Introduction
Esophageal squamous cell carcinoma (ESCC) is the
sixth most common type of cancer in Japan, and ESCC-related
mortality has been steadily increasing in recent years. ESCC is
often diagnosed at an advanced stage, and the 5-year survival rate
of patients who undergo curative surgery is only 20–30%. In our
hospital, we have encountered a number of patients who developed
tumor recurrence or distant metastasis during the early stages of
the disease, within a short period of time after surgery. Recent
molecular biological studies have revealed that esophageal cancer
is caused by the accumulation of multiple genetic defects in
dominant oncogenes and tumor suppressor genes. In order to
elucidate the role of connexin 43 (Cx43) in ESCC, the expression
status of Cx43 in patients with ESCC was evaluated (1–7). In
addition, the associations between Cx43 expression and
clinicopathological characteristics or prognosis were analyzed.
Patients and methods
Patients and tissue samples
Samples were obtained from 98 patients with primary
ESCCs. The patients had undergone radical esophagectomy, without
any preoperative induction therapy, at the Department of Surgery
II, Nagoya City University Medical School (Nagoya, Japan) between
1997 and 2006. The study design was approved by the institutional
review board of our university, and written consent was obtained
from all the patients. The tumors were classified according to the
guidelines for clinical and pathological studies on carcinoma of
the esophagus established by the Japanese Society for Esophageal
Diseases (8). Tissue specimens were
collected from 79 men and 19 women with a mean age of 63.5±8.3
years (range, 46–78 years). Tissues collected for
immunohistochemistry were fixed in formalin and embedded in
paraffin.
Immunohistochemistry
Immunohistochemical staining was performed on
formalin-fixed, paraffin-embedded ESCC tissues. The
paraffin-embedded tumor sections were deparaffinized, rehydrated,
heat-treated by microwaving in 10 mM citrate buffer for 15 min for
antigen retrieval, and cooled to room temperature. The sections
were then treated with 0.3% H2O2 in methanol
for 30 min to neutralize endogenous peroxidase activity, blocked
with Block ace (DS Pharma Biomedical, Osaka, Japan) for 10 min, and
incubated with primary monoclonal anti-Cx43 antibody (cat. no.
sc-59949; 1:500 dilution; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) overnight at 4°C. Immunoreactive protein was detected with
a Dako Envision+ System, horseradish peroxidase (substrate,
diaminobenzidine) and the sections were counterstained with
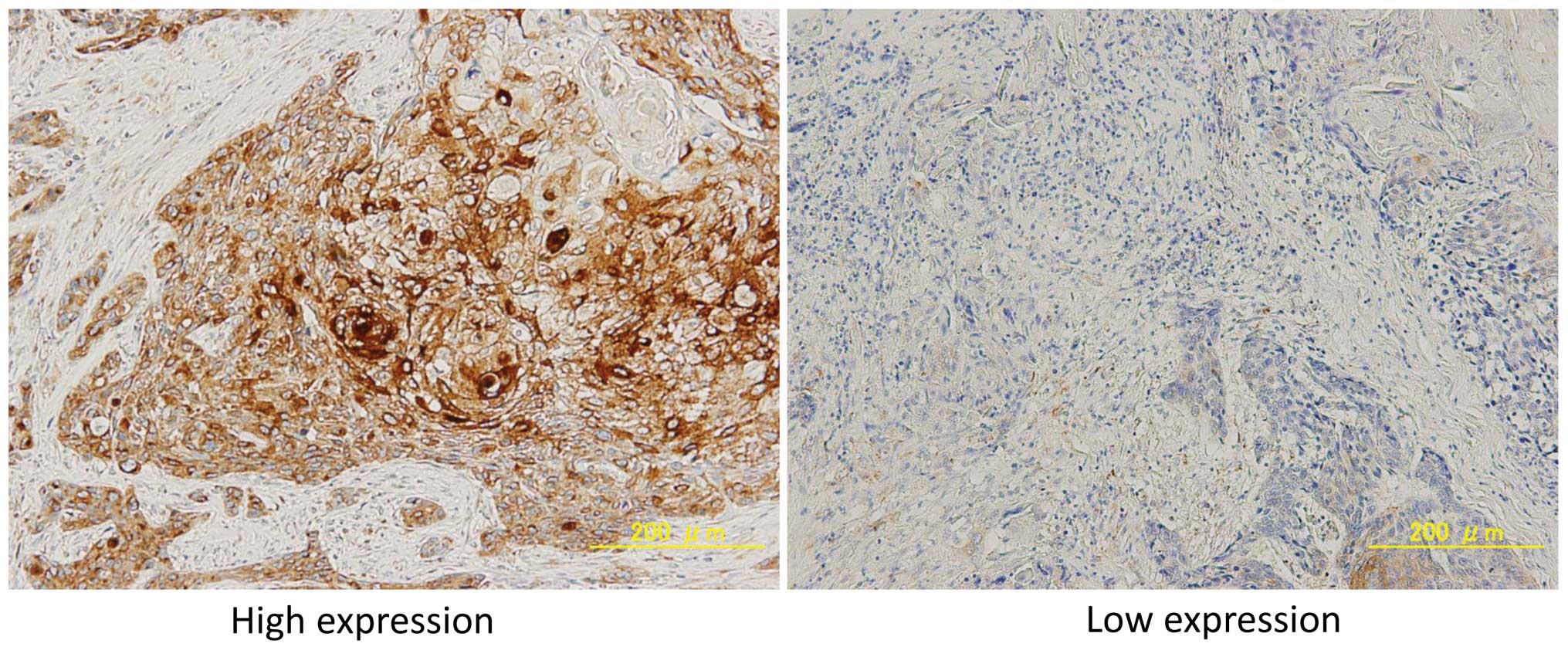
hematoxylin. Cx43 immunostaining was subjectively assessed by two
independent investigators (T.T. and H.I.) using light microscopy.
The intensity of Cx43 immunostaining was classified into 4 grades
(0–3), where grades 0 and 1 were defined as low expression, and
grades 2 and 3 were defined as high expression.
Statistical analysis
Statistical analysis was performed using the EZR
software package (Saitama Medical Center, Jichi Medical University,
Saitama, Japan). The Chi-square test was used to analyze the
association between immunohistochemical analysis and the clinical
and histopathological parameters of the patients. The survival of
ESCC patients following surgery was assessed using the Kaplan-Meier
method, and survival times were compared using the log-rank test.
The data are expressed as mean ± standard deviation. Multivariate
analysis was performed using the Cox regression and logistic
multivariate regression models. In all the analyses, P-values of
<0.05 indicated statistically significant differences.
Results
Cx43 immunostaining
Of the 98 patients enrolled in this study, 62
(63.3%) were high and 36 (37.7%) were low for Cx43 expression. Cx43
expression was observed in epithelial, muscle and tumor cells.
Representative cases of immunostaining are shown in Fig. 1.
Association between
clinicopathological characteristics and Cx43 expression
The correlations between immunostaining for Cx43 and
the clinicopathological characteristics of the patients are
presented in Table I. No significant
correlations were observed between Cx43 expression and
clinicopathological characteristics such as age, gender, T status,
N status, stage, lymphatic invasion, venous invasion and
differentiation (Table I). We
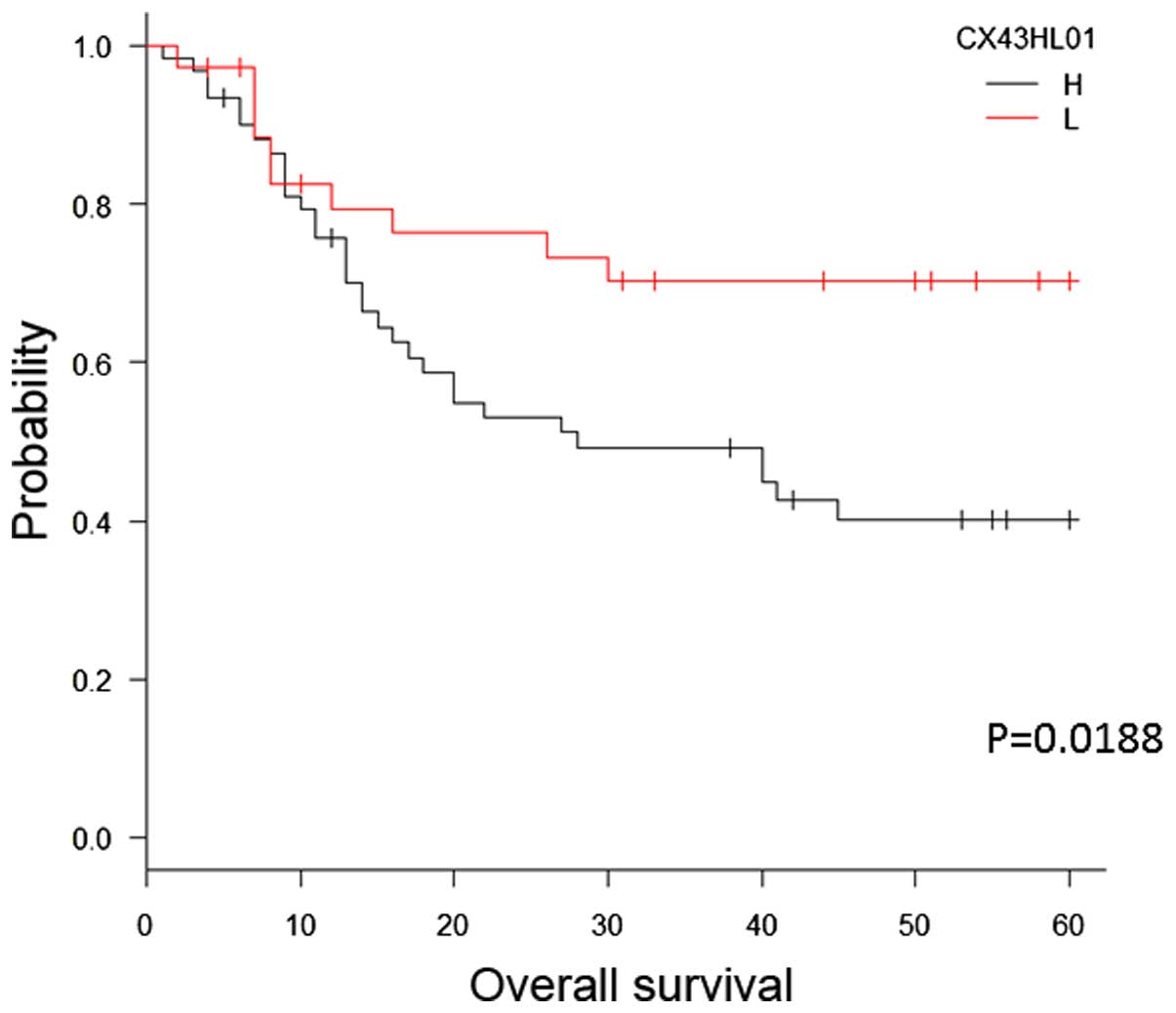
subsequently investigated the correlation between immunostaining
for Cx43 and survival in patients with ESCC after surgery (median
follow-up, 28 months). The patients in the Cx43-high group
exhibited significantly shorter survival following surgery compared
with patients in the Cx43-low group (P=0.0188, log-rank test;
Fig. 2).
 | Table I.Association between
clinicopathological characteristics and connexin 43 expression. |
Table I.
Association between
clinicopathological characteristics and connexin 43 expression.
| Characteristics | Cases (n=98) | High expression
(n=62) | Low expression
(n=36) | P-value |
|---|
| Age, years |
|
|
| 1 |
|
>65 | 44 | 28 | 16 |
|
|
<65 | 54 | 34 | 20 |
|
| Gender |
|
|
| 0.792 |
| Male | 79 | 49 | 30 |
|
|
Female | 19 | 13 | 6 |
|
| Primary tumor |
|
|
|
|
| Tis | 1 | 0 | 1 | 0.00284 |
| 1 | 31 | 12 | 19 |
|
| 2 | 15 | 13 | 2 |
|
| 3 | 25 | 19 | 6 |
|
| 4 | 26 | 18 | 8 |
|
|
Tis-T2 | 47 | 25 | 22 | 0.06 |
| T3,4 | 51 | 37 | 14 |
|
| Lymph node
metastasis |
|
|
| 0.397 |
|
Negative | 42 | 24 | 18 |
|
|
Positive | 55 | 37 | 18 |
|
| Histological
differentiation |
|
|
| 0.481 |
| High | 32 | 23 | 9 |
|
|
Moderate | 52 | 31 | 21 |
|
| Poor | 14 | 8 | 6 |
|
| Lymphatic
invasion |
|
|
| 0.343 |
|
Positive | 72 | 48 | 24 |
|
|
Negative | 26 | 14 | 12 |
|
| Venous invasion |
|
|
| 0.281 |
|
Positive | 64 | 43 | 21 |
|
|
Negative | 34 | 19 | 15 |
|
Uni- and multivariate analysis for
clinicopathological variables of Cx43 expression
The univariate analysis revealed that, among the
clinicopathological factors, tumor status [risk ratio (RR)=6.399;
P<0.01], lymph node status (RR=4.358; P<0.01), lymphatic
invasion (RR=6.952; P=0.001236), venous invasion (RR=6.099;
P=0.000157) and Cx43 expression (RR=2.282; P=0.02324) were all
statistically significant prognostic factors (Table II). The multivariate analysis
revealed that tumor status (RR=4.15; P<0.01) and lymph node
status (RR=3.213; P=0.002) were independently associated with an
unfavorable prognosis in patients with ESCC (Table III). The RR for Cx43 was 2.017.
 | Table II.Univariate analysis for
clinicopathological variables of connexin 43 expression. |
Table II.
Univariate analysis for
clinicopathological variables of connexin 43 expression.
| Variables | Risk ratio | 95% confidence
interval | P-value |
|---|
| Primary tumor |
|
| <0.01 |
|
Tis-T2 | 1 |
|
|
| T3,
4 | 6.399 | 3.025–13.54 |
|
| Lymph node
metastasis |
|
| <0.01 |
|
Negative | 1 |
|
|
|
Positive | 4.358 | 2.078–9.14 |
|
| Lymphatic
invasion |
|
|
0.001236 |
|
Negative | 1 |
|
|
|
Positive | 6.952 | 2.114–22.55 |
|
| Venous invasion |
|
|
0.000157 |
|
Negative | 1 |
|
|
|
Positive | 6.099 | 2.388–15.58 |
|
| Connexin 43
expression |
|
|
0.02324 |
| Low | 1 |
|
|
| High | 2.282 | 1.119–4.653 |
|
 | Table III.Multivariate analysis for
clinicopathological variables of connexin 43 expression. |
Table III.
Multivariate analysis for
clinicopathological variables of connexin 43 expression.
| Variables | Risk ratio | 95% confidence
interval | P-value |
|---|
| Primary tumor |
|
| <0.01 |
|
Tis-T2 | 1 |
|
|
| T3,
4 | 4.15 | 1.9220–8.959 |
|
| Lymph node
metastasis |
|
| 0.002 |
|
Negative | 1 |
|
|
|
Positive | 3.213 | 1.498–6.888 |
|
| Lymphatic
invasion |
|
| 0.8384 |
|
Negative | 1 |
|
|
|
Positive | 1.208 | 0.1967–7.418 |
|
| Venous invasion |
|
| 0.2075 |
|
Negative | 1 |
|
|
|
Positive | 2.534 | 0.5968–10.760 |
|
| Connexin 43
expression |
|
| 0.058 |
| Low | 1 |
|
|
| High | 2.017 | 0.8936–4.1701 |
|
Discussion
Gap junctions, which were first described in the
early 1960s, are cell membrane junctions responsible for
intercellular communication, and are involved in tissue
homeostasis, proliferation and differentiation. Disruption of this
mechanism may facilitate the development of several disorders.
Cx proteins are involved in the function of gap
junctions. Indeed, the primary function of Cx is to form conduits
between neighboring cells; these conduits mediate the intercellular
exchange of small cytoplasmic molecules. Cxs constitute a family of
structurally related transmembrane proteins that connect two
adjacent cells by forming gap junctions. Each gap junction is
composed of two hemichannels or connexons, and each connexon is
composed of six Cx proteins. Cxs coordinate cell-to-cell
communication by allowing the direct transfer of molecules
<1,000 Daltons between cells. Over 20 types of Cxs have been
identified to date. Cx43, which was the focus of this study, is
expressed in the central nervous system, heart, bone and a number
of other organs.
Several studies have demonstrated that Cx proteins
often function as tumor suppressors (1–3).
Additionally, Li et al previously reported the role of Cx43
in metastatic breast cancer cells (9). Their results demonstrated that the
expression of Cx43 in breast cancer cells decreases the metastatic
potential of the cells through a mechanism involving N-cadherin
expression and apoptosis, which is independent of gap junctional
communication. In non-small-cell lung cancer, reduced expression of
Cx43 and E-cadherin has been found to be significantly correlated
with poor differentiation, advanced TNM stage and lymph node
metastasis, suggesting that concurrent reduction of Cx43 and
E-cadherin expression may contribute to the development of lung
cancer (10). Thus, Cx43 may induce
E-cadherin expression, thereby inhibiting cell proliferation and
lung cancer progression. In a study on Cx43 in colon cancer, Ismail
et al analyzed the expression of Cx43 in samples from 80
cases of histopathologically confirmed and clinically diagnosed
human colon cancer and in adjacent control tissues, and assessed
the correlations with the clinicopathological variables (11). The expression of Cx43 was found to be
significantly downregulated (75%) in cancer samples compared with
that in adjacent control tissues. Moreover, Cx43 downregulation was
significantly associated with histological type and tumor invasion
properties of the cancer. Additionally, Bigelow et al
performed gap junction activity assays and protein analysis to
evaluate the effects of Cx43 overexpression in SW480 human
colorectal cancer cells (2) and
demonstrated that overexpression of Cx43 in SW480 cells led to a
6-fold increase in gap junctional activity compared with that in
controls.
In contrast to the abovementioned reports, other
studies have suggested that Cx43 may act as an oncogene (12). Ogawa et al investigated the
expression of Cx43 in rat hepatocellular carcinoma cells (13) and found that suppression of Cx43
expression in tumor cells reduced the migration and invasion
capacity of the cells in vitro and the metastatic ability of
the cells in vivo, indicating that Cx43 may have potential
as a molecular target for the prevention of cancer metastasis in
Cx43-overexpressing tumors. Thus, while Cx43 is often reported to
function as a tumor suppressor, differential functions may be
observed in the context of different tissue types.
In esophageal cancer, the role of Cx43 has not been
sufficiently investigated. Garber et al (7) previously analyzed the expression of Cx43
in neoplastic esophageal epithelial cells in rats. However, the
association between Cx43 expression and the clinicopathological
characteristics of ESCC has not been previously described. In this
study, we aimed to determine the clinicopathological significance
of Cx43 expression in ESCC; three main findings were noted: First,
Cx43 was expressed at a high frequency in patients with ESCC. Of
the 98 ESCC cases, positivity for Cx43 was observed in 62 cases.
Second, the survival of patients with high Cx43 expression was
significantly poorer compared with that of patients with low Cx43
expression. Third, overexpression of Cx43, as measured by
immunohistochemistry, was an independent prognostic indicator in
patients with ESCC.
In summary, the prognosis of patients with
esophageal cancer remains poor. Therefore, it is crucial to
identify prognostic factors for patients with this disease. Several
genes have been reported as prognostic factors in patients with
esophageal cancer. Our data indicated that Cx43 may be a candidate
molecular prognostic marker and molecular target for the
development of an effective therapeutic intervention for patients
with esophageal cancer.
References
|
1
|
Solan JL and Lampe PD: Specific Cx43
phosphorylation events regulate gap junction turnover in vivo. FEBS
Lett. 17:1423–1429. 2014. View Article : Google Scholar
|
|
2
|
Bigelow K and Nguyen TA: Increase of gap
junction activities in SW480 human colorectal cancer cells. BMC
Cancer. 14:5022014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang B, Peng ZH, Yu PW, Yu G, Qian F, Zeng
DZ, Zhao YL, Shi Y, Hao YX and Luo HX: Aberrant expression of Cx43
is associated with the peritoneal metastasis of gastric cancer and
Cx43-mediated gap junction enhances gastric cancer cell diapedesis
from peritoneal mesothelium. PLoS One. 8:e745272013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Oliveira KD, Tedardi MV, Cogliati B and
Dagli ML: Higher incidenceof lung adenocarcinomas induced by DMBA
in connexin 43 heterozygous knockout mice. Biomed Res Int.
2013:6184752013.PubMed/NCBI
|
|
5
|
Yi ZC, Wang H, Zhang GY and Xia B:
Downregulation of connexin 43 in nasopharyngeal carcinoma cells is
related to promoter methylation. Oral Oncol. 43:898–904. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uzu M, Sato H, Yamada R, Kashiba T,
Shibata Y, Yamaura K and Ueno K: Effect of enhanced expression of
connexin 43 on sunitinib-induced cytotoxicity in mesothelioma
cells. J Pharmacol Sci. 128:17–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garber SA, Fernstrom MJ, Stoner GD and
Ruch RJ: Altered gap junctional intercellular communication in
neoplastic rat esophageal epithelial cells. Carcinogenesis.
18:1149–1153. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Japanese Esophageal Society: Japanese
classification of esophageal cancer (10th). Kanehara & Co.,
Ltd. Tokyo: 2008.
|
|
9
|
Li Z, Zhou Z, Welch DR and Donahue HJ:
Expressing connexin 43 in breast cancer cells reduces their
metastasis to lung. Clin Exp Metastasis. 25:893–901. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu HT, Li QC, Zhang YX, Zhao Y, Liu Y,
Yang ZQ and Wang EH: Connexin 43 recruits E-cadherin expression and
inhibits the malignant behavior of lung cancer cells. Folia
histochem Cytobiol. 46:315–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ismail R, Rashid R, Andrabi K, Parray FQ,
Besina S, Shah MA and Ul Hussain M: Pathological implications of
Cx43 down-regulation in human colon cancer. Asian Pac J Cancer
Prev. 15:2987–2991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang A, Hitomi M, Bar-Shain N, Dalimov Z,
Ellis L, Velpula KK, Fraizer GC, Gourdie RG and Lathia JD: Connexin
43 expression is associated with increased malignancy in prostate
cancer cell lines and functions to promote migration. Oncotarget.
10:11640–11651. 2015. View Article : Google Scholar
|
|
13
|
Ogawa K, Pitchakam P, Suzuki S, Chewonarin
T, Tang M, Takahashi S, Naiki-Ito A, Sato S, Takahashi S, Asamoto M
and Shirai T: Silencing of connexin 43 supresses invasion,
migration and lung metastasis of rat hepatocellular carcinoma
cells. Cancer Sci. 103:860–867. 2012. View Article : Google Scholar : PubMed/NCBI
|