Introduction
Breast-conservation surgery (BCS), followed by
adjuvant irradiation of the intact breast, has been used as a
standard treatment for early breast cancer since the mid-1990s
(1). Two randomized studies have
demonstrated an improved local control, with the addition of a
boost to the tumor bed, particularly in younger patients (2,3). Although
breast-conserving therapy is now the most favored option of care
for early breast cancer, 20% of the patients who undergo BCS have a
poor cosmetic result (4). Oncoplastic
(OP)-BCS combines a relatively large volume of breast excision with
optimized breast remodeling, yielding a desirable cosmetic outcome
(5). Immediate plastic remodeling is
indicated for patients with an unfavorable tumor to breast ratio or
an unfavorable tumor location (medial, inferior or central
quadrants), for patients who require re-excision for involved
margins, or for patients with free margins who require the
correction of defects for cosmetic reasons (6,7). Due to
major complications, including infection, wound breakdown, pain,
capsular contraction and instability in the excision bed, silicone
implants have been shown to be unsuccessful at filling breast
defects following a wide excision (8).
Recently, latissimus dorsi mini-flap (LDMF)
reconstruction has become a preferred method in OP-BCS,
particularly for tumors located in the central, upper inner and
upper outer quadrants, where resection of 20–30% of the breast
volume is required (9). Another
frequent cosmetic complaint may be the discrepancy of the size
between the breasts that can be achieved by OP-BCS.
Although the definition of the tumor bed is an
important component of breast radiotherapy (RT), a standard
technique to delineate or define the tumor bed volume remains to be
established (10). The accurate
identification of the tumor bed following OP-BCS is more
challenging compared with standard BCS due to the rearrangement of
breast parenchyma. The aim of present study was to evaluate the
replacement of the tumor bed following OP-BCS with LDMF breast
reconstruction, and to determinate the most accurate method to
delineate the local boost field.
Materials and methods
Patients
This prospective study was conducted between April
2013 and January 2014 with the approval of the Istanbul Bilim
University Research Ethics Committee. Patients were operated on by
a single surgeon, and RT was planned by a single radiation
oncologist. A total of 22 consecutive patients referred from the
surgery department prior to OP-BCS with LDMF breast reconstruction
were included in the present study, and all patients gave informed
consent to be involved in the study.
OP surgical procedures
The surgical technique of LDMF is a one-step
procedure, although it was previously described as a two-stage
procedure by Dixon et al (11). Following a wide local excision of the
tumor with clear margins reported by the breast pathologist
intraoperatively, sentinel lymph node biopsy and/or axillary
dissection is performed. The axillary incision is slightly
lengthened and deepened over the lateral margin of the latissimus
dorsi (LD) muscle while the patient is held in the lateral
decubitus position. The muscle is grasped and retracted from the
chest wall to identify the thoracodorsal vessels. The length of
muscle required to fill the defect is estimated by measuring from
the apex of the axilla to the lower limit of the breast defect. The
LD muscle is mobilized from the surrounding structures by using a
combination of bipolar scissors and electrocautery. When a
sufficient quantity of muscle has been mobilized, the muscle is
divided inferiorly and delivered into the axillary wound. Attention
is subsequently turned to the superior part of the muscle, which is
divided at its insertion into the humerus. At this stage, the LDMF
is ready to be transferred into the breast. At this point, the
cavity is re-evaluated for any hemorrhaging, and is marked with
clips. Depending on the site of the wide local excision, a tunnel
is created from the axillary wound into the breast defect. The flap
is subsequently passed through the tunnel into the cavity. To
remove the tension from the vessels, the flap is secured
superolaterally by suturing the tendonous part of the muscle to
either the edge of the pectoralis major muscle or to adjacent
breast tissue. The muscle is subsequently secured in the breast
defect using absorbable sutures to generate a good shape.
Pre- and postoperative computerized
tomography (CT) imaging and image registration
All patients underwent two sets of planning CT scans
pre- and postoperatively in the treatment position, with a slice
thickness of 2.5 mm. Patients were scanned with an Optima CT580 CT
scanner (GE Healthcare, Buckinghamshire, UK), which had an 80 cm
gantry opening and indexed table (Civco indexed carbon fiber
MT-IL4101; Civco Medical Solutions, Kalona, IA, USA), specific for
RT. Prior to CT scanning, the margins of palpable glandular breast
tissue were marked with a thin CT-compatible Radio Opac wire by the
radiation oncologist. Patients were immobilized in the supine
treatment position; their shoulders and arms were fixed using a
breast board (C-Qual breast inclined plane; Civco Medical
Solutions) with knee support. The breast board index parameters
were recorded in the patients' chart for the purpose of using the
identical parameters in postoperative CT. All CT images were
imported to a treatment planning system (Eclipse version 8.1;
Varian Medical Systems, Palo Alto, CA, USA). Both CTs were fused
using rigid registration on a user-defined region of interest,
superposing the sternum and ipsilateral chest wall. During the
fusion process, the nipple, skin and parenchymal breast tissue were
not assigned priority due to the change in breast configuration
following OP-BCS.
Volume delineation and relative
positions
The breast clinical target volume (CTV) in both CTs
was delineated by a single radiation oncologist, and a 5 mm section
from the skin inwards was excluded. The gross tumor volume (GTV) of
the preoperative tumor, or the excisional biopsy cavity, was
contoured under the supervision of a radiologist using diagnostic
mammography, ultrasonography and/or magnetic resonance images
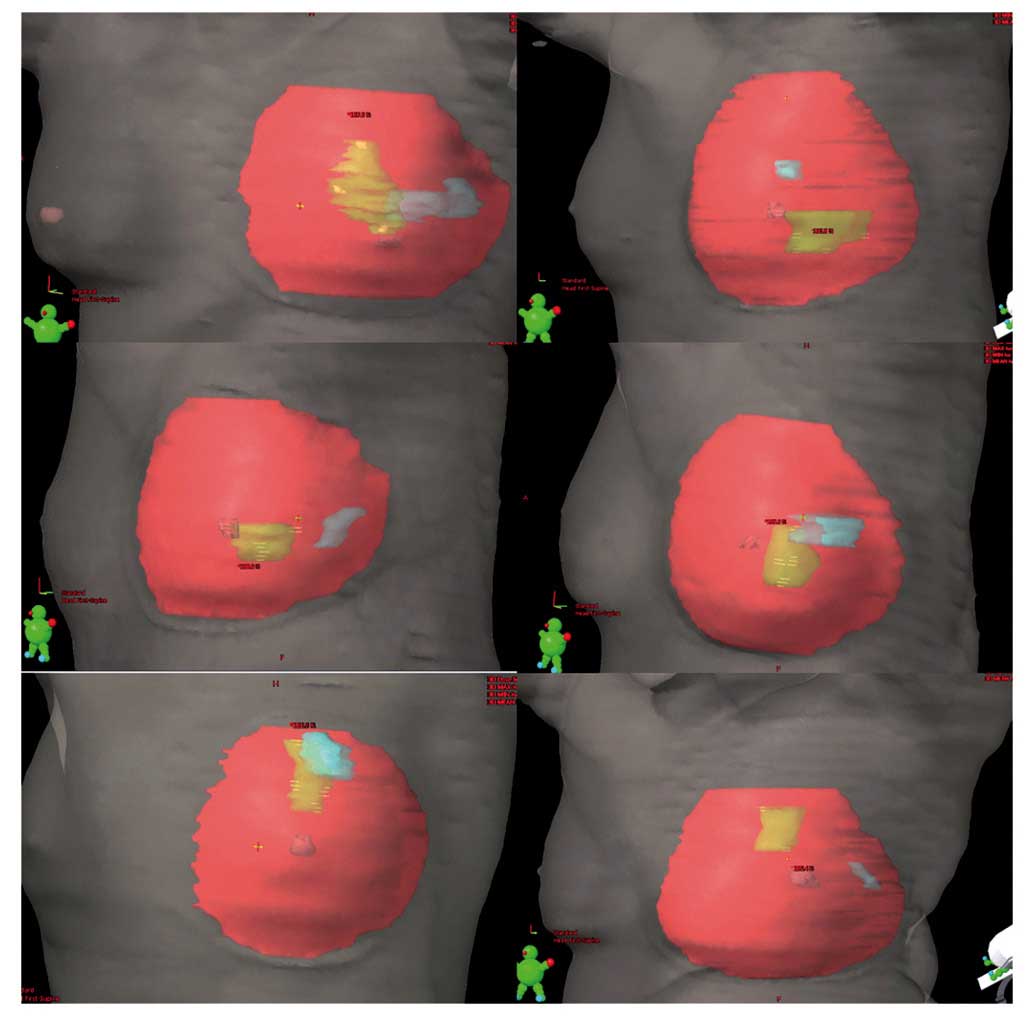
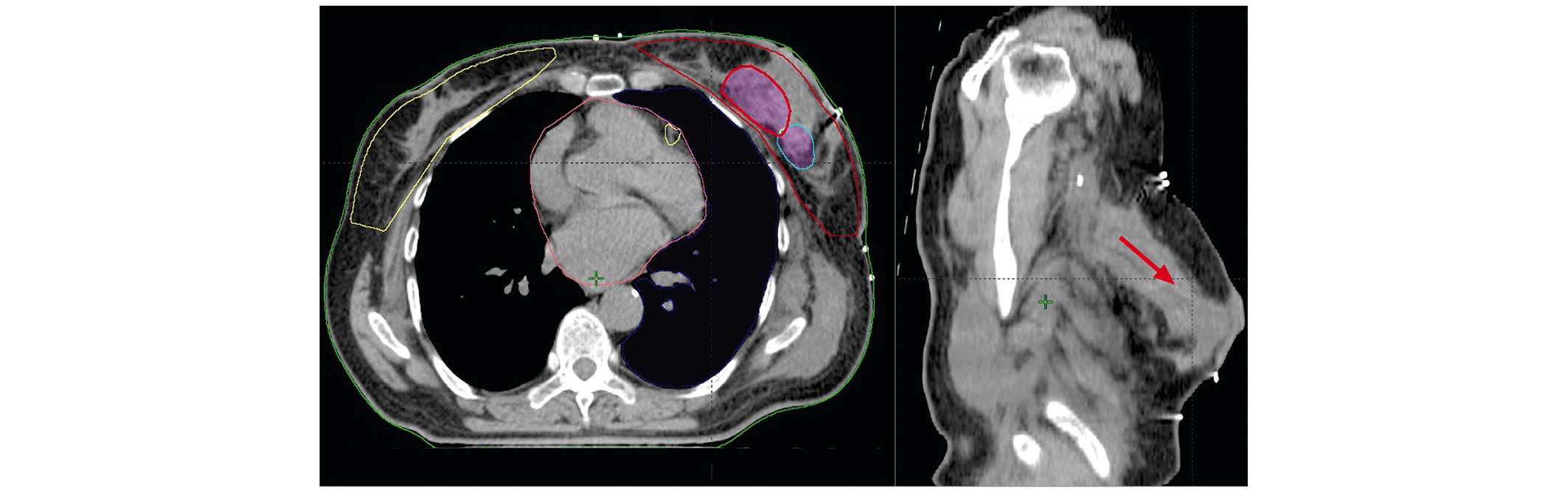
(Fig. 1). All patients were subjected
to mammography and breast ultrasound prior to surgery, and 12
patients were also subjected to magnetic resonance imaging (MRI) of
the breast. The postoperative lumpectomy cavity was contoured as
the area surrounded by the surgical clips. The tumor bed planning
target volume (PTV) was created in accordance with the Radiation
Therapy Oncology Group (RTOG) 1005 protocol (https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1005),
with a 1 cm expansion to the lumpectomy cavity for lumpectomy CTV,
and an additional 0.7 cm expansion to the PTV. An electron boost
field was generated with a 1 cm set-up margin for the PTV
evaluation. Dose-volume histograms were calculated for all
delineated volumes. The conformity index (CI) was calculated using
the formula defined by Struikmans et al (12) as the ratio of overlapping volume to
encompassing total delineated volume. CI was defined between 0 and
1, where a value of 0 indicated no overlap, and 1 indicated 100%
concordance. By using coronal, sagittal and axial CT slices of the
planning system, the shifts of the isocenter of the volumes were
calculated in terms of x, y and z coordinates. Paired sample
t-tests were performed to evaluate the changes in volumes.
Associations between CI and the number of clips, the time interval
between CT scans, pathological tumor size and age were assessed
using an independent samples t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient, tumor and treatment characteristics are
shown in Table I. The median age was
44 years (range, 29–69 years), seven of the patients were ≤40 years
old. The median interval between two CT scans was 90 days (range,
14–170 days). Seven patients had excisional biopsy prior to the
preoperative CT. Six of the patients had right-sided cancer, and 16
were left-sided. The tumor was situated in the upper outer,
retroareolar, lower outer and lower inner quadrants in 16, 2, 3 and
1 patients, respectively. Tumors were resected with clear margins
in all patients; in seven cases, multifocality was present.
 | Table I.Tumor and treatment characteristics
(n=22). |
Table I.
Tumor and treatment characteristics
(n=22).
| Characteristic | n | % | Median | Range |
|---|
| Age, years | 22 | – | 44 | 29–69 |
| ≤40 | 7 | 31.8 |
|
|
|
>40 | 15 | 68.2 |
|
|
| Time from OP-BCS to
RT (day) | 22 | – | 90 | 14–170 |
| Tumor type |
|
|
|
|
| IDC | 19 | 86.3 |
|
|
| ILC | 2 | 9.1 |
|
|
|
Other | 1 | 4.6 |
|
|
| Focality |
|
|
|
|
|
Unifocal | 15 | 68.2 |
|
|
|
Multifocal | 7 | 31.8 |
|
|
| pT stagea |
|
|
|
|
| I | 11 | 50.0 |
|
|
| II | 10 | 45.4 |
|
|
| III | 1 | 4.6 |
|
|
| pN stage |
|
|
|
|
|
0-mic | 14 | 63.6 |
|
|
|
I–III | 8 | 36.4 |
|
|
| p stage |
|
|
|
|
| 0 | 1 | 4.6 |
|
|
| I | 7 | 31.8 |
|
|
| II | 10 | 45.4 |
|
|
| III | 4 | 18.2 |
|
|
| Primary quadrant
location |
|
|
|
|
| Upper
outer | 16 | 72.7 |
|
|
|
Retroareolar | 2 | 9.1 |
|
|
| Lower
outer | 3 | 13.7 |
|
|
| Lower
inner | 1 | 4.5 |
|
|
| Chemotherapy |
|
|
|
|
| No | 4 | 18.2 |
|
|
|
Anthracyclin-based | 3 | 13.7 |
|
|
|
Anthracyclin and
taxan-based | 15 | 68.2 |
|
|
| Trastuzumab |
|
|
|
|
| No | 17 | 77.3 |
|
|
|
Yes | 5 | 22.7 |
|
|
All patients' volumes are shown in Table II. No significant changes were
observed between mean pre- and postoperative whole-breast CTVs [442
cc (range, 276–1,061 cc) vs. 516 cc (range, 243–917 cc); P=0.132].
A median of four surgical clips (range, 2–6) was inserted during
the surgery (superposed clips were counted as one). Median pre- and
postoperative tumor volumes were 6.6 cc (range, 0.4–49 cc) and
22.95 cc (range, 6.2–102.2 cc), respectively (P=0.001). None of the
patients had a seroma or hematoma formation following surgery.
Postoperative lumpectomy cavity volumes were identified completely
outside of the primary quadrant location in eight (36.4%) of the 22
cases, and were distributed in two quadrants in five (22.7%) cases.
Discordance between the volumes was revealed to be high, with a low
CI value (median 0.07; range, 0–1). There was an absence of any
intersection between the preoperative GTV and postoperative
lumpectomy cavity in eight cases (Fig.
2). CI was significantly associated only with the number of
clips (≤3 vs. ≥4; P=0.017; Table
III). No significant correlation was identified between the
time interval between CT scans, pathological tumor size or age and
CI (all P>0.05).
 | Table II.Volume description. |
Table II.
Volume description.
|
|
|
|
|
|
|
|
|
|
| Shift directions
(cm) |
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient number | No. of clips | Time interval
between CT scans (days) | GTV
(cm3) | Lumpectomy cavity
volume (cm3) | Fused volume
(cm3) | Intersection volume
(cm3) | CI | Preoperative
quadrant location | Postoperative
quadrant location | x | y | z |
|---|
| 1 | 2 | 90 |
1.2 |
7.4 |
8.5 |
0.0 | 0.0 | UOQ | RA |
0.47 |
0.47 | 3.25 |
| 2 | 4 | 84 |
5.2 |
20.6 |
25.8 |
0.0 | 0.0 | UOQ | RA |
1.32 |
4.60 | 0.76 |
| 3 | 5 | 127 |
2.7 |
21.7 |
24.4 |
0.0 | 0.0 | UOQ | LOQ |
0.82 |
2.79 | 3.75 |
| 4 | 2 | 148 |
1.8 |
6.2 |
8.0 |
0.0 | 0.0 | LOQ | RA |
2.33 |
1.22 | 1.25 |
| 5 | 4 | 23 |
24.8 |
18.4 |
40.4 |
0.0 | 0.0 | UOQ | RA |
2.44 |
0.90 | 2.25 |
| 6 | 2 | 99 |
3.4 |
16.1 |
19.5 |
0.0 | 0.0 | UOQ | RA |
4.28 |
5.67 | 2.76 |
| 7 | 3 | 14 |
7.7 |
31.5 |
39.2 |
0.0 | 0.0 | LOQ | RA |
2.21 |
1.96 | 1.75 |
| 8 | 3 | 15 |
0.4 |
9.5 |
10.1 |
0.0 | 0.0 | LOQ | LOQ - RA |
1.17 |
0.70 | 1.50 |
| 9 | 3 | 43 |
5.2 |
75.8 |
80.8 |
0.5 | 0.0 | UOQ | UOQ - LOQ |
1.86 |
2.36 | 0.0 |
| 10 | 3 | 30 |
26.8 |
51.3 |
76.1 |
2.1 | 0.0 | UOQ | RA |
4.43 |
3.69 | 1.0 |
| 11 | 4 | 128 |
8.8 |
19.7 |
27.0 |
1.7 | 0.0 | UOQ | UOQ - LOQ |
0.83 |
2.49 | 0.67 |
| 12 | 6 | 170 |
7.3 |
62.6 |
64.8 |
5.4 | 0.08 | UOQ | UOQ |
0.37 |
0.81 | 1.25 |
| 13 | 4 | 48 |
11.7 |
9.8 |
19.9 |
1.9 | 0.09 | UOQ | UOQ |
0.70 |
0.17 | 1.42 |
| 14 | 4 | 119 |
30.3 |
60.7 |
83.7 |
7.6 | 0.09 | UOQ | UOQ - UIQ |
1.86 |
0.74 | 0.19 |
| 15 | 2 | 150 |
38.8 |
12.7 |
45.4 |
6.5 | 0.14 | UOQ | UOQ |
1.06 |
1.07 | 2.25 |
| 16 | 5 | 37 |
13.6 |
50.4 |
54.3 |
10.1 | 0.19 | UOQ | UOQ |
0.51 |
1.07 | 1.44 |
| 17 | 3 | 136 |
49.2 |
43.3 |
74.0 |
18.9 | 0.25 | LIQ | LIQ |
1.41 |
0.78 | 0.75 |
| 18 | 5 | 103 |
2.4 |
20.3 |
20.3 |
2.4 | 1.0 | RA | RA |
0.98 |
1.20 | 0.25 |
| 19 | 4 | 78 |
3.0 |
26.0 |
26.0 |
3.0 | 1.0 | LOQ | LOQ |
0.52 |
0.63 | 0.70 |
| 20 | 4 | 51 |
18.0 | 102.2 | 102.2 |
18.0 | 1.0 | UOQ | UOQ - LOQ |
0.50 |
1.50 | 1.0 |
| 21 | 5 | 42 |
3.0 |
48.1 |
48.1 |
3.1 | 1.0 | UOQ | UOQ |
0.04 |
0.05 | 0.50 |
| 22 | 3 | 120 |
5.9 |
24.2 |
27.4 |
5.9 | 1.0 | RA | RA |
0.24 |
0.41 | 1.0 |
 | Table III.Factors associated with CI
(n=22). |
Table III.
Factors associated with CI
(n=22).
| Factor | n | % | CI mean | P-value |
|---|
| Clip number |
|
|
| 0.017 |
| ≤3 | 10 | 45.4 | 0.14 |
|
| ≥4 | 12 | 54.6 | 0.37 |
|
| Time between CT
scans (day) |
|
|
| 0.31 |
|
<90 | 11 | 50.0 | 0.30 |
|
|
≥90 | 11 | 50.0 | 0.23 |
|
| Age, years |
|
|
| 0.351 |
|
≤44 | 11 | 50.0 | 0.31 |
|
>44 | 11 | 50.0 | 0.22 |
| Tumor size, cm |
|
| 0.22 |
|
| ≤2 | 12 | 54.6 | 0.20 |
|
|
>2 | 10 | 45.4 | 0.34 |
|
The median shifts between the isocenter of volumes
were 1.02 cm (range, 0.4–4.43 cm) in the x, 1.07 cm (range,
0.05–5.67 cm) in the y, and 1.12 cm (range, 0–3.75 cm) in the z
directions, respectively.
Preoperative GTV was found completely outside of the
electron boost field in one of the 22 patients, and partially
outside in five of the 22 patients. In those six patients, the
median volume of the preoperative GTV receiving 95% of the
prescribed dose (V95) was 67.75% (range, 0–86.8%). In the remaining
16 patients, the primary tumor area was inside the electron boost
field, and received 100% of the prescribed dose of 60 Gy.
Discussion
Although previously published studies have
investigated the accuracy of a boost technique following OP surgery
(13–16), to the best of our knowledge, the
present study is the only prospective study following LDMF
reconstruction, showing a marked tumor bed shift. The shift between
pre- and postoperative geometric isocenter coordinates was shown to
be >1 cm in all directions (up to 5.67 cm). Postoperative boost
volumes were identified completely outside of the primary quadrant
location in eight (36.4%) of the 22 patients, and discordance
between the volumes was revealed to be high (median CI=0.07). In
only five of the 22 patients was the pre- and postoperative volume
concordance 100% (CI=1). The tumor bed electron field did not cover
the preoperative GTV location in six of the 22 patients following
OP-BCS, causing underdosage in this area.
The advantage of using a boost to lower in-breast
recurrences has been decisively demonstrated in randomized trials
(2,3).
However, how to delineate the boost volume remains a matter of
controversy in the radiation oncology community (10). Historically, presurgical scars have
been used to assist the location of the tumor bed; however, to
achieve improved cosmetic results, surgical scars are frequently
being placed at a distance from the original site, particularly
following OP-BCS. Clinical methods that take account of information
on preoperative imaging, clinical palpation of seroma and surgical
scars of the tumor bed delineation are not commonly used, due to
numerous inaccuracies. In order to overcome the problem of missing
the target, postoperative three-dimensional imaging has replaced
the clinical methods in planning the boost volumes. However, in the
era of OP-BCS, accurate boost volume localization has become more
complicated when the tumor is far away from its primary
location.
Despite the more widespread use of OP-BCS
techniques, few published data have addressed the pitfalls of
variability in the postoperative tumor bed shift following these
surgical procedures. The use of extensive remodeling for preserving
breast shape results in a considerable displacement of breast
tissue, which modifies the original position of the tumor bed. In
the review of Schaverien et al (17), the use of boost RT was reported in 11
of 24 OP-BCS studies, and marking of the tumor bed was discussed in
only eight of them. In this review, none of the studies reported on
the number of patients where the tumor bed could not be
localized.
After OP tissue manipulation, the clips ultimately
end up in various different locations within the breast. Two
retrospective studies were published in order to analyze tumor
cavity replacement following OP-BCS (18,19). In
these studies, 43–73% of the patients were shown to have clips
outside of the original tumor quadrant, as revealed in the
postoperative CT images. The breast quadrant location of the
primary tumor was identified retrospectively on the basis of a
preoperative diagnostic mammography, breast ultrasound or MRI: A
high rate of discordance between the location of the primary tumor
and the surgical clips following surgery was demonstrated in the
two studies. In the study of Pezner et al (18), 11 of 25 patients (26 tumors) had ≥4
clips inserted, and in eight of them (73%) the tumor bed was beyond
the original quadrant; in three patients (27%), the CTV was located
in two or three separated quadrants. The findings of the present
study are consistent with their results, showing quadrant
dislocation in eight (36.4%) of the 22 patients, with a
distribution in two quadrants for five (22.7%) of the patients.
For an accurate tumor bed definition following
plastic breast remodeling, Kirova et al (14) described the optimal multidisciplinary
approach using pre- and postoperative CT scans, image registration
and clips in the tumorectomy region. During surgery, between one
and five clips (mean ± standard deviation, 3±1) were inserted into
the tumor bed, and a larger discrepancy was observed in the
right-left axis (1.4 cm) between the presurgery GTV and
postsurgical clips. Their tumor bed PTV included clips in the
postoperative CT, GTV in the preoperative CT, and a surgical scar
with an overall margin of 5–10 mm in all directions. In a study
published by González-Sanchis et al (15), including the patients' reconstructive
mammoplasty, a total of four or five titanium clips were inserted
in all cases, and pre- and postsurgery CT scans were performed in
all patients in order to quantify the tumor bed deviation from the
original tumor site, and to determine the required margin to cover
the tumor bed (15). Variations
between the geometric isocenters were identified of between 0.5 and
3 cm, with the largest being in the upper-outer quadrant, of up to
4.5 cm. In the present study, the electron boost fields were
created according to RTOG 1005 protocol. However, this protocol
requires generous margins for tumor bed delineation, and in six
(27.3%) of our patients, the preoperative GTV was outside of the
electron field and remained underdosed.
Although the guidelines clearly suggest the use of
surgical clips marking the tumor bed, Kirwan et al (20) demonstrated that more than one-third of
patients do not have tumor bed clips inserted at the time of the
surgery. Ideally, it is recommended that at least six surgical
clips should be placed prior to closing the cavity (18,21).
Studies by Kirova et al (14)
and Furet et al (16)
demonstrated that the use of three or more clips during tumorectomy
increased the accuracy of tumor bed delineation following image
registration. In addition, Pezner et al (18) reported that the superposition of the
pre- and postoperative volumes was significantly lower in patients
with two clips compared with patients with three or more clips
(0.73 vs.35.45%; P=0.028). The results of the present study are in
line with those studies, showing a positive correlation between the
number of clips inserted and the CI (P=0.017). Despite the use of
clips, the study by Kirova et al (14) and the present study revealed a similar
volumetric analysis, demonstrating an intersection between the
initial tumor site and the clip zone in 32 and 36.4% of the
patients, respectively (18).
Another controversial issue is the image
registration. Although rigid pre- and postoperative CT image
registration has become standard practice in several clinics, the
use of deformable image fusion to allow an improved definition of
the boost volume following OP surgery is currently a topic for
investigation (22,23). The external soft tissue of the breast
would present a deformation following surgery due to postoperative
edema, and a changed breast configuration as a result of
remodeling. Therefore, a deformable registration would lead to an
improved positioning of the preoperative GTV and postoperative clip
locations. A Korean study, by Cho et al (23), used an initial diagnostic positron
emission tomography-CT fusion with a postoperative CT scan for
deformable image registration. The mean preoperative
18F-fluorodeoxyglucose-avid tumor volume inside the
postoperative tumor bed volume was revealed to be 94.8% (range,
60.9–100%). In contrast with the Korean study, which studied
patients who had been subjected to standard BCS, in the present
study, following LDMF OP-BCS, the concordance of pre- and
postoperative volumes were shown to be very low (median
CI=0.07).
In conclusion, the present study has demonstrated
that the tumor bed is markedly replaced following OP-BCS with LDMF,
with a quadrant dislocation in 36.4% of the patients. Variability
in the geometric shift may occur in three dimensions, and the
isocenter differed by >1 cm in all directions. Pre- and
postoperative CT scans considered in isolation lack accuracy. The
use of four or more clips is required to identify changes in the
position of tumor bed. Since the lumpectomy cavity may be more
extensive and relocated, special care should be taken in terms of
defining the tumor bed in RT planning. For an accurate
determination of the tumor bed localization, pre- and postsurgery
image registration, and marking the lumpectomy cavity with an
adequate number of clips following resection and prior to the OP
reconstruction, is essential. Therefore, a multidisciplinary
approach involving OP breast surgeons and radiotherapists is
necessary in order to treat these patients, as was described by
Kirova et al (13) previously,
and as has been supported by the findings in the present study.
References
|
1
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Romestaing P, Lehingue Y, Carrie C,
Coquard R, Montbarbon X, Ardiet JM, Mamelle N and Gérard JP: Role
of a 10-Gy boost in the conservative treatment of early breast
cancer: Results of a randomized clinical trial in Lyon, France. J
Clin Oncol. 15:963–968. 1997.PubMed/NCBI
|
|
3
|
Barthelink H, Horiot JC, Poortmans PM,
Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad
WJ, Oei SB, Wárlám-Rodenhuis CC, et al: Impact of a higher
radiation dose on local control and survival in breast-conserving
therapy of early breast cancer: 10-year results of the randomized
boost versus no boost EORTC 22881–10882 trial. J Clin Oncol.
25:3259–3265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Ghazal SK and Blamey RW: Cosmetic
assessment of breast-conserving surgery for primary breast cancer.
Breast. 8:162–168. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clough KB, Lewis JS, Couturaud B, Fitoussi
A, Nos C and Falcou MC: Oncoplastic techniques allow extensive
resections for breast-conserving therapy of breast carcinomas. Ann
Surg. 237:26–34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cochrane RA, Valasiadou P, Wilson AR,
Al-Ghazal SK and Macmillan RD: Cosmesis and satisfaction after
breast-conserving surgery correlates with the percentage of breast
volume excised. Br J Surg. 90:1505–1509. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bulstrode NW and Shrotria S: Prediction of
cosmetic outcome following conservative breast surgery using breast
volume measurements. Breast. 10:124–126. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomas PR, Ford HT and Gazet JC: Use of
silicone implants after wide local excision of the breast. Br J
Surg. 80:868–870. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rainsbury RM: Breast-sparing
reconstruction with latissimus dorsi miniflaps. Eur J Surg Oncol.
28:891–895. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benda RK, Yasuda G, Sethi A, Gabram SG,
Hinerman RW and Mendelhall NP: Breast boost: Are we missing the
target? Cancer. 97:905–909. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dixon JM, Venizelos B and Chan P:
Latissimus dorsi mini-flap: A technique for extending breast
conservation. Breast. 11:58–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Struikmans H, Wárlám-Rodenhuis C, Stam T,
Stapper G, Tersteeg RJ, Bol GH and Raaijmakers CP: Interobserver
variability of clinical target volume delineation of glandular
breast tissue and of boost volume in tangential breast irradiation.
Radiother Oncol. 76:293–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirova YM, Fournier-Bidoz N, Servois V,
Laki F, Pollet GA, Salmon R, Thomas A, Dendale R, Bollet MA,
Campana F and Fourquet A: How to boost the breast tumor bed? A
multidisciplinary approach in eight steps. Int J Radiat Oncol Biol
Phys. 72:494–500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kirova YM Castro, Pena P, Hijal T,
Fournier-Bidoz N, Laki F, Sigal-Zafrani B, Dendale R, Bollet MA,
Campana F and Fourquet A: Improving the definition of tumor bed
boost with the use of surgical clips and image registration in
breast cancer patients. Int J Radiat Oncol Biol Phys. 78:1352–1355.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
González-Sanchis A Brualla, González L,
Diana C Fuster, Partearroyo JC Gordo, Comas A Garcia-Vilanova,
Torrecilla JL Lopez and Ferrando J Roselló: Tumor bed segmentation:
First step for partial breast irradiation. Clin Transl Oncol.
15:39–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Furet E, Peurien D, Fournier-Bidoz N,
Servois V, Reyal F, Fourquet A, Rouzier R and Kirova YM: Plastic
surgery for breast conservation therapy: How to define the volume
of the tumor bed for the boost? Eur J Surg Oncol. 40:830–834. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schaverien MV, Stallard S, Dodwell D and
Doughty JC: Use of boost radiotherapy in oncoplastic
breast-conserving surgery - A systematic review. Eur J Surg Oncol.
39:1179–1185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pezner RD, Tan MC, Clancy SL, Chen YJ,
Joseph T and Vora NL: Radiation therapy for breast cancer patients
who undergo oncoplastic surgery: Localization of the tumor bed for
the local boost. Am J Clin Oncol. 36:535–539. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eaton BR, Losken A, Okwan-Duodu D,
Schuster DM, Switchenko JM, Mister D, Godette K and Torres MA:
Local recurrence patterns in breast cancer patients treated with
oncoplastic reduction mammaplasty and radiotherapy. Ann Surg Oncol.
21:93–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kirwan CC, Al Sarakbi W, Loncaster J, Chan
HY, Thompson AM and Wishart GC: Tumor bed clip localisation for
targeted breast radiotherapy: Compliance is proportional to
trial-related research activity: Tumour bed clip localisation in
breast radiotherapy. Eur J Surg Oncol. 40:158–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coles CE, Wilson CB, Cumming J, Benson JR,
Forouhi P, Wilkinson JS, Jena R and Wishart GC: Titanium clip
placement to allow accurate tumour bed localization following
breast conserving surgery: Audit on behalf of the IMPORT Trial
Management group. Eur J Surg Oncol. 35:578–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kirova YM, Servois V, Reyal F, Peurien D,
Fourquet A and Fournier-Bidoz N: Use of deformable image fusion to
allow better definition of tumor bed boost volume after oncoplastic
breast surgery. Surg Oncol. 20:e123–e125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho O, Chun M, Oh YT, Kim MH, Park HJ, Heo
JS and Noh OK: Can initial diagnostic PET-CT aid to localize tumor
bed in breast cancer radiotherapy: Feasibility study using
deformable image registration. Radiat Oncol. 8:1632013. View Article : Google Scholar : PubMed/NCBI
|