
| Hgb | 0.929
(0.536–1.608) | 0.792 | 1.330
(0.646–2.740) | 0.439 |
| HCT | 0.726
(0.417–1.264) | 0.258 | 1.164
(0.568–2.386) | 0.678 |
| MCV | 1.585
(0.907–2.770) | 0.106 | 1.644
(0.792–3.416) | 0.182 |
| MCH | 1.959
(1.114–3.445) | 0.020 | 1.848
(0.885–3.858) | 0.102 |
| MCHC | 1.108
(0.640–1.920) | 0.714 | 1.015
(0.495–2.082) | 0.967 |
| NLR | 1.808
(1.021–3.202) | 0.042 | 1.230
(0.591–2.559) | 0.579 |
 | Table III.Multivariate model predicting DFS
using MCH. |
Table III.
Multivariate model predicting DFS
using MCH.
|
| DFS |
|---|
|
|
|
|---|
| Factor | Hazard ratio (95%
CI) | P-value |
|---|
| Tumor size, T2-T4
vs. T1 | 2.800
(1.146–6.841) | 0.024 |
| Lymph node N1–3 vs.
N0 | 1.525
(0.625–3.725) | 0.354 |
| Histology grade,
III vs. I/II | 1.405
(0.776–2.544) | 0.261 |
| Clinical stage, III
vs. I/II | 2.244
(1.042–4.830) | 0.039 |
| MCH | 1.975
(1.118–3.487) | 0.019 |
 | Table IV.Multivariate model predicting DFS
using NLR. |
Table IV.
Multivariate model predicting DFS
using NLR.
|
| DFS |
|---|
|
|
|
|---|
| Factor | Hazard ratio (95%
CI) | P-value |
|---|
| Tumor size, T2-T4
vs. T1 | 2.503
(1.023–6.126) | 0.044 |
| Lymph node N1–3 vs.
N0 | 1.453
(0.596–3.541) | 0.411 |
| Histology grade,
III vs. I/II | 1.440
(0.792–2.618) | 0.232 |
| Clinical stage, III
vs. I/II | 2.253
(1.041–4.876) | 0.039 |
| NLR | 1.435
(0.803–2.563) | 0.223 |
Predictive value of NLR
A Cox proportional hazards model yielded a
univariate HR=1.808 (95% CI: 1.021–3.202, P=0.042) of high compared
with low NLR for DFS, whereas NLR was not significantly associated
with OS (P=0.579) (Table III). a
multivariate Cox proportional hazards model was constructed for DFS
by incorporating the following variables: NLR, tumor size, lymph
node status, clinical stage and histological grade. The
multivariate HR of high compared with low NLR was 1.435 (95% CI:
0.803–2.563, P=0.223) (Table
IV).
Discussion
In this study, we performed a systematic evaluation
of the prognostic significance of RCIs as well as of NLR in breast
cancer and revealed three major findings: i) MCH values were
positively associated with HER2 status; ii) patients with higher
MCH and NLR values prior to treatment were found to be associated
with a poor prognosis; and iii) the multivariate Cox analysis
demonstrated that MCH was an independent factor associated with DFS
in our cohort, while NLR did not exhibit any independent prognostic
significance regarding OS and DFS.
Using median values as cut-off points, hematological
parameters were stratified into high and low groups. Other than MCH
and NLR, no parameters were found to be significantly correlated
with DFS and OS in our cohort. As markers of anemia, HGB and MCV
have been evaluated in a number of studies. Anemia is a frequent
complication of cancer and cancer therapy, which has been shown to
be highly associated with patient energy levels and quality of life
scores, but also to exert a prognostic effect in several types of
cancer, including breast cancer (17,24). From
the European Cancer Anemia Survey prospective study in 2001, 62% of
the 3,278 patients with breast cancer developed anemia at least
once during the study follow-up period (25). A number of investigators have
suggested an association between HGB level during chemotherapy and
local relapse-free survival in predicting breast cancer outcome
(17,21). However, HGB did not exhibit
significant prognostic value in this study. Previous studies have
reported the effect of MCV increase on prognosis in patients with
metastatic breast cancer treated with capecitabine (9,22). In this
study, MCV only exhibited a trend of higher mortality in patients
(data not shown). A possible explanation is that the test
parameters were recorded prior to treatment in our study, compared
with those collected during the treatment process in those
studies.
Clinical stage, tumor size and ER status are known
predictors of prognosis in the patients with breast cancer.
However, OS and DFS may differ widely in patients with the same
status who receive the same treatment, suggesting that other, as
yet undetermined, factors may affect prognosis. as regards
pretreatment hematological parameters that have been predictive of
patient prognosis, we next performed univariate and multivariate
analyses of factors predictive of DFS and OS in patients with
breast cancer. We found that clinical stage, tumor size,
histological grade and pretreatment MCH were prognostic factors for
DFS in our patient cohort. MCH represents the absolute amount of
hemoglobin in the average red cell in a sample. Since the
correlation between MCH and tumors has not been extensively
investigated, the mechanisms underlying the association of high MCH
with poor DFS in breast cancer patients is poorly understood. One
potential mechanism underlying the prognostic impact of MCH may be
the association of elevated MCH with metabolism. Tarocco et
al (26) reported that elevated
MCH was found to be negatively correlated with low level of serum
folic acid and vitamin B12, which are important cofactors in DNA
synthesis, repair and methylation (27,28).
Deficient folate and vitamin B12 levels may affect the prognosis of
breast cancer patients by reducing the availability of
S-adenosylmethionine for DNA methylation (29). a population-based case-control study
of breast cancer conducted in urban Shanghai during 1996–1998
(28) reported evidence of a
decreased risk of breast cancer associated with high consumption of
folate. Several reports also revealed an association between higher
plasma levels of folate and reduced risk of developing breast
cancer (30–32).
A number of recent studies have suggested that an
elevated NLR is associated with poor survival of patients with
cancer (33). The mechanisms
underlying the association of high NLR with poor outcome of cancer
patients are poorly understood, but may be explained by the
following facts: an association with inflammation may be underlying
the prognostic impact of NLR (7). The
contribution of host inflammatory reactions to cancer development
has been reported. Immunocompetent lymphocytes and neutrophils may
play an important role in the systemic inflammatory response
(34). Azab et al (10) first evaluated NLR in predicting
mortality in breast cancer patients and found that NLR was an
independent predictor of mortality in breast cancer patients with
NLR >3.3. Noh et al reported that patients with an
elevated pretreatment NLR exhibited poorer DFS compared with
patients without elevated NLR, which was most evident in the
luminal A subtype (14). Similar
results were reported in several other studies on breast cancer
(11,13,15,16). In
this study, the breast cancer patients with elevated NLR exhibited
shorter DFS times compared with those without elevated NLR
(P=0.039; Fig. 1C). This was
consistent with previously reported results (11,14), while
there was no significant trend for elevated NLR to be associated
with worse patient OS, and NLR was not found to be an independent
predictor of DFS or OS in breast cancer patients. The possible
reasons for this may be as follows: First, the median value of NLR
was used as a cut-off point to stratify high and low NLR groups,
which is different from other reports; and second, our small sample
size may reflect a selection bias to some extent.
There were certain limitations and potential biases
to this study. First, only Chinese breast cancer patients were
included in our study. Due to small sample size, larger scale and
multicenter studies should be performed to elucidate the accuracy
and clinical value of MCH. Second, due to its retrospective nature,
some records of clinical details, such as body mass index,
carbohydrate antigen 15–3 and folic acid concentration, were not
available in this study. Thus, the correlation between MCH and
these parameters remains unclear. Furthermore, we were unable to
determine whether preoperative MCH was a better predictor of DFS
compared with conventional prognostic factors.
In conclusion, we demonstrated that patients with
high pretreatment MCH may be predicted to exhibit shorter DFS time
compared with those with low pretreatment MCH. MCH is an
independent predictor associated with DFS. Additional studies,
however, are required for further validation in a larger population
from different races and regions.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81472497, 81572620 and
31100960) and the Shandong Provincial Natural Science Foundation of
China (no. ZR2015HM003).
Glossary
Abbreviations
Abbreviations:
|
BMI
|
body mass index
|
|
CBC
|
complete blood count
|
|
DFS
|
disease-free survival
|
|
HGB
|
hemoglobin
|
|
HCT
|
hematocrit
|
|
MCV
|
mean corpuscular volume
|
|
MCH
|
mean corpuscular hemoglobin
|
|
MCHC
|
mean corpuscular hemoglobin
concentration
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
RBC
|
red blood cell
|
|
RCI
|
red cell indices
|
|
OS
|
overall survival
|
References
|
1
|
Eichelser C, Flesch-Janys D, Chang-Claude
J, Pantel K and Schwarzenbach H: Deregulated serum concentrations
of circulating cell-free microRNAs miR-17, miR-34a, miR-155, and
miR-373 in human breast cancer development and progression. Clin
Chem. 59:1489–1496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei C, Cao Y, Yang X, Zheng Z, Guan K,
Wang Q, Tai Y, Zhang Y, Ma S, Cao Y, et al: Elevated expression of
TANK-binding kinase 1 enhances tamoxifen resistance in breast
cancer. Proc Natl Acad Sci USA. 111:E601–E610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Zhang BN, Fan JH, Pang Y, Zhang P,
Wang SL, Zheng S, Zhang B, Yang HJ, Xie XM, et al: A nation-wide
multicenter 10-year (1999–2008) retrospective clinical
epidemiological study of female breast cancer in China. BMC cancer.
11:3642011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loi S, Sirtaine N, Piette F, Salgado R,
Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, et al:
Prognostic and predictive value of tumor-infiltrating lymphocytes
in a phase III randomized adjuvant breast cancer trial in
node-positive breast cancer comparing the addition of docetaxel to
doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin
Oncol. 31:860–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molina R, Auge JM, Farrus B, Zanón G,
Pahisa J, Muñoz M, Torne A, Filella X, Escudero JM, Fernandez P and
Velasco M: Prospective evaluation of carcinoembryonic antigen (CEA)
and carbohydrate antigen 15.3 (CA 15.3) in patients with primary
locoregional breast cancer. Clin Chem. 56:1148–1157. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arslan C, Aksoy S, Dizdar O, Kurt M, Güler
N, Ozisik Y, Güllü I and Altundag K: Increased mean corpuscular
volume of erythrocytes during capecitabine treatment: A simple
surrogate marker for clinical response. Tumori. 97:711–716.
2011.PubMed/NCBI
|
|
10
|
Azab B, Bhatt VR, Phookan J, Murukutla S,
Kohn N, Terjanian T and Widmann WD: Usefulness of the
neutrophil-to-lymphocyte ratio in predicting short- and long-term
mortality in breast cancer patients. Ann Surg Oncol. 19:217–224.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakano K, Hosoda M, Yamamoto M and
Yamashita H: Prognostic significance of pre-treatment neutrophil:
Lymphocyte ratio in Japanese patients with breast cancer.
Anticancer Res. 34:3819–3824. 2014.PubMed/NCBI
|
|
12
|
Ishizuka M, Nagata H, Takagi K, Iwasaki Y
and Kubota K: Combination of platelet count and neutrophil to
lymphocyte ratio is a useful predictor of postoperative survival in
patients with colorectal cancer. Br J Cancer. 109:401–407. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dirican A, Kucukzeybek BB, Alacacioglu A,
Kucukzeybek Y, Erten C, Varol U, Somali I, Demir L, Bayoglu IV,
Yildiz Y, et al: Do the derived neutrophil to lymphocyte ratio and
the neutrophil to lymphocyte ratio predict prognosis in breast
cancer? Int J Clin Oncol. 20:70–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noh H, Eomm M and Han A: Usefulness of
pretreatment neutrophil to lymphocyte ratio in predicting
disease-specific survival in breast cancer patients. J Breast
Cancer. 16:55–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Azab B, Shah N, Radbel J, Tan P, Bhatt V,
Vonfrolio S, Habeshy A, Picon A and Bloom S: Pretreatment
neutrophil/lymphocyte ratio is superior to platelet/lymphocyte
ratio as a predictor of long-term mortality in breast cancer
patients. Med Oncol. 30:4322013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koh YW, Lee HJ, Ahn JH, Lee JW and Gong G:
Prognostic significance of the ratio of absolute neutrophil to
lymphocyte counts for breast cancer patients with ER/PR-positivity
and HER2-negativity in neoadjuvant setting. Tumour Biol.
35:9823–9830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dubsky P, Sevelda P, Jakesz R,
Hausmaninger H, Samonigg H, Seifert M, Denison U, Mlineritsch B,
Steger G, Kwasny W, et al: Anemia is a significant prognostic
factor in local relapse-free survival of premenopausal primary
breast cancer patients receiving adjuvant
cyclophosphamide/methotrexate/5-fluorouracil chemotherapy. Clin
Cancer Res. 14:2082–2087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bottini A, Berruti A, Brizzi MP, Bersiga
A, Generali D, Allevi G, Aguggini S, Bolsi G, Bonardi S, Bertoli G,
et al: Pretreatment haemoglobin levels significantly predict the
tumour response to primary chemotherapy in human breast cancer. Br
J Cancer. 89:977–982. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vaupel P, Mayer A, Briest S and Höckel M:
Hypoxia in breast cancer: Role of blood flow, oxygen diffusion
distances, and anemia in the development of oxygen depletion. Adv
Exp Med Biol. 566:333–342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peters-Engl C, Cassik P, Schmidt I,
Denison U, Medl M, Pokieser W and Sevelda P: Impact of haemoglobin
levels during adjuvant chemotherapy on the survival of patients
with primary breast cancer. Acta Oncol. 44:129–133. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boehm DU, Lebrecht A, Schmidt M, Siggelkow
W, Lindner C, Litz A, Ulbrich E and Koelbl H: Prognostic impact of
haemoglobin levels in breast cancer. Anticancer Res. 27:1223–1226.
2007.PubMed/NCBI
|
|
22
|
Karvellas CJ, Sawyer M, Hamilton M and
Mackey JR: Effect of capecitabine on mean corpuscular volume in
patients with metastatic breast cancer. Am J Clin Oncol.
27:364–368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bozkurt O, Berk V, Kaplan MA, Cetin B,
Ozaslan E, Karaca H, Inanc M, Duran AO and Ozkan M: Lack of
prognostic value of mean corpuscular volume with capecitabine
therapy in metastatic breast cancer. Asian Pac J Cancer Prev.
15:2501–2504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ludwig H, Müldür E, Endler G and Hübl W:
Prevalence of iron deficiency across different tumors and its
association with poor performance status, disease status and
anemia. Ann Oncol. 24:1886–1892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ludwig H, Van Belle S, Barrett-Lee P,
Birgegård G, Bokemeyer C, Gascón P, Kosmidis P, Krzakowski M,
Nortier J, Olmi P, et al: The european cancer anaemia survey
(ECAS): A large, multinational, prospective survey defining the
prevalence, incidence, and treatment of anaemia in cancer patients.
Eur J Cancer. 40:2293–2306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tarocco RP, Faro G, Sargiotto A and
Ansermin A: Folate and vitamin B12 deficiency. Characterization of
parameters for early diagnosis. Recenti Prog Med. 80:547–550.
1989.(In Italian). PubMed/NCBI
|
|
27
|
Toprak B, Yalcin HZ and Colak A: Vitamin
B12 and folate deficiency: Should we use a different cutoff value
for hematologic disorders? Int J Lab Hematol. 36:409–414. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shrubsole MJ, Jin F, Dai Q, Shu XO, Potter
JD, Hebert JR, Gao YT and Zheng W: Dietary folate intake and breast
cancer risk: Results from the Shanghai breast cancer study. Cancer
Res. 61:7136–7141. 2001.PubMed/NCBI
|
|
29
|
Blount BC, Mack MM, Wehr CM, MacGregor JT,
Hiatt RA, Wang G, Wickramasinghe SN, Everson RB and Ames BN: Folate
deficiency causes uracil misincorporation into human DNA and
chromosome breakage: Implications for cancer and neuronal damage.
Proc Natl Acad Sci USA. 94:3290–3295. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang SM, Willett WC, Selhub J, Hunter DJ,
Giovannucci EL, Holmes MD, Colditz GA and Hankinson SE: Plasma
folate, vitamin B6, vitamin B12, homocysteine, and risk of breast
cancer. J Natl Cancer Inst. 95:373–380. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin J, Lee IM, Cook NR, Selhub J, Manson
JE, Buring JE and Zhang SM: Plasma folate, vitamin B-6, vitamin
B-12, and risk of breast cancer in women. Am J Clin Nutr.
87:734–743. 2008.PubMed/NCBI
|
|
32
|
Li B, Lu Y, Wang L and Zhang CX: Folate
intake and breast cancer prognosis: A meta-analysis of prospective
observational studies. Eur J Cancer Prev. 24:113–121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Inanc M, Duran AO, Karaca H, Berk V,
Bozkurt O, Ozaslan E and Ozkan M: Haematologic parameters in
metastatic colorectal cancer patients treated with capecitabine
combination therapy. Asian Pac J Cancer Prev. 15:253–256. 2014.
View Article : Google Scholar : PubMed/NCBI
|