Introduction
Prostate cancer is the most common cancer among
European men, comprising 12% of all new cancer cases and 5% of all
cancer deaths in 2012 (1). There is
no evidence that one radical treatment for localized disease is
more effective compared with another (2). In a recently published randomized
trial, it was demonstrated that radical prostatectomy and
radiotherapy (RT) achieved comparable disease control after a
10-year follow-up (3). External beam
radiation therapy (EBRT) is a treatment option available at
different stages of non-metastatic prostate cancer (4). Androgen-deprivation therapy has also
been used as primary treatment for localized prostate cancer
(5), leading to apoptotic regression
of androgen-dependent tumors. Possible androgen-deprivation therapy
regimens include orchiectomy, gonadotropin hormone-releasing
hormone (GnRH) agonists, GnRH antagonists and androgen receptor
antagonists (6). Use of early
androgen-deprivation therapy for prostate cancer may lead to the
development of castration-resistant prostate cancer. The median
time to bone metastasis in this group of patients is 9–30 months
(7,8); the wide time range indicates the
heterogeneity of this patient group. Additionally, the treatments
and outcomes vary. No consensus on the treatment of
non-metastasized castration-resistant prostate cancer has yet been
reached. Few published studies have evaluated RT for
castration-resistant prostate cancer (9–16). The
aim of the present study was to evaluate the outcome of RT among
patients who underwent androgen-deprivation therapy as a primary
therapy for non-metastatic prostate cancer with progression to
castration-resistant disease at our institution between 2000 and
2010.
Patients and methods
Patient chart review
Patient data were obtained through retrospective
chart reviews from 2000 to 2010 and based on RT treatment codes. In
total, 1,463 patients were treated with EBRT during this period. Of
those, 68 had castration-resistant prostate cancer. Furthermore, 21
of those patients received a prostate radiation dose of >45 Gy.
For these patients, RT was administered as salvage treatment for
non-metastatic, castration-resistant prostate cancer. Nodal status
was systematically assessed only by RT dose planning native
computed tomography. Patients with evidently enlarged pelvic lymph
nodes were excluded from higher (>45 Gy) prostate radiation
doses. Castration-resistant prostate cancer was defined as a
progressive increase in the prostate-specific antigen (PSA)
concentration in repeated measurements during surgical or chemical
(i.e., GnRH agonist therapy) castration. Baseline characteristics,
radiation dose, lowest PSA concentration after RT, time to PSA
increase (PSA nadir + 2 µg/l) and survival were recorded. The gross
tumor volume (GTV) for RT was the prostate gland. The clinical
target volume (CTV) was defined as GTV + 1 cm. The seminal vesicles
were included in the CTV. The planning target volume (PTV) was
defined as the CTV + 1 cm, except for 0.5 cm towards the rectum. If
fiducial markers were used in the prostate, the CTV was GTV + 0.5
cm and the PTV was CTV + 0.5 cm, except 0 cm towards the rectum.
Only conventional fractionation (2 Gy/fraction) was applied.
Following written assurance that patient information
would remain coded and anonymous, this retrospective chart review
was exempted from formal Institutional Review Board approval
according to Finnish legislation and directions from Finnish ethics
committees. The study was conducted according to the principles of
the Helsinki Declaration.
Statistical analysis
Data were analyzed using SPSS software, version 22.0
(IBM Corp., Armonk, NY, USA). Between-group comparisons were
performed using a t-test. Two-tailed P-values were reported, and
P-values <0.05 were considered to indicate statistically
significant differences. Prostate cancer-specific survival rates
were calculated using the Kaplan-Meier method, and statistical
significance between groups was analyzed using the log-rank test.
Multivariate prognostic factor analyses were performed using Cox
regression analyses.
Results
Patient characteristics
In total, 21 patients with castration-resistant
prostate cancer were treated with EBRT with a prostate dose of
>45 Gy. The median age of the patients at diagnosis was 68 years
(range, 58–75 years). The median age of the patients at the start
of EBRT was 74 years (range, 62–80 years). Another concurrent
malignancy was present in 3 (14.3%) patients (lung cancer, n=1;
malignant melanoma, n=1; and renal cancer, n=1). The Gleason score
at diagnosis, clinical T-class [TNM classification (17)], and primary hormonal therapy are
presented in Table I. None of the
patients had histologically confirmed lymph node metastases at
diagnosis. A bone scan was performed to exclude bone metastases at
the time of diagnosis in 20 of the 21 patients. The median PSA
concentration at the time of diagnosis was 50.2 µg/l (range,
4.8–335.0 µg/l).
 | Table I.Gleason score and clinical T stage at
diagnosis and primary hormonal therapy among the study population
(n=21). |
Table I.
Gleason score and clinical T stage at
diagnosis and primary hormonal therapy among the study population
(n=21).
| TNM
classification | N (%) |
|---|
| Gleason score at
diagnosis |
|
| ≤6 | 5 (23.8) |
| 7 | 11 (52.4) |
| 8–10 | 5 (23.8) |
| Clinical T stage |
|
| 1a-c | 3 (14.3) |
| 2a-c | 5 (23.8) |
| 3a,b | 9 (42.9) |
| 4 | 4 (19.0) |
| Primary hormonal
therapy |
|
|
Antiandrogens | 4 (19.0) |
| Chemical
castration | 13 (61.9) |
| Chemical
castration + antiandrogen | 3 (14.3) |
| Surgical
castration | 1 (4.8) |
The median time until the PSA nadir was reached was
10 weeks (range, 2–96 weeks). Biochemical progression developed in
a median of 41 months (range, 8–110 months). The median lowest PSA
concentration was 0.8 µg/l (range, 0.2–25.0 µg/l). All the patients
underwent a bone scan prior to RT to exclude metastatic disease; 6
patients (28.6%) also underwent a computed tomography scan and 6
patients (28.6%) underwent abdominal ultrasonography, whereas 1
patient (4.8%) underwent all three diagnostic modalities. The
individual patient baseline characteristics are presented in
Table II.
 | Table II.Baseline characteristics of the
patients (n=21). |
Table II.
Baseline characteristics of the
patients (n=21).
| Patient no. | Age at diagnosis,
years | PSA at diagnosis,
µg/l | Gleason score | Clinical T stage | Lowest PSA prior to
RT, µg/l | PSA prior to RT,
µg/l | Time to nadir PSA
prior to RT, months | Time to biochemical
progression prior to RT, months |
|---|
| 1 | 68 | 12.0 | 6 | 3 | 0.8 | 2.1 | 12 | 98 |
| 2 | 73 | 28.6 | 8 | 1 | 2.5 | 1.0a | 10 | 24 |
| 3 | 64 | 22.4 | 6 | 2 | 0.4 | 2.4 | 12 | 82 |
| 4 | 69 | 16.2 | 7 | 3 | 1.3 | 3.0 | 4 | 41 |
| 5 | 74 | 36.7 | 10 | 4 | 1.4 | 26.7 | 4 | 8 |
| 6 | 58 | 30.7 | 7 | 3 | 0.4 | 1.5 | 10 | 39 |
| 7 | 68 | 214.0 | 8 | 4 | 5.6 | 68.2 | 11 | 27 |
| 8 | 73 | 45.1 | 7 | 3 | 0.7 | 15.4 | 12 | 43 |
| 9 | 63 | 335.0 | 7 | 4 | 25.0 | 219.0 | 10 | 25 |
| 10 | 68 | 10.8 | 4 | 1 | 0.4 | 40.1 | 3 | 55 |
| 11 | 66 | 35.4 | 7 | 3 | 0.8 | 8.6 | 5 | 35 |
| 12 | 65 | 37.7 | 7 | 4 | 0.4 | 16.1 | 2 | 55 |
| 13 | 75 | 21.1 | 7 | 3 | 3.2 | 32.7 | 3 | 39 |
| 14 | 70 | 53.3 | 6 | 2 | 12.3 | 106.8 | 96 | 110 |
| 15 | 72 | 10.3 | 7 | 3 | 3.9 | 5.9 | 19 | 34 |
| 16 | 70 | 42.3 | 7 | 2 | 0.4 | 27.4 | 31 | 52 |
| 17 | 62 | 97.6 | 8 | 3 | 0.4 | 59.6 | 5 | 25 |
| 18 | 70 | 35.8 | 7 | 3 | 0.2 | 9.7 | 28 | 49 |
| 19 | 73 | 39.9 | 9 | 2 | 3.6 | 10.0 | 8 | 11 |
| 20 | 62 | 4.8 | 6 | 1 | 1.2 | 14.4 | 13 | 48 |
| 21 | 65 | 11.6 | 7 | 2 | 0.2 | 130.0 | 37 | 84 |
The median PSA concentration prior to RT was 15.4
µg/l (range, 1.0–219.0 µg/l). The median RT dose was 66 Gy (range,
46–72 Gy) and 10 patients (43.5%) received a dose of 72 Gy.
Additionally, 19 patients received pelvic lymph node irradiation,
with a median dose of 46 Gy (range, 46–56 Gy).
The mean follow-up duration was 108 months (range,
35–219 months). A total of 18 patients died during the follow-up
period: 14 patients (66.7%) succumbed to prostate cancer, whereas 4
(19%) died from other causes, namely chronic obstructive pulmonary
disease, intracerebral hemorrhage, gastric cancer and myocardial
infarction. Three (14.3%) patients remained alive at the time of
the chart review. The individual patient RT dose data and follow-up
data are presented in Table
III.
 | Table III.Radiation therapy dose data and
follow-up data of the patients (n=21). |
Table III.
Radiation therapy dose data and
follow-up data of the patients (n=21).
| Patient no. | Prostate radiation
dose, Gy | Pelvic lymph node
radiation dose, Gy | Lowest PSA after
RT, µg/l | Time to PSA nadir
after RT, months | Time to biochemical
recurrence after RT, months | Cause of death | Survival after RT,
months | Overall
survival/follow-up months |
|---|
| 1 | 72 | 46 | 0.8 | 6.0 | 12.4 | Prostate
cancer | 47.8 | 158 |
| 2 | 66 | 56 | 0.1 | 4.8 |
| Other
diseasea | 9.5 | 36 |
| 3 | 72 | 50 | 0.1 | 7.9 |
| Alive |
| 154 |
| 4 | 72 | 46 | 0.4 | 4.7 | 59.9 | Alive |
| 174 |
| 5 | 66 | 46 | 1.9 | 3.0 | 9.9 | Prostate
cancer | 30.5 | 40 |
| 6 | 72 | 46 | 0.2 | 5.6 |
| Alive |
| 131 |
| 7 | 50 | 50 | 72.9 | 5.4 | 7.3 | Prostate
cancer | 45.2 | 95 |
| 8 | 72 | 46 |
|
|
| Other
diseaseb | 3.8 | 62 |
| 9 | 66 | 46 | 105.0 | 4.6 | 9.0 | Prostate
cancer | 19.4 | 62 |
| 10 | 72 | 56 | 38.7 | 1.9 | 4.9 | Prostate
cancer | 38.6 | 131 |
| 11 | 72 | 46 | 0.4 | 9.7 | 35.0 | Prostate
cancer | 86.8 | 115 |
| 12 | 50 | 50 | 86.2 | 4.0 | 4.7 | Prostate
cancer | 6.1 | 64 |
| 13 | 50 |
| 65.4 | 7.7 | 9.6 | Prostate
cancer | 21.9 | 81 |
| 14 | 50 | 50 | 126.9 | 3.5 | 6.6 | Prostate
cancer | 16.1 | 128 |
| 15 | 50 |
| 1.6 | 3.7 | 7.9 | Prostate
cancer | 59.2 | 106 |
| 16 | 50 | 50 | 0.9 | 17.0 |
| Other
diseasec | 27.8 | 120 |
| 17 | 72 | 46 | 3.4 | 4.0 | 9.5 | Prostate
cancer | 57.8 | 85 |
| 18 | 72 | 46 | 0.2 | 8.8 | 33.4 | Prostate
cancer | 89.6 | 145 |
| 19 | 50 | 50 | 3.0 | 7.3 | 10.5 | Prostate
cancer | 20.3 | 35 |
| 20 | 72 | 46 | 0.4 | 5.7 | 35.8 | Other
diseased | 218.8 | 219 |
| 21 | 46 | 46 | 158.7 | 4.1 | 9.3 | Prostate
cancer | 32.9 | 129 |
The median nadir PSA concentration after RT was 1.8
µg/l (range, 0.1–158 µg/l).
The mean time to the PSA nadir after RT was 5.9
months (range, 1.9–17.0 months). Among patients who developed
biochemical recurrence after RT (n=16), the mean time to
biochemical recurrence was 17 months [range, 4.7–60.0; 95%
confidence interval (CI), 8.4–24.0 months]. Among patients who died
during follow-up (n=18), the mean survival after RT was 36 months
(range, 3.8–90.0 months).
The radiation dose was associated with the lowest
PSA concentration after RT, time to biochemical recurrence after
RT, and survival. For patients treated with a prostate radiation
dose of ≤66 vs. >66 Gy, the mean lowest PSA concentration after
RT was 56.6 and 5.0 µg/l, respectively (P=0.18). The time to
biochemical recurrence and survival after RT for patients treated
with a prostate radiation dose of ≤66 vs. >66 Gy were 8.3 vs. 27
months (P=0.011) and 26 vs. 54 months (P=0.028), respectively.
After RT, the mean overall survival was 42 months
(95% CI: 29–55 months). The overall survival did not depend on the
primary hormonal treatment. There were no significant differences
between different hormonal treatments (data not shown).
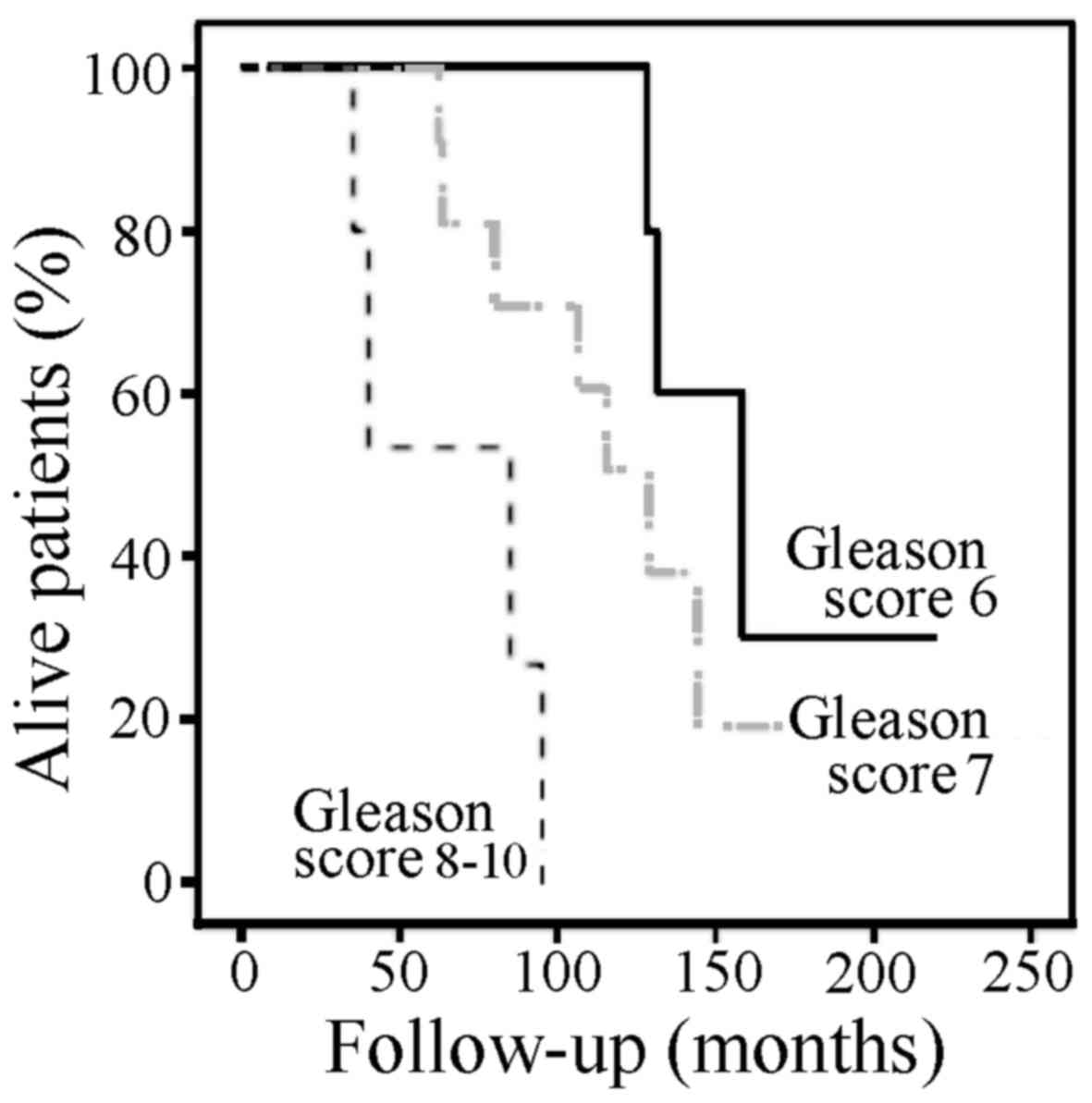
The mean prostate-specific survival was negatively
associated with the Gleason score at diagnosis (Fig. 1). The mean (95% CI) survival among
patients with a Gleason score of ≤6, 7 and ≥8 was 165 (130–201),
119 (95–144), and 66 (36–95) months, respectively (P=0.002).
The PSA level prior to RT was a prognostic factor
for biochemical recurrence of prostate cancer after RT [hazard
ratio (HR)=1.02; 95% CI: 1.01–1.04; P=0.002). Furthermore, it was a
prognostic factor for prostate cancer-specific survival (HR=1.01;
95% CI: 1.00–1.02; P=0.03).
A multivariate analysis was performed, including
age, PSA concentration prior to RT and the Gleason score groups.
Age, PSA concentration prior to RT and a high Gleason score were
independent prognostic factors for prostate cancer-specific
survival (Table IV).
 | Table IV.Cox proportional multivariate
analysis of patient age at diagnosis, PSA concentration prior to RT
and Gleason score at diagnosis as risk factors for prostate cancer
death. |
Table IV.
Cox proportional multivariate
analysis of patient age at diagnosis, PSA concentration prior to RT
and Gleason score at diagnosis as risk factors for prostate cancer
death.
| Variables | HR | 95% CI | P-value |
|---|
| Age at
diagnosis | 1.25 | 1.01–1.54 | 0.04 |
| PSA value prior to
RT | 1.02 | 1.00–1.03 | 0.02 |
| Gleason score |
|
|
|
| ≤6 | 1 (ref.) |
|
|
| 7 | 1.94 | 0.49–7.74 | 0.35 |
| ≥8 | 15.9 | 2.38–106 | 0.004 |
Discussion
Castration-resistant prostate cancer without
metastases represents a challenge for physicians. Medical or
surgical castration has been historically used to treat prostate
cancer in patients unfit for radical prostatectomy. Castration is
currently not recommended for asymptomatic patients with
non-metastatic prostate cancer (18). Furthermore, castration may increase
the risk of cardiovascular side effects (19). Thus, there must be clear indications,
such as severe obstructive voiding symptoms in a patient unsuitable
for transurethral resection of the prostate, for castration to be
applied; however, some patients are still primarily treated with
castration, and this may lead to the need for radical EBRT when
primary hormonal therapy fails.
Previous studies have reported the outcome of RT in
patients with non-metastatic castration-resistant prostate cancer:
To the best of our knowledge, the earliest patient series with a
number of patients sufficient for statistical analyses was
performed by Lankford et al (9), reporting the outcomes of 29 patients:
At 4 years, 80% exhibited disease progression or an increasing PSA
concentration (9). Botticella et
al (20) reported that, during a
5-year follow-up, 60% of the 42 patients benefited from EBRT. This
was the case for patients with a lower Gleason score, lower T stage
and low PSA concentration prior to RT. Moreover, at a median
follow-up of 53 months after EBRT, 21 of 42 (50%) patients
developed biochemical failure, defined as the nadir PSA + 2 µg/l
(20). Among Japanese patients, 66
of 140 (47%) exhibited clinical progression after EBRT during a
median follow-up of 20.7 months (12). Another Japanese study presented
results from 84 patients with a 3-year progression-free survival
(PFS) rate of 61% (21). In an
earlier Japanese report containing data from 61 patients (14), the 5-year PFS rate was 43.5%. Another
study from Japan (11) presented
results from 53 patients: The 3-year clinical relapse-free survival
rate was 78%, and 15 patients developed clinical metastases during
a median follow-up of 35 months (11). An Italian study published at the same
time reported the data from 29 patients, 24 (83%) of whom had
developed biochemical failure after a median of 9.2 months from
EBRT (13). A study from Australia
reported an actuarial median locoregional PFS duration of 43 months
in 34 patients (15); in that study,
however, 14 of the 34 patients had metastatic disease at baseline,
and the outcome of patients with non-metastatic disease was not
specified (15).
In our cohort, 16 of 21 (76%) patients developed
biochemical recurrence after RT with a mean time to biochemical
recurrence of 17 months. Among patients who died during follow-up
(n=18), the mean survival following RT was 3 years.
Our study included a limited population and was
retrospective in nature. Possible toxicities were not recorded. The
outcomes in our institutional cohort are comparable with those
published earlier. However, the benefit of EBRT for
castration-refractory prostate cancer appears to be limited, and
similar survival may be reached in this population with the novel
hormonal treatments abiraterone and enzalutamide. Currently, the
use of castration is limited among patients without metastasis, and
the need for EBRT with curative intent may be on the decrease.
In conclusion, disease progression following EBRT
for castration-resistant prostate cancer was common and survival
was limited in the present study. However, certain patients, more
likely those who are younger and have low-risk disease at
diagnosis, may benefit significantly from RT.
Glossary
Abbreviations
Abbreviations:
|
CI
|
confidence interval
|
|
CTV
|
clinical target volume
|
|
GnRH
|
gonadotropin hormone-releasing
hormone
|
|
GTV
|
gross tumor volume
|
|
HR
|
hazard ratio
|
|
PSA
|
prostate-specific antigen
|
|
PTV
|
planning target volume
|
References
|
1
|
Prostate (PRC) cancer factsheet.
http://www.encr.eu/images/docs/factsheets/ENCR_Factsheet_Prostate_2014.pdfAccessed.
September 28–2016.
|
|
2
|
Wilt TJ, MacDonald R, Rutks I, Shamliyan
TA, Taylor BC and Kane RL: Systematic review: Comparative
effectiveness and harms of treatments for clinically localized
prostate cancer. Ann Intern Med. 148:435–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamdy FC, Donovan JL, Lane JA, Mason M,
Metcalfe C, Holding P, Davis M, Peters TJ, Turner EL, Martin RM, et
al: Study Group: 10-Year Outcomes after Monitoring, Surgery, or
Radiotherapy for Localized Prostate Cancer. N Engl J Med. Epub
ahead of print. https://doi.org/10.1056/NEJMoa1606220
|
|
4
|
Guideline for the management of clinically
localized prostate cancer. 2007.https://www.auanet.org/education/guidelines/prostate-cancer.cfmAccessed
September 28, 2016.
|
|
5
|
Kawakami J, Cowan JE, Elkin EP, Latini DM,
DuChane J and Carroll PR: CaPSURE Investigators:
Androgen-deprivation therapy as primary treatment for localized
prostate cancer: Data from Cancer of the Prostate Strategic
Urologic Research Endeavor (CaPSURE). Cancer. 106:1708–1714. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tammela T: Endocrine treatment of prostate
cancer. J Steroid Biochem Mol Biol. 92:287–295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith MR, Kabbinavar F, Saad F, Hussain A,
Gittelman MC, Bilhartz DL, Wynne C, Murray R, Zinner NR, Schulman
C, et al: Natural history of rising serum prostate-specific antigen
in men with castrate nonmetastatic prostate cancer. J Clin Oncol.
23:2918–2925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dotan ZA, Bianco FJ Jr, Rabbani F, Eastham
JA, Fearn P, Scher HI, Kelly KW, Chen HN, Schöder H, Hricak H, et
al: Pattern of prostate-specific antigen (PSA) failure dictates the
probability of a positive bone scan in patients with an increasing
PSA after radical prostatectomy. J Clin Oncol. 23:1962–1968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lankford SP, Pollack A and Zagars GK:
Radiotherapy for regionally localized hormone refractory prostate
cancer. Int J Radiat Oncol Biol Phys. 33:907–912. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furuya Y, Akakura K, Akimoto S, Ichikawa T
and Ito H: Radiotherapy for local progression in patients with
hormone-refractory prostate cancer. Int J Urol. 6:187–191. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akimoto T, Kitamoto Y, Saito J, Harashima
K, Nakano T, Ito K, Yamamoto T, Kurokawa K, Yamanaka H, Takahashi
M, et al: External beam radiotherapy for clinically node-negative,
localized hormone-refractory prostate cancer: Impact of
pretreatment PSA value on radiotherapeutic outcomes. Int J Radiat
Oncol Biol Phys. 59:372–379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sasaki T, Nakamura K, Ogawa K, Onishi H,
Okamoto A, Koizumi M, Shioyama Y, Mitsumori M and Teshima T:
Japanese Patterns of Care Study Working Subgroup on Prostate
Cancer: Radiotherapy for patients with localized hormone-refractory
prostate cancer: Results of the Patterns of Care Study in Japan.
BJU Int. 104:1462–1466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanguineti G, Marcenaro M, Franzone P,
Tognoni P, Barra S and Vitale V: Is there a “curative” role of
radiotherapy for clinically localized hormone refractory prostate
cancer? Am J Clin Oncol. 27:264–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura K, Teshima T, Takahashi Y, Imai
A, Koizumi M, Mitsuhashi N, Shioyama Y and Inoue T: Japanese PCS
Working Subgroup of Prostate Cancer: Radiotherapy for localized
hormone-refractory prostate cancer in Japan. Anticancer Res.
24:3141–3145. 2004.PubMed/NCBI
|
|
15
|
Gogna NK, Baxi S, Hickey B, Baumann K,
Burmeister E and Holt T: Split-course, high-dose palliative pelvic
radiotherapy for locally progressive hormone-refractory prostate
cancer. Int J Radiat Oncol Biol Phys. 83:e205–e211. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
White R, Khor R, Bressel M, Duchesne G,
Williams S, Bowden P, Tai K and Foroudi F: Efficacy of high-dose
palliative radiotherapy for localised, castration-resistant
prostate cancer. Clin Oncol (R Coll Radiol). 27:16–21. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobin L and Wittekind C: Union for
international cancer control: TNM classification of malignant
tumours. 6th. Wiley; New York: 2002
|
|
18
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: European Association of Urology: EAU guidelines
on prostate cancer. Part II: Treatment of advanced, relapsing, and
castration-resistant prostate cancer. Eur Urol. 65:467–479. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bosco C, Bosnyak Z, Malmberg A, Adolfsson
J, Keating NL and Van Hemelrijck M: Quantifying observational
evidence for risk of fatal and nonfatal cardiovascular disease
following androgen deprivation therapy for prostate cancer: A
meta-analysis. Eur Urol. 68:386–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Botticella A, Guarneri A, Filippi AR,
Levra NG, Munoz F, Ragona R, Gontero P and Ricardi U: May
non-metastatic clinically localized castration-resistant prostate
cancer after primary androgen ablation benefit from salvage
prostate radiotherapy? J Cancer Res Clin Oncol. 139:1955–1960.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogawa K, Nakamura K, Sasaki T, Onishi H,
Koizumi M, Shioyama Y, Araya M, Mukumoto N, Mitsumori M and Teshima
T: Japanese Patterns of Care Study Working Subgroup of Prostate
Cancer: External beam radiotherapy for clinically localized
hormone-refractory prostate cancer: Clinical significance of Nadir
prostate-specific antigen value within 12 months. Int J Radiat
Oncol Biol Phys. 74:759–765. 2009. View Article : Google Scholar : PubMed/NCBI
|