Colorectal cancer is one of the most common types of
cancer worldwide. Due to the advances in endoscopic treatment,
particularly endoscopic submucosal dissection, several T1
colorectal cancers are resected endoscopically with negative
margins (1–3). Lymph node metastasis (LNM) occurs in
~10% of patients with T1 colorectal cancer, with these patients
requiring surgical resection with lymph node dissection (4–7).
Therefore, determining risk factors associated with LNM in patients
with T1 colorectal cancer is crucial.
A number of studies have assessed factors predictive
of LNM in patients with T1 colorectal cancer. Previously identified
risk factors for LNM include lymphovascular invasion, histological
grade, tumor budding and degree of submucosal invasion (8–10). These
factors are included in various diagnostic and treatment
guidelines, including those of the National Comprehensive Cancer
Network, the European Society for Medical Oncology and the Japanese
Society for Cancer of the Colon and Rectum (8–10).
However, the majority of the studies identifying these guidelines
were retrospective in design and included small numbers of
patients. In addition, these analyses were limited to pathological
factors. The indications for additional surgery plus lymph node
dissection following endoscopic resection remain unclear.
A recent retrospective, single-center study, which
included a large number of patients, reported that female gender
was associated with LNM in patients with T1 colorectal cancer
(4). Other studies also reported
higher rates of LNM in female compared with male patients, although
these differences were not statistically significant (11,12).
Several systematic reviews and meta-analyses have investigated risk
factors for LNM; however, none has focused on patient gender as a
predictive factor for LNM to date (13–17). The
aim of the present systematic review and meta-analysis was to
assess whether the gender of patients with T1 colorectal cancer is
predictive of LNM.
This systematic review and meta-analysis was
performed according to the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) statement, was conducted in
accordance with the Cochrane Handbook (18,19) and
was pre-registered (CRD42015024588). MEDLINE, EMBASE and the
Cochrane Central Register of Controlled Trials were searched from
the earliest date of indexing through July 11, 2015. The search
terms included ‘T1’, ‘early’, ‘colorectal’, ‘colonic’, ‘rectal’,
‘adenocarcinoma’, ‘neoplasm’, ‘lymph node’, ‘N1’ and ‘N2’ in
various combinations. Additional searches were performed by manual
cross-referencing. Only studies published in English were included.
The meta-analysis was restricted to studies reporting the adjusted
odds ratio (aOR) or risk ratio (RR) of dissection-diagnosed LNM in
relation to gender in patients with T1 colorectal cancer. Patients
with familial adenomatous polyposis, Lynch syndrome and ulcerative
colitis were excluded, as were patients who underwent only
endoscopic treatment or transanal endoscopic microsurgery.
Two authors (K.I. and Y.K.) independently reviewed
the abstracts and titles identified by the searches. All studies
rated as possible candidates by either of these two reviewers were
included in the preliminary list, and their full texts were
retrieved. The two authors independently reviewed the full texts to
determine whether the studies met the review criteria.
Disagreements were resolved by discussion, or if necessary by a
third reviewer (Y.K.). Information extracted from studies deemed to
have met the review criteria included name of first author, year of
publication, country, study design, number of patients, study
inclusion and exclusion criteria, study quality, demographic data
and outcome events.
Two authors (K.I. and Y.K.) independently assessed
the risk of bias using the Quality in Prognostic Studies (QUIPS)
tool (20). Each domain was rated as
being at low, high or unclear risk of bias, based on whether the
study sample adequately represented the population of interest;
whether the participants not lost to follow-up adequately
represented the study sample; whether prognostic factors and
outcomes of interest were measured similarly for all participants;
and whether there were other sources of bias. Disagreements between
reviewers were resolved by discussion.
Data were analyzed by a single investigator (Y.K.).
All studies included in the meta-analysis reported the frequency of
LNM in men and women, either in the text or in the tables. Data
were synthesized using Stata software, version 13.0 (Stata Corp.,
College Station, TX, USA). A meta-analysis was performed to
summarize the prognostic effects of gender, with results reported
as RR and 95% confidence interval (CI). A random-effects model was
used. The quality of evidence was evaluated using the Grading of
Recommendations Assessment, Development and Evaluation (GRADE)
approach (21). Heterogeneity was
assessed by visual inspection of the forest plots. I2
statistics were calculated and analyzed based on the
recommendations of the Cochrane Handbook, in which I2
values of 0–40, 30–60, 50–90 and 75–100% represent little,
moderate, substantial and considerable heterogeneity, respectively
(19). A sensitivity analysis was
conducted to pool all 36 studies reporting unadjusted relative risk
of gender. A subgroup analysis could not be conducted due to data
insufficiency. P-values <0.05 were considered to indicate
statistically significant differences.
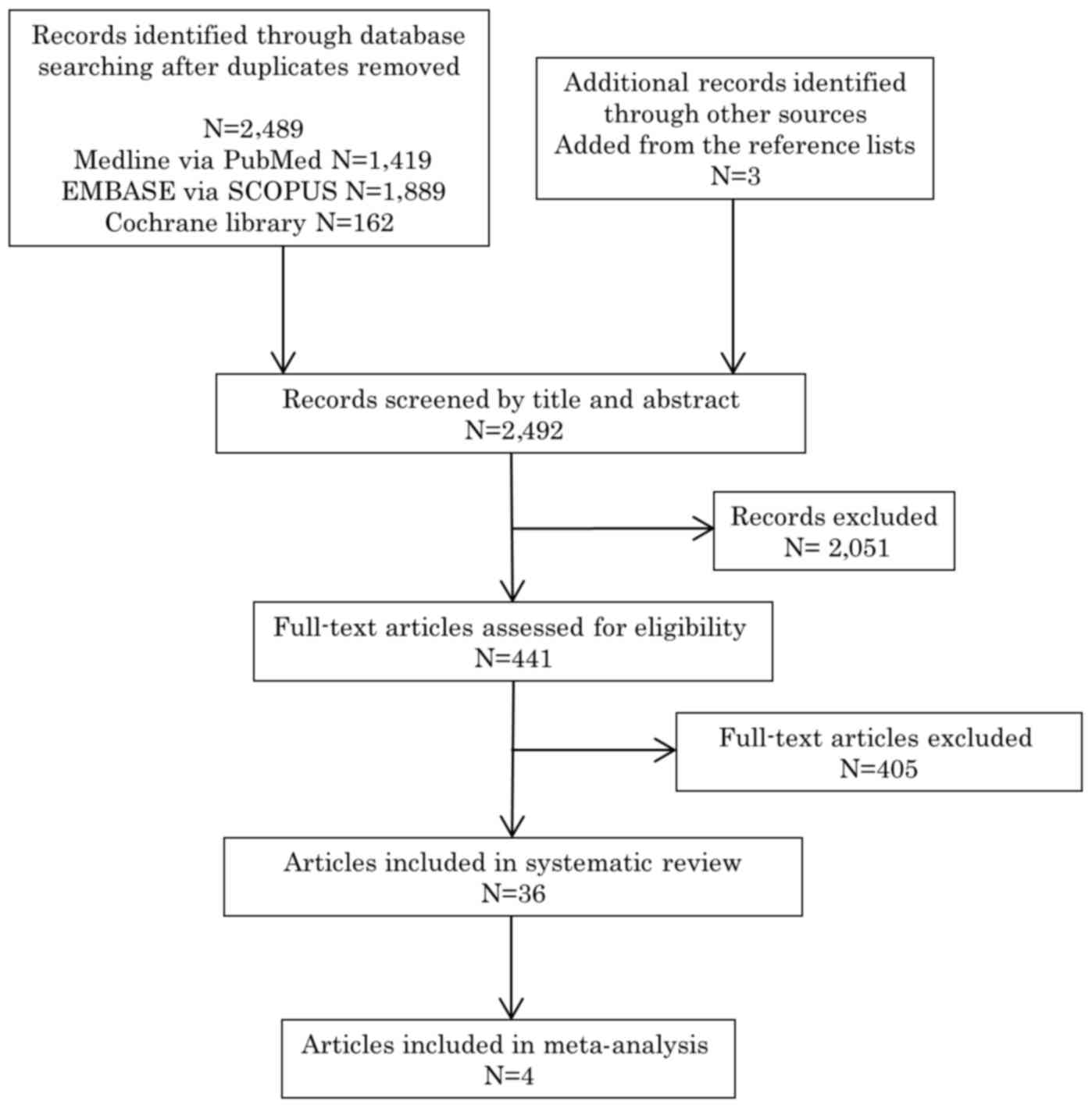
The initial database search identified 2,492
publications. Following removal of duplicates, 2,489 unique
publications were identified, 1,419 on PubMed, 1,889 on EMBASE and
162 on the Cochrane Library. Three additional publications were
identified through other sources or from the references lists of
the included publications. After screening the titles and
abstracts, 441 full-text articles were assessed for eligibility
(first review). Of those, 36 studies reported unadjusted results
and were included for systematic review (second review). Of the 36
studies, 4 (4,22–24)
reported adjusted results and fulfilled the predetermined inclusion
criteria for the meta-analysis (Fig.
1).
The 4 studies included in this meta-analysis were
retrospective in design and involved 1,329 patients with T1
colorectal cancer. Of the 4 studies, 3 were single-center and 1 was
a multicenter study. As regards bias, 3 studies were graded as
having a moderate risk of bias and 1 as having a low risk of bias.
The median number of patients per study was 332 (range, 142–653).
Of the 1,329 included patients, 864 (65.0%) were male and 465
(35.0%) were female; 558 (42.0%) had rectal carcinomas and 771
(58.0%) had colon carcinomas. The characteristics of the 4 included
studies are presented in Table
I.
Of the 1,329 patients, 113 (8.5%; 95% CI: 7.1–10.1)
were positive for LNM, with the number per study ranging from 6.3
to 9.9%. The incidence of LNM was 6.4% (55/864, 95% CI: 4.8–8.2) in
male and 12.5% (58/465, 95% CI: 9.6–15.8) in female patients.
Publication bias could not be evaluated using funnel
plots or Egger's regression test. Only 4 of 36 studies reported
adjusted outcomes, suggesting a selective outcome reporting bias
(25). The risk of bias was serious,
as the number of studies with a low risk of bias was limited. The
I2 statistic was 0.901, classified as very low (+OOO),
and was downgraded by the risk of bias, inconsistency and
publication bias (Table II).
Of the 4 studies, 3 reported a higher rate of LNM in
female compared with male patients with T1 colorectal cancer (10.8
vs. 4.6%, 15.7 vs. 4.2% and 12.7 vs. 7.1%, respectively), whereas 1
study reported a lower rate of LNM in female patients (8.3 vs.
10.6%) (4,22–24). Of
the 4 studies, 2 (4,23) reported that female gender was an
independent risk factor for LNM in patients with T1 colorectal
cancer (OR=5.68 and 2.22, respectively), whereas the remaining 2
studies (22,24) reported no significant difference
between male and female patients on the univariate as well as the
multivariate analyses. The result of the meta-analysis for
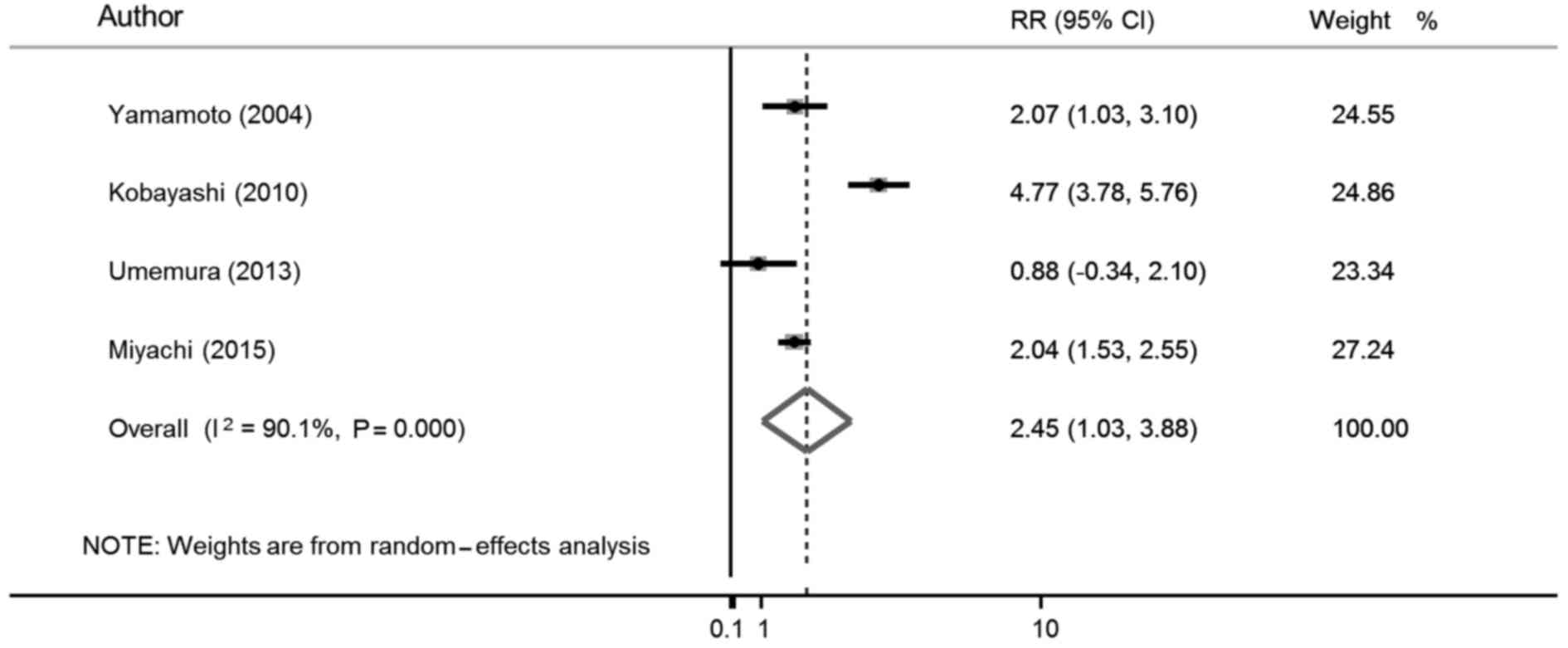
multivariate risk ratio is shown in Fig.
2. The weights were from the random-effects analysis. The
meta-analysis demonstrated that female gender was associated with
LNM in patients with T1 colorectal cancer (RR=2.45, 95% CI:
1.03–3.88).
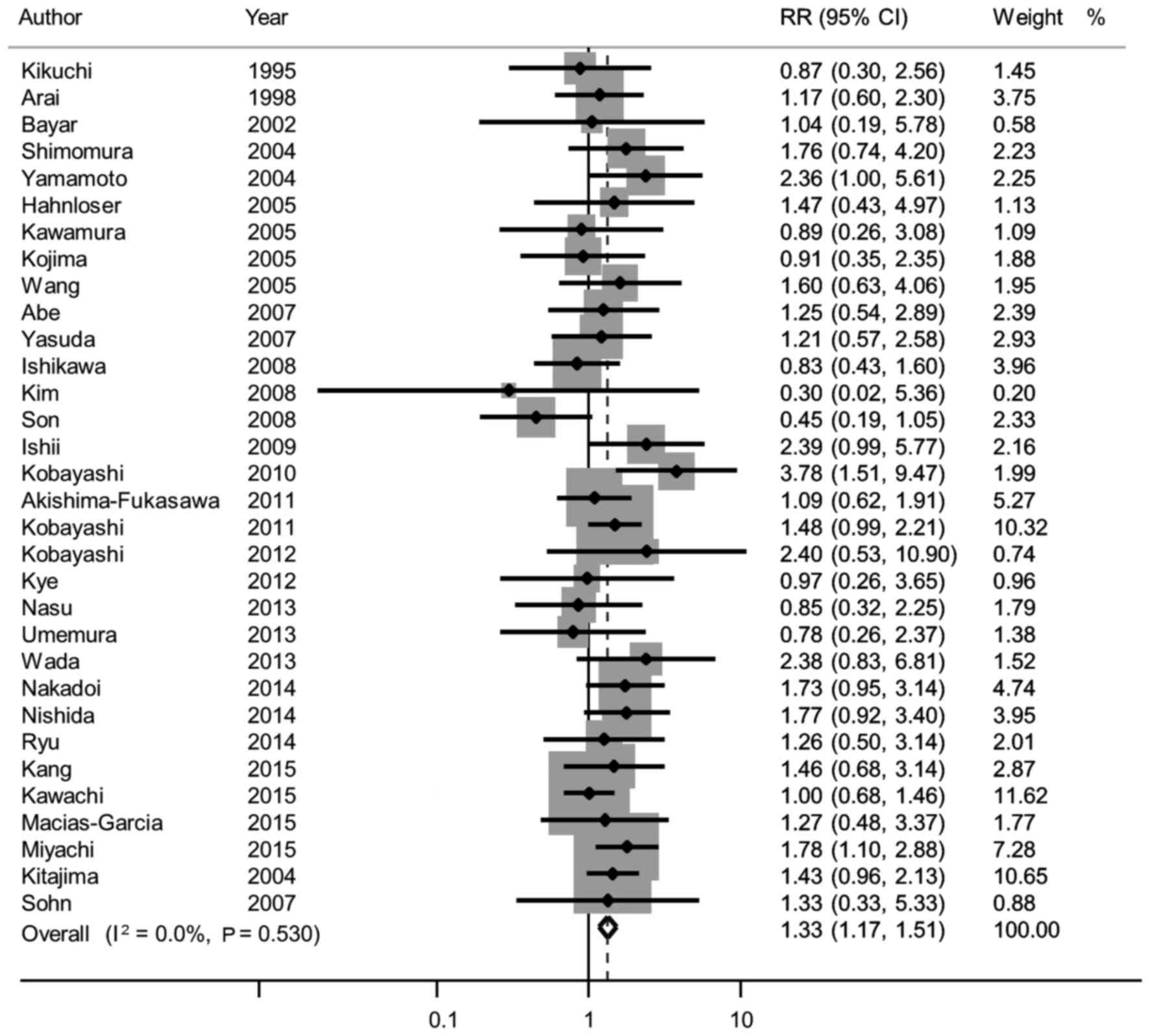
The pooled sensitivity analysis of the 36 studies
revealed that female gender was associated with LNM (RR=1.33, 95%
CI: 1.17–1.51; Fig. 3), which was
consistent with the main results (4–6,11,12,22–24,26–49).
In the present study, the association between
patient gender and LNM in patients with T1 colorectal cancer was
systematically reviewed. Our meta-analysis revealed that female
gender was associated with LNM in T1 colorectal cancer. To the best
of our knowledge, this is the first such analysis showing that
patient gender is predictive of LNM in patients with T1 colorectal
cancer.
Overall, ~10% of patients with T1 colorectal cancers
have LNM, thereby requiring more invasive surgery along with lymph
node dissection (4–7). Operative treatments are relatively
invasive and costly, making local excision an attractive treatment
option. However, local excision is oncologically safe only in the
absence of LNM. As LNM is difficult to assess preoperatively, the
decision to perform radical surgery following endoscopic resection
is based on the results of clinicopathological analysis. Several
previous systematic reviews of small, retrospective studies have
identified reliable pathological factors associated with the risk
of LNM in T1 colorectal cancer (13–17).
These meta-analyses reported that depth of submucosal invasion
>1,000 µm, lymphovascular invasion, poorly differentiated tumors
and tumor budding were all risk factors for LNM. The diagnosis of
pathological factors may differ among observers (38,50).
Moreover, pathological diagnoses may depend on the
immunohistochemical assay used, such as D2-40, Victoria Blue and
CAM 5.2. For example, lymphatic invasion is more accurately
diagnosed using an anti-human podoplanin antibody rather than by
hematoxylin and eosin staining (51–53). By
contrast, our meta-analysis was the first to demonstrate that
patient gender as a new clinical risk factor was predictive of LNM.
Moreover, in contrast to the other meta-analyses, ours assessed the
risk of bias of each study using the QUIPS tool and evaluated the
quality of evidence using the GRADE approach.
A recent study of 653 patients with T1 colorectal
cancer demonstrated that female gender was an independent risk
factor for LNM (4). Stratification
of patients according to the status of the muscularis mucosae
(whether the muscle fibers were maintained or
fragmented/disappeared), pathological factors and patient gender
provides more appropriate indications for additional surgery along
with lymph node dissection in this patient population, and may help
reduce the incidence of unnecessary surgery. Several other studies
also reported that the rate of LNM was higher in female compared
with male patients (5,11), and that female gender was an
independent risk factor for LNM in patients with T1 lower rectal
cancer (23).
Although the mechanism underlying the higher rate of
LNM in women with T1 colorectal cancer has not been fully
elucidated, epidemiological studies have reported a potential
association between gender hormones and colorectal cancer (54–56).
Some studies indicate a role for estrogen in the protection against
colorectal cancer (55–58). The effects of estrogen are mediated
by estrogen receptors (ERs), namely ERα and ERβ (59,60). ERβ
expression was found to be significantly reduced in adenomatous
tissues with high levels of dysplasia as well as in carcinomatous
tissues compared with normal mucosa (61). In addition, the degree of ERβ
expression loss appears to be correlated with more advanced stage
and higher tumor grade (62,63). The degree of reduction in ERβ level
may also be correlated with LNM. Nussler et al reported that
ERβ levels were significantly reduced in colorectal cancer in both
men and women compared with normal colonic mucosa, and this
reduction in ERβ level was different by gender (64). Other studies were unable to detect
such gender differences (62,65).
However, the samples of all those studies were very limited and
investigation using larger sample sizes would be required to
demonstrate the difference in ERβ levels by gender. Furthermore,
not only a reduction of the ERβ levels, but more importantly, a
change in the ERα:ERβ ratio, may determine the susceptibility of a
given tissue to carcinogenesis (66,67).
This is only one plausible reason and there may be other possible
explanations for the association between LNM and gender in T1
colorectal cancer; therefore, further investigation is
required.
This meta-analysis had several limitations. The main
limitation was the lack of randomized controlled trials, as
confounding factors may be more effectively removed from a
randomized trial rather than from an observational study. As the
patients in these studies were not randomized by gender, our
analysis may have been sensitive to confounding variables.
Therefore, only studies with adjusted results were included.
Second, only 4 of the 36 studies reported adjusted results. Thus, a
selective outcome reporting bias may have led to the gender-related
difference in LNM rate. Our sensitivity analysis included all 36
studies, with the results not differing markedly. Third, all the
studies in this meta-analysis originated in Japan, which may have
affected our results. Only 3 of the 36 studies were from western
countries, none of which reported adjusted results, and were thus
excluded from the current criteria (28,30,48). In
fact, these 3 studies showed a tendency of higher LNM rate in
female rather than in male patients, but the difference was not
significant due to insufficient number (<100) of study subjects.
The association between female gender and LNM may differ by race.
However, such a meta-analysis including western populations cannot
be conducted at present; thus, this risk factor requires
larger-scale validation in western countries. In our meta-analysis,
1 of the 4 included studies reported a higher LNM rate in male
rather than female patients, although the difference was not
statistically significant. There was little clinical heterogeneity.
Thus, this difference may be due to chance by small sample
size.
In conclusion, the gender of patients with T1
colorectal cancer was found to be predictive of LNM. This finding
may help select patients who may be spared radical resection,
thereby preventing unnecessary surgery without compromising
oncological safety. Further prospective randomized studies with
larger patient populations are required to confirm this result.
The authors would like to thank Yoko Tanaka for
assisting with the English composition of the manuscript, and all
members of the Digestive Disease Center and the Department of
Pathology of Showa University Northern Yokohama Hospital for their
excellent assistance.
|
1
|
Lee EJ, Lee JB, Lee SH and Youk EG:
Endoscopic treatment of large colorectal tumors: Comparison of
endoscopic mucosal resection, endoscopic mucosal
resection-precutting and endoscopic submucosal dissection. Surg
Endosc. 26:2220–2230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanaka S, Oka S, Kaneko I, Hirata M, Mouri
R, Kanao H, Yoshida S and Chayama K: Endoscopic submucosal
dissection for colorectal neoplasia: Possibility of
standardization. Gastrointest Endosc. 66:100–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kobayashi N, Saito Y, Uraoka T, Matsuda T,
Suzuki H and Fujii T: Treatment strategy for laterally spreading
tumors in Japan: Before and after the introduction of endoscopic
submucosal dissection. J Gastroenterol Hepatol. 24:1387–1392. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyachi H, Kudo SE, Ichimasa K, Hisayuki
T, Oikawa H, Matsudaira S, Kouyama Y, Kimura YJ, Misawa M, Mori Y,
et al: Management of T1 colorectal cancers after endoscopic
treatment based on the risk stratification of lymph node
metastasis. J Gastroenterol Hepatol. 31:1126–1132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi H, Mochizuki H, Morita T, Kotake
K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K, Oya M, et
al: Characteristics of recurrence after curative resection for T1
colorectal cancer: Japanese multicenter study. J Gastroenterol.
46:203–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Son HJ, Song SY, Lee WY, Yang SS, Park SH,
Yang MH, Yoon SH and Chun HK: Characteristics of early colorectal
carcinoma with lymph node metastatic disease.
Hepatogastroenterology. 55:1293–1297. 2008.PubMed/NCBI
|
|
7
|
Hackelsberger A, Fruhmorgen P, Weiler H,
Heller T, Seeliger H and Junghanns K: Endoscopic polypectomy and
management of colorectal adenomas with invasive carcinoma.
Endoscopy. 27:153–158. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
AB III Benson, Bekaii-Saab T, Chan E, Chen
YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton
MJ, et al: Localized colon cancer, version 3.2013: Featured updates
to the NCCN Guidelines. J Natl Compr Canc Netw. 11:519–528.
2013.PubMed/NCBI
|
|
9
|
Labianca R, Nordlinger B, Beretta GD,
Mosconi S, Mandalà M, Cervantes A and Arnold D; ESMO Guidelines
Working Group, : Early colon cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 24
Suppl 6:vi64–vi72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
Guidelines 2014 for treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakadoi K, Oka S, Tanaka S, Hayashi N,
Terasaki M, Arihiro K, Shimamoto F and Chayama K: Condition of
muscularis mucosae is a risk factor for lymph node metastasis in T1
colorectal carcinoma. Surg Endosc. 28:1269–1276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kitajima K, Fujimori T, Fujii S, Takeda J,
Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T, et
al: Correlations between lymph node metastasis and depth of
submucosal invasion in submucosal invasive colorectal carcinoma: A
Japanese collaborative study. J Gastroenterol. 39:534–543. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wada H, Shiozawa M, Katayama K, Okamoto N,
Miyagi Y, Rino Y, Masuda M and Akaike M: Systematic review and
meta-analysis of histopathological predictive factors for lymph
node metastasis in T1 colorectal cancer. J Gastroenterol.
50:727–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi JY, Jung SA, Shim KN, Cho WY, Keum B,
Byeon JS, Huh KC, Jang BI, Chang DK, Jung HY, et al: Meta-analysis
of predictive clinicopathologic factors for lymph node metastasis
in patients with early colorectal carcinoma. J Korean Med Sci.
30:398–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beaton C, Twine CP, Williams GL and
Radcliffe AG: Systematic review and meta-analysis of
histopathological factors influencing the risk of lymph node
metastasis in early colorectal cancer. Colorectal Dis. 15:788–797.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bosch SL, Teerenstra S, de Wilt JH,
Cunningham C and Nagtegaal ID: Predicting lymph node metastasis in
pT1 colorectal cancer: A systematic review of risk factors
providing rationale for therapy decisions. Endoscopy. 45:827–834.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mou S, Soetikno R, Shimoda T, Rouse R and
Kaltenbach T: Pathologic predictive factors for lymph node
metastasis in submucosal invasive (T1) colorectal cancer: A
systematic review and meta-analysis. Surg Endosc. 27:2692–2703.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. PLoS Med.
6:e10000972009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higgins J and Green S: Cochrane Handbook
for Systematic Reviews of Interventions Version 5.1.0 [updated
March 2011]. 2008. The Cochrane Collaboration; 2011
|
|
20
|
Hayden JA, van der Windt DA, Cartwright
JL, Côté P and Bombardier C: Assessing bias in studies of
prognostic factors. Ann Intern Med. 158:280–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iorio A, Spencer FA, Falavigna M, Alba C,
Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, et al:
Use of GRADE for assessment of evidence about prognosis: Rating
confidence in estimates of event rates in broad categories of
patients. BMJ. 350:h8702015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Umemura K, Takagi S, Shimada T, Masuda T,
Shiga H, Takahashi S, Takahashi S, Kinouchi Y, Shibuya D and
Shimosegawa T: Prognostic and diagnostic significance of tumor
budding associated with β-catenin expression in submucosal invasive
colorectal carcinoma. Tohoku J Exp Med. 229:53–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kobayashi H, Mochizuki H, Kato T, Mori T,
Kameoka S, Shirouzu K, Saito Y, Watanabe M, Morita T, Hida J, et
al: Is total mesorectal excision always necessary for T1-T2 lower
rectal cancer? Ann Surg Oncol. 17:973–980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamamoto S, Watanabe M, Hasegawa H, Baba
H, Yoshinare K, Shiraishi J and Kitajima M: The risk of lymph node
metastasis in T1 colorectal carcinoma. Hepatogastroenterology.
51:998–1000. 2004.PubMed/NCBI
|
|
25
|
Chan AW, Hróbjartsson A, Haahr MT,
Gøtzsche PC and Altman DG: Empirical evidence for selective
reporting of outcomes in randomized trials: Comparison of protocols
to published articles. J Am Med Assoc. 291:2457–2465. 2004.
View Article : Google Scholar
|
|
26
|
Kikuchi R, Takano M, Takagi K, Fujimoto N,
Nozaki R, Fujiyoshi T and Uchida Y: Management of early invasive
colorectal cancer. Risk of recurrence and clinical guidelines. Dis
Colon Rectum. 38:1286–1295. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arai T, Akiyama Y, Yamamura A, Hosoi T,
Shibata T, Saitoh K, Okabe S and Yuasa Y: Allelotype analysis of
early colorectal cancers with lymph node metastasis. Int J Cancer.
79:418–423. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bayar S, Saxena R, Emir B and Salem RR:
Venous invasion may predict lymph node metastasis in early rectal
cancer. Eur J Surg Oncol. 28:413–417. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimomura T, Ishiguro S, Konishi H,
Wakabayashi N, Mitsufuji S, Kasugai T, Manou M and Kodama T: New
indication for endoscopic treatment of colorectal carcinoma with
submucosal invasion. J Gastroenterol Hepatol. 19:48–55. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hahnloser D, Wolff BG, Larson DW, Ping J
and Nivatvongs S: Immediate radical resection after local excision
of rectal cancer: An oncologic compromise? Dis Colon Rectum.
48:429–437. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawamura YJ, Sakuragi M, Togashi K, Okada
M, Nagai H and Konishi F: Distribution of lymph node metastasis in
T1 sigmoid colon carcinoma: Should we ligate the inferior
mesenteric artery? Scand J Gastroenterol. 40:858–861. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kojima M, Shiokawa A, Ohike N, Ohta Y,
Kato H, Iwaku K, Hayasi R and Morohoshi T: Clinical significance of
nuclear morphometry at the invasive front of T1 colorectal cancer
and relation to expression of VEGF-A and VEGF-C. Oncology.
68:230–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang HS, Liang WY, Lin TC, Chen WS, Jiang
JK, Yang SH, Chang SC and Lin JK: Curative resection of T1
colorectal carcinoma: Risk of lymph node metastasis and long-term
prognosis. Dis Colon Rectum. 48:1182–1192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abe A, Fukui H, Fujii S, Fujita M, Mukawa
K, Ichikawa K, Tomita S, Ono Y, Imai Y, Imura J, et al: Involvement
of cyclooxygenase-2 and vascular endothelial growth factor in
vascularization and lymph node metastasis of colorectal cancers
with submucosal invasion. J Gastroenterol Hepatol. 22:1071–1077.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yasuda K, Inomata M, Shiromizu A,
Shiraishi N, Higashi H and Kitano S: Risk factors for occult lymph
node metastasis of colorectal cancer invading the submucosa and
indications for endoscopic mucosal resection. Dis Colon Rectum.
50:1370–1376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ishikawa Y, Akishima-Fukasawa Y, Ito K,
Akasaka Y, Yokoo T and Ishii T: Toho Study Group for Cancer
Biological Behavior: Histopathologic determinants of regional lymph
node metastasis in early colorectal cancer. Cancer. 112:924–933.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim JH, Cheon JH, Kim TI, Baik SH, Kim NK,
Kim H and Kim WH: Effectiveness of radical surgery after incomplete
endoscopic mucosal resection for early colorectal cancers: A
clinical study investigating risk factors of residual cancer. Dig
Dis Sci. 53:2941–2946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ishii M, Ota M, Saito S, Kinugasa Y,
Akamoto S and Ito I: Lymphatic vessel invasion detected by
monoclonal antibody D2-40 as a predictor of lymph node metastasis
in T1 colorectal cancer. Int J Colorectal Dis. 24:1069–1074. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Akishima-Fukasawa Y, Ishikawa Y, Akasaka
Y, Uzuki M, Inomata N, Yokoo T, Ishii R, Shimokawa R, Mukai K,
Kiguchi H, et al: Histopathological predictors of regional lymph
node metastasis at the invasive front in early colorectal cancer.
Histopathology. 59:470–481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kobayashi H, Higuchi T, Uetake H, Iida S,
Ishikawa T, Ishiguro M and Sugihara K: Resection with en bloc
removal of regional lymph node after endoscopic resection for T1
colorectal cancer. Ann Surg Oncol. 19:4161–4167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kye BH, Jung JH, Kim HJ, Kang SG, Cho HM
and Kim JG: Tumor budding as a risk factor of lymph node metastasis
in submucosal invasive T1 colorectal carcinoma: A retrospective
study. BMC Surg. 12:2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nasu T, Oku Y, Takifuji K, Hotta T,
Yokoyama S, Matsuda K, Tamura K, Ieda J, Yamamoto N, Takemura S, et
al: Predicting lymph node metastasis in early colorectal cancer
using the CITED1 expression. J Surg Res. 185:136–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wada H, Shiozawa M, Sugano N, Morinaga S,
Rino Y, Masuda M, Akaike M and Miyagi Y: Lymphatic invasion
identified with D2-40 immunostaining as a risk factor of nodal
metastasis in T1 colorectal cancer. Int J Clin Oncol. 18:1025–1031.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nishida T, Egashira Y, Akutagawa H, Fujii
M, Uchiyama K, Shibayama Y and Hirose Y: Predictors of lymph node
metastasis in T1 colorectal carcinoma: An immunophenotypic analysis
of 265 patients. Dis Colon Rectum. 57:905–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ryu HS, Kim WH, Ahn S, Kim DW, Kang SB,
Park HJ, Park YS, Lee CH and Lee HS: Combined morphologic and
molecular classification for predicting lymph node metastasis in
early-stage colorectal adenocarcinoma. Ann Surg Oncol.
21:1809–1816. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kang J, Lee HW, Kim IK, Kim NK, Sohn S-K
and Lee KY: Clinical implications of microsatellite instability in
T1 colorectal cancer. Yonsei Med J. 56:175–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kawachi H, Eishi Y, Ueno H, Nemoto T,
Fujimori T, Iwashita A, Ajioka Y, Ochiai A, Ishiguro S, Shimoda T,
et al: A three-tier classification system based on the depth of
submucosal invasion and budding/sprouting can improve the treatment
strategy for T1 colorectal cancer: A retrospective multicenter
study. Mod Path. 28:872–879. 2015. View Article : Google Scholar
|
|
48
|
Macias-Garcia F, Celeiro-Muñoz C,
Lesquereux-Martinez L, Gude-Sampedro F, Uribarri-Gonzalez L,
Abdulkader I, Alvarez-Castro A and Dominguez-Muñoz JE: A clinical
model for predicting lymph node metastasis in submucosal invasive
(T1) colorectal cancer. Int J Colorectal Dis. 30:761–768. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sohn DK, Chang HJ, Park JW, Choi DH, Han
KS, Hong CW, Jung KH, Kim DY, Lim SB, Choi HS and Jeong SY:
Histopathological risk factors for lymph node metastasis in
submucosal invasive colorectal carcinoma of pedunculated or
semipedunculated type. J Clin Pathol. 60:912–915. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Morodomi T, Isomoto H, Shirouzu K,
Kakegawa K, Irie K and Morimatsu M: An index for estimating the
probability of lymph node metastasis in rectal cancers. Lymph node
metastasis and the histopathology of actively invasive regions of
cancer. Cancer. 63:539–543. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Walgenbach-Bruenagel G, Tolba RH, Varnai
AD, Bollmann M, Hirner A and Walgenbach KJ: Detection of lymphatic
invasion in early stage primary colorectal cancer with the
monoclonal antibody D2-40. Eur Surg Res. 38:438–444. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kahn HJ and Marks A: A new monoclonal
antibody, D2-40, for detection of lymphatic invasion in primary
tumors. Lab Invest. 82:1255–1257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kahn HJ, Bailey D and Marks A: Monoclonal
antibody D2-40, a new marker of lymphatic endothelium, reacts with
Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol.
15:434–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
La Vecchia C and Franceschi S:
Reproductive factors and colorectal cancer. Cancer Causes Control.
2:193–200. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fernandez E, La Vecchia C, Balducci A,
Chatenoud L, Franceschi S and Negri E: Oral contraceptives and
colorectal cancer risk: A meta-analysis. Br J Cancer. 84:722–727.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Grodstein F, Newcomb PA and Stampfer MJ:
Postmenopausal hormone therapy and the risk of colorectal cancer: A
review and meta-analysis. Am J Med. 106:574–582. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hendifar A, Yang D, Lenz F, Lurje G, Pohl
A, Lenz C, Ning Y, Zhang W and Lenz HJ: Gender disparities in
metastatic colorectal cancer survival. Clin Cancer Res.
15:6391–6397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Newcomb PA, Zheng Y, Chia VM, Morimoto LM,
Doria-Rose VP, Templeton A, Thibodeau SN and Potter JD: Estrogen
plus progestin use, microsatellite instability, and the risk of
colorectal cancer in women. Cancer Res. 67:7534–7539. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Walter P, Green S, Greene G, Krust A,
Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M,
et al: Cloning of the human estrogen receptor cDNA. Proc Natl Acad
Sci USA. 82:7889–7893. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kuiper GG, Enmark E, Pelto-Huikko M,
Nilsson S and Gustafsson JA: Cloning of a novel receptor expressed
in rat prostate and ovary. Proc Natl Acad Sci USA. 93:5925–5930.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Barone M, Scavo MP, Papagni S, Piscitelli
D, Guido R, Di Lena M, Comelli MC and Di Leo A: ERβ expression in
normal, adenomatous and carcinomatous tissues of patients with
familial adenomatous polyposis. Scand J Gastroenterol.
45:1320–1328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Konstantinopoulos PA, Kominea A, Vandoros
G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G
and Papavassiliou AG: Oestrogen receptor beta (ERbeta) is
abundantly expressed in normal colonic mucosa, but declines in
colon adenocarcinoma paralleling the tumour's dedifferentiation.
Eur J Cancer. 39:1251–1258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jassam N, Bell SM, Speirs V and Quirke P:
Loss of expression of oestrogen receptor beta in colon cancer and
its association with Dukes' staging. Oncol Rep. 14:17–21.
2005.PubMed/NCBI
|
|
64
|
Nüssler NC, Reinbacher K, Shanny N,
Schirmeier A, Glanemann M, Neuhaus P, Nussler AK and Kirschner M:
Gender-specific differences in the expression levels of estrogen
receptor subtypes in colorectal cancer. Gend Med. 5:209–217. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Foley EF, Jazaeri AA, Shupnik MA, Jazaeri
O and Rice LW: Selective loss of estrogen receptor beta in
malignant human colon. Cancer Res. 60:245–248. 2000.PubMed/NCBI
|
|
66
|
Roger P, Sahla ME, Mäkelä S, Gustafsson
JA, Baldet P and Rochefort H: Decreased expression of estrogen
receptor beta protein in proliferative preinvasive mammary tumors.
Cancer Res. 61:2537–2541. 2001.PubMed/NCBI
|
|
67
|
Shaw JA, Udokang K, Mosquera JM, Chauhan
H, Jones JL and Walker RA: Oestrogen receptors alpha and beta
differ in normal human breast and breast carcinomas. J Pathol.
198:450–457. 2002. View Article : Google Scholar : PubMed/NCBI
|