Introduction
In Japan, cetuximab has been established as a
non-surgical treatment option for head and neck cancer in
combination with radiotherapy since December, 2012. Cetuximab is
expected to be effective in the long-term management of patients
with recurrent or metastatic head and neck cancer; however, adverse
reactions such as sudden death, arrhythmia, degeneration of
interstitial pneumonia and hypomagnesaemia are commonly observed
(1). Therefore, it is crucial to
identify and address those symptoms in the early stages. We herein
present a case of infusion reactions (IR) induced by cetuximab
(Erbitux®) from an anesthesiologist's viewpoint. The
patient was treated for the IR by administration of ephedrine
alone, which is an uncommon method for treating IR (2). The difficulties of diagnosing and
managing IR are also discussed, with a brief review of the
literature.
Case report
The patient was a 77-year-old man (160 cm in height,
57 kg in weight, body surface area 1.591 m2). The
patient had previously undergone tracheotomy, resection of the left
part of the tongue, left radical neck dissection and reconstructive
surgery with pectoralis major myocutaneous flaps for the treatment
of left-sided tongue cancer, and had received 3 cycles of
chemotherapy with cisplatin and TS-1. A lymph node metastasis was
found in the right neck within 6 months after the surgery, and
intravenous infusion of cetuximab in the outpatient clinic was
planned. The patient had been medicated with voglibose, glimepiride
and clopidogrel for diabetes (HbA1c 6.0) and atherosclerosis
obliterans of the lower extremities.
Upon arrival at the outpatient clinic, the patient's
blood pressure (BP) was 140/76 mmHg, the heart rate (HR) was 60 bpm
and the blood oxygen saturation (SpO2) was 96%.
Peripheral venous access was obtained with an 22G indwelling needle
on the forearm, and D-chlorpheniramine maleate 5 mg, dexamethasone
sodium phosphate 13.2 mg and azasetron hydrochloride 10 mg were
infused intravenously over 1 h. The infusion of cetuximab 600 mg,
which represents the loading dose of 400 mg/m2, was then
initiated at a rate of 5 mg/min. Ten minutes after starting the
infusion, facial flushing developed and the patient complained of
pruritus on the abdomen. The vital signs were stable (BP, 124/68
mmHg; HR, 75 bpm; and SpO2, 96%). According to the
instruction manual, cetuximab infusion was continued at a reduced
rate of 2 mg/min. A few minutes later, diaphoresis developed and
the pruritus worsened, with severe erythema and raised rash on the
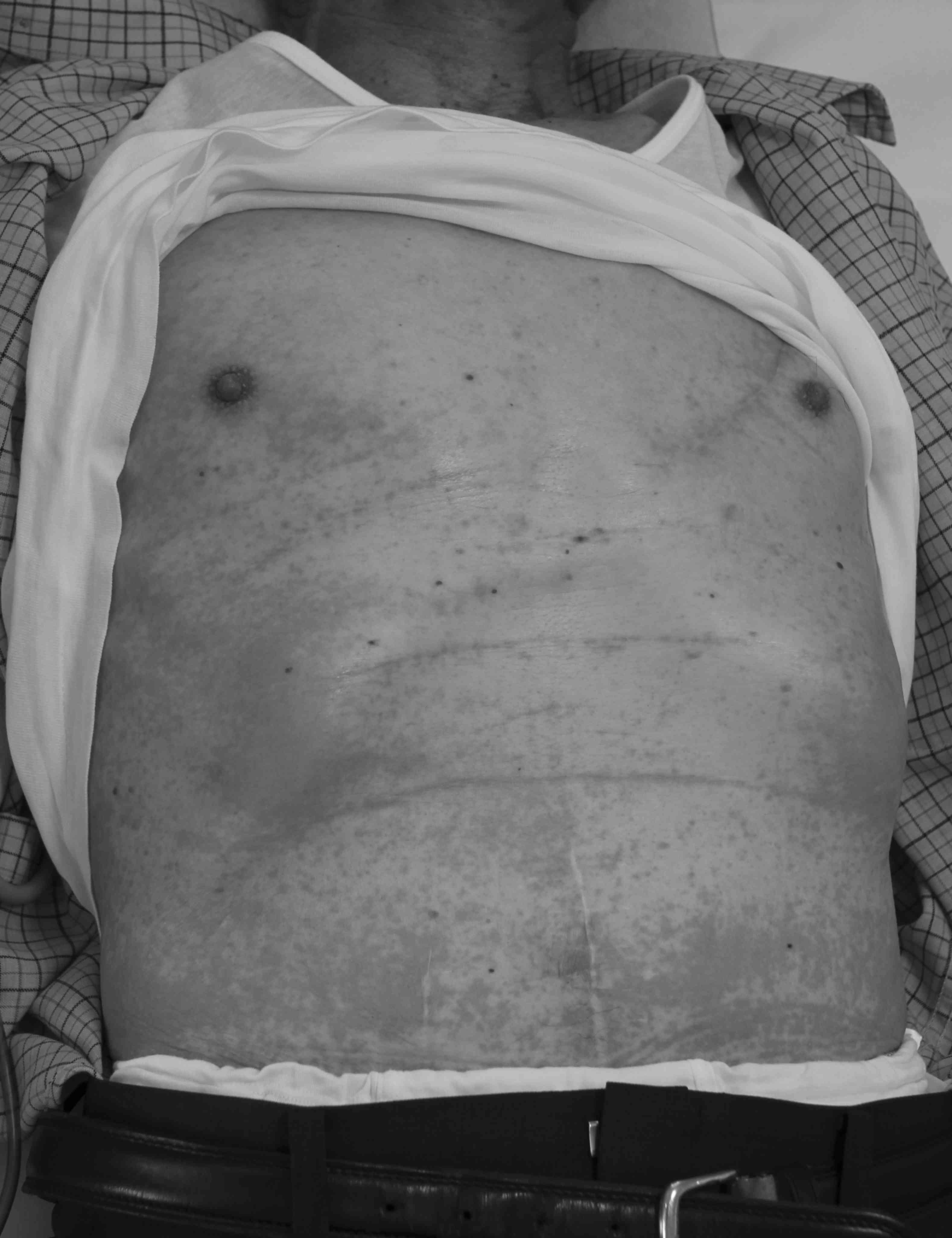
trunk and extremities (Fig. 1). The
BP had decreased to 62/36 mmHg and the HR to 48 bpm; therefore, an
anaphylactoid reaction was suspected. Cetuximab infusion was
discontinued immediately, and the infusion solution was replaced
with Ringer's acetate solution. A total of 16 mg methylephedrine
was administered. The patient was sufficiently lucid to breath
normally without nausea. Although no decrease in SpO2
was noted, he was administered oxygen through a Polymask at 6 l/min
to prevent hypoxia. After methylephedrine hydrochloride
administration and rapid fluid infusion of 500 ml, the patient
recovered from the circulatory collapse with a BP of 119/63 mmHg
and a HR of 63 bpm. The vital signs were stabilized (BP,
122–148/66-78 mmHg; HR, 62–76 bpm; SpO2, 99% in room
air), and the patient was hospitalized at the Osaka Dental
University Hospital (Osaka, Japan), whereas a dermatologist at an
affiliated hospital was consulted. In addition to prescription of
olopatadine hydrochloride and betamethasone butyrate propionate,
the patient received an intravenous infusion of 250 mg
methylprednisolone sodium succinate. During hospitalization, mild
erythema persisted on the back, foreneck and chest, but the raised
rash had disappeared before discharge on the following day.
Discussion
Our case presented two interesting clinical points:
Methylephedrine may be useful for the treatment of anaphylactoid
reactions when administered alone, and the patient's living
environment is significantly associated with the incidence of
IR.
This case provided us with a valuable opportunity to
determine whether ephedrine hydrochloride administration was
effective to reverse the grade 3–4 IR, as epinephrine, rather than
ephedrine, has been commonly used for the treatment of IR.
Ephedrine is a vasoconstrictor generally used to restore the BP to
normal levels. The incidence of IR has been reported to be 15% for
all grades of IR, and 3% for IR of grade ≥3 (3). Pre-treatment with antihistamines and
corticosteroids is often performed as a standard method to reduce
the risk of IR associated with cetuximab treatment; in colorectal
cancer patients, the incidence of grade 3–4 IR was found to be
lower, namely 1.1% (4). However,
symptoms associated with IR may occur despite prophylaxis, as this
patient developed a reaction to cetuximab despite premedication. As
regards the development of IR, previous reports suggested that IR
developed within 1 h after starting the cetuximab infusion,
particularly within the first 15 min (4). The patient in the present case
experienced the development of IR within 20 min after treatment
initiation. The treatment of IR depends on the severity, and grade
≥3 symptoms require treatment similar to that for anaphylaxis
(1,4). In this case, the severity of the
symptoms increased even after reducing the infusion rate of
cetuximab according to the instruction manual. It was crucial that
the surgeon as well as the dental anesthesiologist carefully
observed the patient in order to assess the situation in the early
stage. The key charateristic was circulatory collapse following
dermatological reactions. Respiratory events, such as airway
obstruction and edema, were not evident. Ephedrine, which was ready
for emergency use, was initially administered, and the BP and HR
gradually increased following additional dosage of ephedrine with
rapid infusion of acetate Ringer's solution. The vital signs were
stabilized by administering a total dose of 16 mg ephedrine.
D-chlorpheniramine and dexamethasone as prophylaxis may be helpful
in restoring BP and HR, enhancing the vasopressor effect of
ephedrine. In case the BP and HR were unresponsive to ephedrine, a
different vasopressor would have to be considered; an α- and
β-stimulator, such as epinephrine, is considered to be a safe
method for controlling respiratory events.
This case also suggested a correlation between the
patient's living environment and high incidence of IR. Certain
factors in the living environment may act as antigen towards
cetuximab, eventually increasing the risk of IR by sensitizing the
patient. For example, reports from the United States have described
that the prevalence of anti-cetuximab IgE antibodies varies in
different geographical areas, and hypothesized that the incidence
of allergic reactions to cetuximab may be related to the incidence
of allergic reactions to red meat and Rocky Mountain spotted fever,
and the habit distribution of ticks carrying Rickettsia rickettsii,
the bacterium causing Rocky Mountan spotted fever (5). It has been reported that IgE antibodies
against galactose-α-1, 3-galactose (α-gal) elicit anaphylaxis in
patients receiving cetuximab (5,6). The
fact that patients who develop IR during the first infusion of
cetuximab were found to have IgE antibodies specific to cetuximab,
indicates that they had been previously exposed to sensitizers
(5,6); α-gal is present in cows and pigs, and
is believed to cause late-onset urticaria and anaphylaxis that
develop in 3–6 h after eating beef or pork. It is also believed
that the level of beef-specific IgE antibodies is correlated with
the level of cetuximab-specific IgE antibodies (5,7,8). The level of α-gal-specific IgE
antibodies is also correlated with the level of IgE antibodies
specific to the tick Amblyomma americanum, a vector of Rocky
Mountain spotted fever that is commonly found in the southwestern
to eastern part of the United States (4). These findings indicate that antibodies
in patients with a meat allergy, or those with a history of tick
bite, may cross-react against cetuximab (5–8). In
Japan, mites are drawing attention as a cause of a syndrome
combining severe fever with thrombocytopenia in recent years.
However, a report from the Shimane Prefecture suggests that there
are overlaps in areas where spotted fever is prevalent and those
where several patients with beef allergy reside (9). That study also reported that 75% of
patients with beef allergy are also allergic to flounder eggs,
which indicates that anti-beef antibodies may cross-react with
flounder egg antigens (9). As a
number of these patients have lived with dogs and have IgE
antibodies specific to dog's skin, the authors of that study
concluded that mites, which bite dogs as well as humans, may induce
beef allergy (9). Altogether,
reports from the United States as well as Japan suggest that
several factors in the living environment are associated with the
incidence of IR to cetuximab.
Therefore, physicians must question the patients on
past illnesses prior to administering cetuximab. Unfortunately, the
patient in the present case received cetuximab infusion without a
detailed consultation on the risk factors for IR. A consultation
after the onset of IR revealed that he was from Izumo (Shimane,
Japan). In addition, male gender and smoking are factors known to
increase the risk of IR (10),
indicating that this patient was at a higher risk of developing
IR.
In conclusion, this case illustrated the usefulness
of ephedrine in managing IR symptoms during the first
administration of cetuximab for the treatment of a metastatic lymph
node in the neck. The present case has also highlighted the
importance of interviewing the patient on past and present places
of residence, history of allergic reactions to beef and/or flounder
eggs, history of tick bites and history of living with dogs prior
to initiating cetuximab therapy.
Informed consent was obtained from the patient
regarding the publication of the details of this case and
associated images.
References
|
1
|
Lenz HJ: Management and preparedness for
infusion and hypersensitivity reactions. Oncologist. 12:601–609.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Enokibori M, Kuge M and Mori K:
Anaphylactoid reaction to maltose 5% solution during spinal
anaesthesia. Can J Anaesth. 45:52–55. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamaguchi K, Watanabe T, Satoh T, Ishiguro
M, Izawa M, Inoshiri S, Sugihara K and Sakata Y: Severe infusion
reactions to cetuximab occur within 1 h in patients with metastatic
colorectal cancer: Results of a nationwide, multicenter,
prospective registry study of 2126 patients in Japan. Jpn J Clin
Oncol. 44:541–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Commins SP, James HR, Kelly LA, Pochan SL,
Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark
E, et al: The relevance of tick bites to the production of IgE
antibodies to the mammalian oligosaccharide
galactose-α-1,3-galactose. J Allergy Clin Immunol. 127:1286–93.e6.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung CH, Mirakhur B, Chan E, Le QT,
Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, et
al: Cetuximab-induced anaphylaxis and IgE specific for
galactose-alpha-1,3-galactose. N Engl J Med. 358:1109–1117. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Commins SP and Platts-Mills TA:
Anaphylaxis syndromes related to a new mammalian cross-reactive
carbohydrate determinant. J Allergy Clin Immunol. 124:652–657.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Commins SP, Satinover SM, Hosen J, Mozena
J, Borish L, Lewis BD, Woodfolk JA and Platts-Mills TA: Delayed
anaphylaxis, angioedema, or urticaria after consumption of red meat
in patients with IgE antibodies specific for
galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 123:426–433.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chinuki Y, Takahashi H and Morita E:
Clinical and biochemical evaluation of twenty patients with red
meat allergies. Jpn J Dermatol. 123:1807–1814. 2013.
|
|
10
|
Hopps S, Medina P, Pant S, Webb R, Moorman
M and Borders E: Cetuximab hypersensitivity infusion reactions:
Incidence and risk factors. J Oncol Pharm Pract. 19:222–227. 2013.
View Article : Google Scholar : PubMed/NCBI
|