Introduction
Solitary pulmonary caseating granulomas (SPCGs) are
benign lesions that occur as mycobacterial pulmonary nodules (MPN)
in the majority of cases (1);
however, they have also been reported in some extremely rare cases
of infection caused by fungi (2),
Mycoplasma spp. (3), or
Leishmania spp. (4). Among
MPNs, tuberculomas caused by Mycobacterium tuberculosis
(M. tuberculosis) account for 5–24% of all benign solitary
pulmonary nodules (SPNs). Furthermore, 77–85% of all tuberculomas
manifest as SPNs, with ≥2 accompanying nodules or satellite lesions
in 15–22% of the cases. These nodules most frequently occur in the
upper lobes of the lungs. Clinically, 77% of the cases are
asymptomatic and are discovered incidentally during health
examinations (5,6). Furthermore, it is extremely difficult
to differentiate such nodules from lung cancer using computed
tomography or the standardized uptake value (SUV) on positron
emission tomography (PET) (7,8).
Currently, the only practicable method for the detection of PCGs is
histopathological examination of specimens excised via
video-assisted thoracoscopic surgery (VATS) or other surgical
methods (9).
The post-VATS treatment of SPCG varies by case.
Anti-tuberculosis drug treatment for ~6 months is currently the
standard therapy for tuberculomas. However, this therapy is
associated with a high frequency of drug-induced hepatotoxicity
(DIH), with the subsequent requirement for hyposensitization
therapy (10). Furthermore, a
diagnosis of PCG by histopathological examination does not
necessarily indicate the presence of a tuberculoma: It may also
represent a non-tuberculous mycobacterial pulmonary nodule (NTMPN).
The Mycobacterium avium (M. avium) complex accounts for the
largest percentage of NTMPN cases, which reportedly occur more
frequently in older age groups compared with tuberculomas (11,12),
although the precise age distribution is unknown. Furthermore,
cases of PCG and concurrent lung cancer have also been reported
(6,13); therefore, a meticulous pathological
examination of excised lesion tissue is required.
The aim of the present study was to perform a
retrospective analysis of SPN cases histopathologically diagnosed
as PCGs following VATS at the Tokyo Medical University Ibaraki
Medical Center (Inashiki, Japan), in order to determine the
clinical characteristics of patients with PCGs in terms of
diagnosis (M. tuberculosis, NTM and others), presence of
lung cancer and treatment status (clinical response and
recurrence).
Patients and methods
Patients
The present retrospective study included a review
and analysis of data from 17 patients aged ≥18 years who presented
with SPNs that were histopathologically diagnosed as PCGs following
VATS at the Tokyo Medical University Ibaraki Medical Center between
2011 and 2015. All the patients were negative for human
immunodeficiency virus on serological tests, and had no history of
anti-tuberculosis drug treatment. The Tokyo Medical University
Ibaraki Medical Center is a teaching hospital with ~400 beds, which
is located in the southern part of Ibaraki Prefecture and serves a
population of ~400,000. The study was conducted with the approval
of the Tokyo Medical University Ibaraki Medical Center Ethics
Committee (approval no. 15–33). Individual informed consent was not
required given the retrospective nature of the study.
All 17 patients were assessed in terms of the
following parameters: Age; gender; diagnostic modality; clinical
manifestations; underlying disease; location, size and number of
nodules; microbial isolates; treatment status; clinical course; SUV
on PET; history of undergoing an interferon-γ release assay (IGRA),
namely the T-spot tuberculosis (TB) test; and histopathological
analysis, including simultaneous occurrence of a cancer lesion.
Statistical considerations
All statistical analyses were performed with
StatMate IV software (ATMS Co., Ltd., Tokyo, Japan). A descriptive
analysis was performed for demographic and clinical
characteristics, and results are presented as the mean ± standard
deviation (SD) for quantitative variables and numbers (percentages)
for qualitative variables. For comparisons, the statistical
significance of differences between tuberculoma, NTMPN and
concurrent lung cancer groups was measured by one-way analysis of
variance. All P-values were two-sided and P<0.05 was considered
to indicate statistically significant differences.
Results
Clinical background of the
patients
The study population consisted of 10 men and 7
women, ranging in age from 29 to 79 years (mean, 59.1±14.4 years)
(Table I). The pulmonary nodules of
8 of the patients were discovered incidentally during a physical
examination. In 6 patients, the nodules were discovered during
follow-up for underlying diseases. No significant respiratory
symptoms were observed upon diagnosis in 14 patients (82.4%),
whereas 3 patients (17.6%) had overt symptoms (including bloody
sputum in 1 patient, and cough and fever in 2 patients). Underlying
diseases were present in 8 patients, including chronic renal
failure (n=2), diabetes mellitus (n=2), hypertension (n=1), chronic
obstructive pulmonary disease (n=2), sick sinus syndrome (n=1), and
postoperative lung cancer (under follow-up; n=1). Of the 17
patients, 6 had tuberculomas, including 1 male and 5 female
patients, and the remaining 11 patients (9 men and 2 women,
including the 3 patients with concurrent lung cancer) had
NTMPNs.
 | Table I.Background characteristics of the 17
patients included in the study. |
Table I.
Background characteristics of the 17
patients included in the study.
|
|
|
|
|
| Nodule |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Case no. | Gender | Age, years | Underlying
disease | Discovery and
symptoms | Number | Location | Size, mm | Historical
culture | T-spot TB test | PET SUV | Final diagnosis | Treatment |
|---|
| 1 | M | 51 |
| Health check | Solitary | RS2 | 15 | M. avium | NP | NP | NTMPN | Observation |
| 2 | M | 63 | Post-lung cancer | Follow-up check | Solitary | RS2 | 15 | (−) | NP | NP | Cancer + NTMPN | Chemotherapy |
| 3 | M | 70 | CRF DM | Follow-up check | 2 | LS6S9 | 20 | (−) | NP | NP | NTMPN | Observation |
| 4 | M | 56 | CKD | Follow-up
check | Solitary | RS6 | 10 | M. spp | NP | NP | NTMPN | Observation |
| 5 | F | 70 |
| Health check | Solitary | RS2 | 15 | M.
kansasii | NP | NP | Cancer + NTMPN | Chemotherapy |
| 6 | F | 57 |
| Health check | Solitary | LS6 | 8 | (−) | (+) | NP | Tuberculoma | INH, RFP, EB,
PZA |
| 7 | F | 31 |
| Health check | Solitary | LS10 | 23 | M.
tuberculosis | (+) | 9.2 | Tuberculoma | INH, RFP, EB,
PZA |
| 8 | M | 58 |
| Health check | Solitary | LS1+2 | 20 | M.
kansasii | NP | 2.1 | NTMPN | Observation |
| 9 | M | 79 | DM, CRF | Hemoptysis | 2 | RS4/5 | 27 | M.
avium | (−) | 12.5 | Cancer + NTMPN | Palliative
medicine |
| 10 | F | 38 |
| Health check | Solitary | LS6 | 8 | (−) | (±) | 4.8 | Tuberculoma | INH, RFP, EB,
PZA |
| 11 | M | 74 | COPD, HT | Health check | Solitary | LS3 | 13 | (−) | (−) | 7.3 | NTMPN | Observation |
| 12 | M | 61 |
| Health check | Solitary | RS2 | 15 | (−) | (+) | 12 | Tuberculoma | INH, RFP, EB,
PZA |
| 13 | F | 74 | CHF, SSS | Follow-up
check | Solitary | RS3 | 20 | M.
tuberculosis | (+) | NP | Tuberculoma | INH, RFP, EB,
PZA |
| 14 | F | 55 |
| Health check | Solitary | Hilar | 20 | (−) | (−) | NP | NTMPN | Observation |
| 15 | M | 66 |
| Pneumonia | Solitary | LS6 | 17 | M.
kansasii | (−) | 7.3 | NTMPN | Observation |
| 16 | F | 32 |
| Bronchitis | Solitary | RS2 | 15 | (−) | (+) | NP | Tuberculoma | INH, RFP, EB,
PZA |
| 17 | M | 70 | COPD | Follow-up
check | Solitary | LS3 | 15 | M.
kansasii | (−) | NP | NTMPN | Observation |
Characteristics of SPNs
The main locations of the nodules were the right
upper lobe (n=6) and the left lower lobe (n=4). In all patients
with nodules in the left lower lobe, they were located in the S6
segment. The nodules were located in sites with a propensity for
tuberculosis in 12 patients (70.6%). The nodules were solitary in
15 patients, and 2 patients had SPCGs accompanied by a small
satellite nodule. The occurrence of a small lesion was suspected
upon re-examination in these 2 patients, whereas an infiltrative
shadow was present in 1 patient, and 1 patient presented with a
hilar nodule. The mean ± SD diameter of the nodules was 16.2±5.1 mm
(range, 8–27 mm). PET scans were performed in 7 patients, with the
mean ± SD SUV of the nodules measured as 7.9±3.7 (range, 2.1–12.0).
The mean SUV was 8.7±3.6 in the 3 patients from whom M.
tuberculosis was isolated, and 5.6±3.0 in the 3 patients from
whom NTMs were isolated (excluding the patients with concurrent
lung cancer) (Table II).
 | Table II.Comparison of cases by type. |
Table II.
Comparison of cases by type.
| Variables | Tuberculoma | Lung cancer +
NTMPN | NTMPN | Total |
|---|
| No. of cases | 6 | 8 | 3 | 17 |
| Gender, no.
male/female | 1/5 | 7/1 | 2/1 | 10/7 |
| Age, years; mean ±
SD | 48.8±17.7 | 62.5±8.51 |
70.7±8.0a | 59.1±14.4 |
| Size, mm; mean ±
SD | 13.8±7.7 | 16.3±3.7 | 19.0±6.9 | 16.1±5.4 |
| PET SUV; mean ± SD
(n) | 8.7±3.6 (3) | 5.6±3.0 (3) | 12.0 (1) | 7.9±3.7 (7) |
| Isolation | 2 | 5 | 2 | 9 |
| No. positive T-spot
TB/tests | 6/6 | 0/4 | 0/1 | 6/11 |
| performed |
| No. with/without
underlying disease | 1/5 | 4/4 | 2/1 | 7/10 |
Bacterial culture test and IGRA
(T-spot TB test)
Mycobacteria were isolated by culture in 9 patients
(52.9%), including M. tuberculosis in 2 patients (11.8%),
M. avium in 2 patients (11.8%), Mycobacterium
kansasii (M. kansasii) in 4 patients (23.5%), and other
NTM (unidentifiable as the 12 tested Mycobacterium spp.) in
1 patient (5.9%). IGRA was performed in 11 patients, of whom 5
demonstrated positive test results, and 1 result was indeterminate.
IGRA was positive in the 2 patients with culture-proven M.
tuberculosis and among the 3 patients in whom no mycobacteria
could be isolated. The result of the IGRA was indeterminate for 1
patient without mycobacteria, and negative for 1 patient with M.
avium infection and for 2 patients with M. kansasii
infection.
Histopathological findings and final
diagnosis
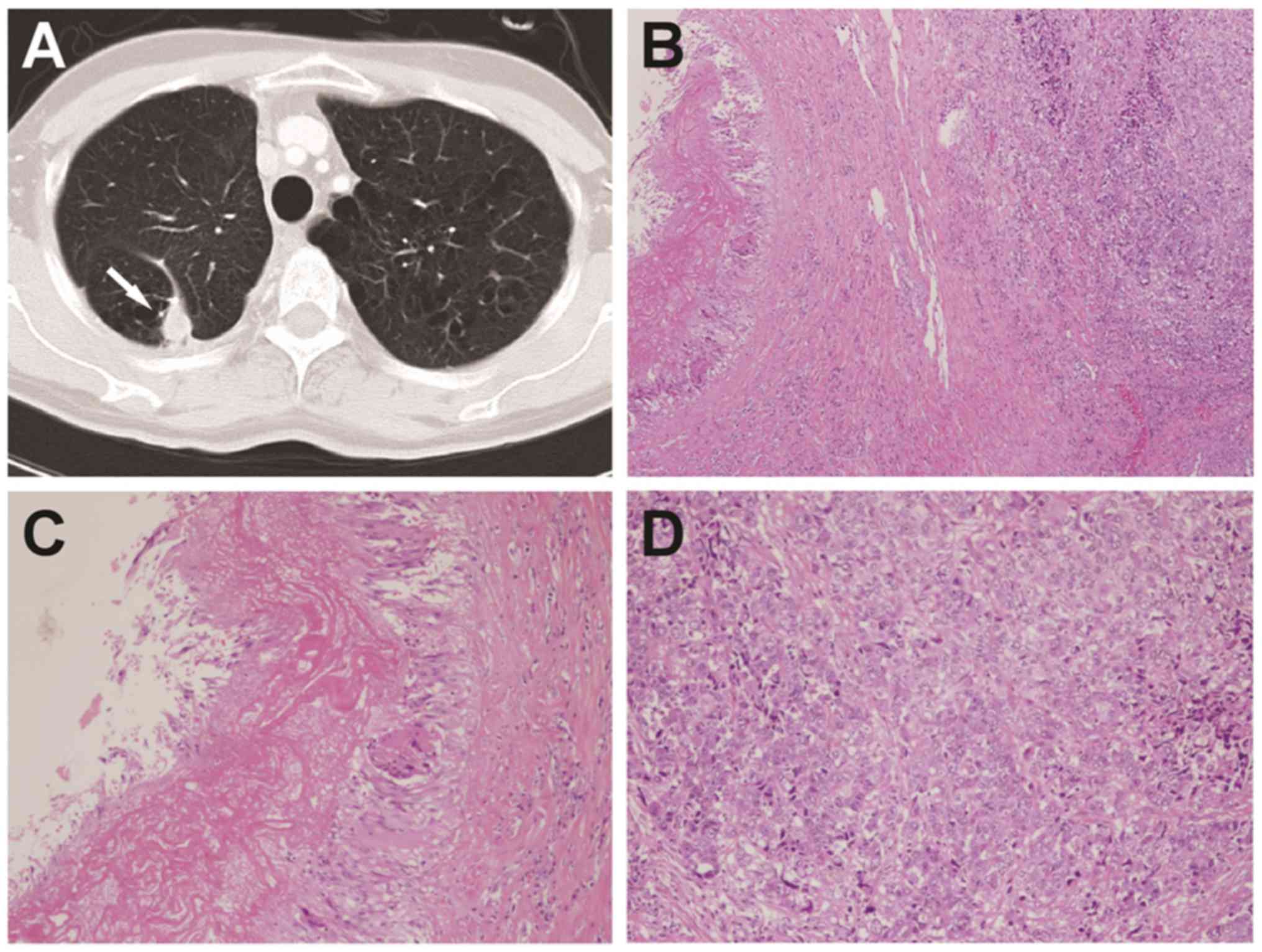
In all 17 patients enrolled, the PCGs were diagnosed
histopathologically. The final diagnosis was tuberculoma in 6
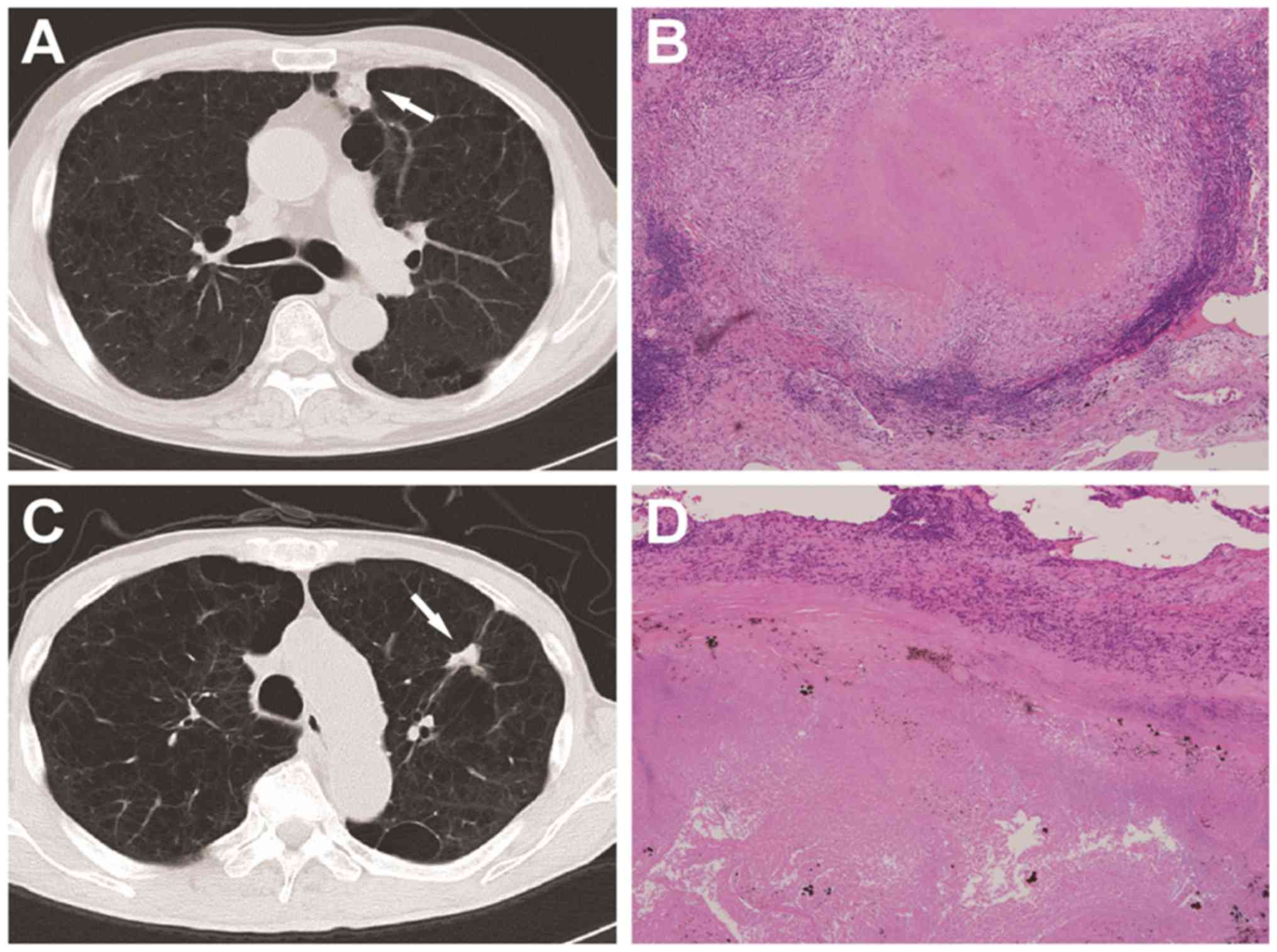
patients (35.3%) and NTMPN in 11 patients (64.7%) (Fig. 1). Lung cancer was also present in 3
patients (17.6%); morphologically, the major lesion in all 3 cases
was adenocarcinoma of the lung, and the mycobacterial nodules were
located in the vicinity of the major malignant lesion (Fig. 2). NTMs were isolated from all 3
patients with lung cancer, including M. avium in 1 patient,
M. kansasii in 1 patient, and an unknown species in 1
patient.
Treatment status
A total of 6 patients with tuberculoma received
standard anti-tuberculosis drug therapy for 6 months. For 1 of
these patients, the overall treatment period was extended to 12
months, as the patient developed DIH and required hyposensitization
therapy, which required the anti-tuberculosis drugs to be increased
gradually from a low initial dose. In all 3 patients with
concurrent lung cancer, including 2 patients with T1aN0M0 stage Ia
cancer and 1 with stage IV cancer, the nodules were
non-tuberculous. The 2 patients with stage Ia cancer were treated
by complete resection and followed up, while the patient with stage
IV disease (with distant metastasis) only received palliative care
at his own request. Of the 14 patients (6 with tuberculoma and 8
with NTMPN) without concurrent lung cancer, the follow-up period
after treatment was >3 years in 6 patients, 2 years in 4
patients, and 1 year in 4 patients. None of the patients exhibited
any evidence of relapse during follow-up.
Discussion
Tuberculomas reportedly account for 5–24% of all
cases of SPN and are generally recognized as benign lesions
(14). In an estimated 77.3% of the
cases, tuberculomas are asymptomatic and are discovered
incidentally during routine health examinations. It has been
reported that tuberculomas manifest as SPNs in 77–85% of the cases,
and that ≥2 nodules or satellite lesions are present in 15–22% of
the cases. The nodules predominantly occur in the upper lobes of
the lungs and have a mean diameter of 2–5 cm (5,6). It has
also been reported that the likelihood of malignancy of an SPN
increases with the increasing size of the nodule (15). In the present case series, the mean
diameter of the nodules was 17.1±4.9 mm; the estimated incidence of
malignancy in nodules of this size range is 33–64% (15). However, lung cancer (including
metastatic cancer) was suspected in all the present cases upon
imaging diagnosis prior to VATS. PET has been reported to be useful
for the diagnosis of malignancy in patients presenting with an SPN
(15,16). However, in the present cases, the
mean SUV was 7.9±3.7 in the patients that were examined by PET (7
of 17). Therefore, in all cases, the maximum SUV exceeded the
reported SUV cutoff value of ~2.5 (17) for differentiating malignancy among
nodular lesions. However, it is extremely difficult to
differentiate between PCGs and lung cancer based on SUV alone
(10,16). In the 3 patients with NTMPNs and
concurrent lung cancer, the mean SUV was 5.6±3.0, which was not
sufficient for the diagnostic differentiation of malignancy. No
significant difference in the SUV was observed between tuberculomas
and NTMPNs (8.7±3.6 vs. 5.6±3.0, respectively). It may, therefore,
be hypothesized that there is no difference in SUV between
tuberculomas and NTMPNs, which both consist of local inflammatory
reactions that produce nodular lesions. Generally, NTMs are more
commonly encountered in postmenopausal women, and a number of
theories have been postulated to explain this association,
including low physical respiratory clearance (18), postmenopausal functional depression
of macrophages (19), depressed
villous movement (20) and a
decrease in airway-epithelial ciliary movement caused by
progesterone (21). According to
previous reports, tuberculomas are more likely to occur in men
compared with women (5,6). Additionally, in the present study,
patients with NTMPNs were older than those with tuberculomas, which
is consistent with a previous report (11). Regarding gender, patients with NTMPNs
comprised more men than women (Table
II). These results are of considerable interest as they may
suggest that the mechanism underlying nodular lesion formation
differs from the mechanism of mycobacterial settlement in the case
of NTMs. Further investigation, with a larger number of cases, is
required.
In the present case series, the major lesion in all
3 patients (17.6%) with concurrent lung cancer was pulmonary
adenocarcinoma. Regarding the association between lung cancer and
mycobacteria, various theories have been postulated, such as the
post-mycobacteriosis-healing cicatrization theory and the theory of
mycobacterial activation by cancer (22). More recently, latent tuberculosis has
been associated with the risk of developing lung cancer (23). In the present study, SPCGs were
detected in the vicinity of the lung cancer in all 3 cases. The
mean age of the patients with concurrent lung cancer was 70.7±8.0
years, which was significantly higher compared with that of the
patients diagnosed with tuberculoma (48.8±17.7 years) (P<0.05).
Our findings are highly suggestive of a potential association
between lung cancer and latent mycobacterial infection. However, it
is also possible that a breakdown of the local immune balance
around the cancer lesion facilitated the emergence of the
mycobacterial infection.
Mycobacterial species were identified in only 9 of
the 17 patients (52.9%) by detection/isolation from the excised
tissues. In the remaining cases, no mycobacterial species were
identified. The T-spot TB test, which is widely used for the
diagnosis of tuberculosis, was employed in addition to the
bacteriological diagnosis. The IGRA test is comparable with the
QuantiFERON TB test regarding assay sensitivity and specificity,
and it is also procedurally simpler (24,25). The
T-spot TB test was performed in 11 of the 17 patients in the
present study. Of the 6 patients undergoing this test in whom
mycobacteria could not be identified, 2 exhibited negative test
results, while the test was positive in the remaining 4 patients,
who were then finally diagnosed with tuberculoma. It has been
reported that the T-spot TB test may also yield positive results in
patients with infections caused by other mycobacteria, including
M. kansasii, M. szulgai, M. marinum and M. gordonae
(26). In the present study,
however, the T-spot TB test was negative in all patients from whom
NTM species, including M. kansasii, were isolated. Although
it remains unclear why the T-spot TB test was negative in patients
with M. kansasii infection, this test was considered useful
for differentiating between tuberculoma and NTMPNs in patients with
SPCGs identified by histopathology when no mycobacteria could be
detected or isolated. Based on the present findings, it was
concluded that no etiological factors for SPCGs other than
mycobacteria were present in the current case series.
Standard anti-tuberculosis therapy is administered
for the treatment of tuberculomas (5,6,27). All 6 patients diagnosed with
tuberculoma in the present study received anti-tuberculosis
therapy. It has been reported that an estimated 15% of patients
with tuberculoma who receive anti-tuberculosis treatment develop
DIH (9). In the present study, 1 of
the patients who received anti-tuberculosis treatment developed
DIH, and hyposensitization therapy was undertaken, which proved
efficacious. Regarding the management of NTMPNs, a previous study
documented the case of a patient with an SPN caused by M.
kansasii, who was followed up without anti-tuberculosis therapy
following VATS and remained free from recurrence (28). None of the 8 patients with NTMPNs in
the present study (excluding the 3 patients with NTMPNs associated
with concurrent lung cancer) received anti-tuberculosis therapy,
and no relapse was detected in any of these patients during the
course of follow-up for >1 year. These results indicate that
careful periodic follow-up may be sufficient for the management of
NTMPNs, provided that the lesions are solitary.
The limitations of this study include a disparity in
the process with which VATS was performed, the single-center design
of the study and the limited number of cases, which did not allow
for adequate assessment of SPCG. Accordingly, accumulation of data
from multiple institutions, using pathological and microbiological
analysis from VATS, is required for the study of SPCG.
In summary, a clinical evaluation was conducted for
17 patients with pulmonary nodules in whom SPCGs were
histopathologically identified. Mycobacteria were identified
following VATS in 9 of the patients (52.9%). Finally, 6 patients
were diagnosed with tuberculoma, 8 patients were diagnosed with
NTMPNs and 3 patients were diagnosed with NTMPNs with concurrent
lung cancer. Thus, NTMPNs accounted for >60% of the patients
with SPCGs, which also occurred more frequently in men. Lung cancer
was suspected in all patients prior to VATS, and PET proved
ineffective for diagnostic differentiation. Although, these results
suggested that close periodic observation was sufficient for
patients in whom SPCG from NTM (NTMPN) was identified by VATS, they
also emphasize the necessity for aggressive VATS to obtain an
accurate diagnosis. Furthermore, even if the central region of a
solitary nodule is a malignancy, a caseous necrotic lesion may
exist in the peripheral area. Therefore, it is necessary to examine
the isolated tissue carefully.
References
|
1
|
Asakura T, Ishii M, Haraguchi M, Kamiyama
I, Kohno M, Sakamaki H, Emoto K, Hayashi Y, Sugiura H, Kawada I, et
al: Dry pleurisy complicating solitary pulmonary nodules caused by
Mycobacterium avium: A case report. J Med Case Rep. 9:2382015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scott RS, Sutton DA and Jagirdar J: Lung
infection due to opportunistic fungus, Phialemonium obovatum, in a
bone marrow transplant recipient: An emerging infection with
fungemia and Crohn disease-like involvement of the gastrointestinal
tract. Ann Diagn Pathol. 9:227–230. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Linz D, Kessler S and Kane J: Pulmonary
caseating granulomas associated with Mycoplasma pneumoniae
pneumonia. South Med J. 76:1429–1432. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aoun J, Habib R, Charaffeddine K, Taraif
S, Loya A and Khalifeh I: Caseating granulomas in cutaneous
leishmaniasis. PLoS Negl Trop Dis. 8:e32552014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee HS, Oh JY, Lee JH, Yoo CG, Lee CT, Kim
YW, Han SK, Shim YS and Yim JJ: Response of pulmonary tuberculomas
to anti-tuberculous treatment. Eur Respir J. 23:452–455. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsu KY, Lee HC, Ou CC and Luh SP: Value of
video-assisted thoracoscopic surgery in the diagnosis and treatment
of pulmonary tuberculoma: 53 cases analysis and review of
literature. J Zhejiang Univ Sci B. 10:375–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chun EJ, Lee HJ, Kang WJ, Kim KG, Goo JM,
Park CM and Lee CH: Differentiation between malignancy and
inflammation in pulmonary ground-glass nodules: The feasibility of
integrated (18)F-FDG PET/CT. Lung Cancer. 65:180–186. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawate E, Yamazaki M, Kohno T, Fujimori S
and Takahashi H: Two cases with solitary pulmonary nodule due to
non-tuberculous mycobacterial infection showing intense
18F-fluorodeoxyglucose uptake on positron emission tomography scan.
Geriatr Gerontol Int. 10:251–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen S, Zhou J, Zhang J, Hu H, Luo X,
Zhang Y and Chen H: Video-assisted thoracoscopic solitary pulmonary
nodule resection after CT-guided hookwire localization: 43 cases
report and literature review. Surg Endosc. 25:1723–1729. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watanabe H, Uruma T, Seita I, Chikasawa Y,
Kikuchi R, Itoh M, Aoshiba K, Nakamura H and Oishi T: Successful
desensitization therapy involving fluoroquinolone for the treatment
of a solitary tuberculoma: A case report and literature review. Mol
Clin Oncol. 5:117–120. 2016.PubMed/NCBI
|
|
11
|
Lim J, Lyu J, Choi CM, Oh YM, Lee SD, Kim
WS, Kim DS, Lee H and Shim TS: Non-tuberculous mycobacterial
diseases presenting as solitary pulmonary nodules. Int J Tuberc
Lung Dis. 14:1635–1640. 2010.PubMed/NCBI
|
|
12
|
Hahm CR, Park HY, Jeon K, Um SW, Suh GY,
Chung MP, Kim H, Kwon OJ and Koh WJ: Solitary pulmonary nodules
caused by Mycobacterium tuberculosis and Mycobacterium avium
complex. Lung. 188:25–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ashizawa K, Matsuyama N, Okimoto T,
Hayashi H, Takahashi T, Oka T, Nagayasu T and Hayashi K:
Coexistence of lung cancer and tuberculoma in the same lesion:
Demonstration by high resolution and contrast-enhanced dynamic CT.
Br J Radiol. 77:959–962. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andreu J, Cáceres J, Pallisa E and
Martinez-Rodriguez M: Radiological manifestations of pulmonary
tuberculosis. Eur J Radiol. 51:139–149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhan P, Xie H, Xu C, Hao K, Hou Z and Song
Y: Management strategy of solitary pulmonary nodules. J Thorac Dis.
5:824–829. 2013.PubMed/NCBI
|
|
16
|
Sathekge MM, Maes A, Pottel H, Stoltz A
and van de Wiele C: Dual time-point FDG PET-CT for differentiating
benign from malignant solitary pulmonary nodules in a TB endemic
area. S Afr Med J. 100:598–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Opoka L, Kunikowska J, Podgajny Z,
Szołkowska M, Błasińska-Przerwa K, Burakowska B, Oniszh K,
Rudziński P, Bestry I and Roszkowski-Śliż K: Accuracy of FDG PET/CT
in the evaluation of solitary pulmonary lesions-own experience.
Pneumonol Alergol Pol. 82:198–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chalermskulrat W, Gilbey JG and Donohue
JF: Nontuberculous mycobacteria in women, young and old. Clin Chest
Med. 23:675–686. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miller L and Hunt JS: Sex steroid hormones
and macrophage function. Life Sci. 59:1–14. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Comer MT, Leese HJ and Southgate J:
Induction of a differentiated ciliated cell phenotype in primary
cultures of Fallopian tube epithelium. Hum Reprod. 13:3114–3120.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jain R, Ray JM, Pan JH and Brody SL: Sex
hormone-dependent regulation of cilia beat frequency in airway
epithelium. Am J Respir Cell Mol Biol. 46:446–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim YI, Goo JM, Kim HY, Song JW and Im JG:
Coexisting bronchogenic carcinoma and pulmonary tuberculosis in the
same lobe: Radiologic findings and clinical significance. Korean J
Radiol. 2:138–144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su VY, Yen YF, Pan SW, Chuang PH, Feng JY,
Chou KT, Chen YM, Chen TJ and Su WJ: Latent tuberculosis infection
and the risk of subsequent cancer. Medicine (Baltimore).
95:e23522016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Higuchi K, Sekiya Y, Igari H, Watanabe A
and Harada N: Comparison of specificities between two
interferon-gamma release assays in Japan. Int J Tuberc Lung Dis.
16:1190–1192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mancuso JD, Mazurek GH, Tribble D, Olsen
C, Aronson NE, Geiter L, Goodwin D and Keep LW: Discordance among
commercially available diagnostics for latent tuberculosis
infection. Am J Respir Crit Care Med. 185:427–434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuznetcova TI, Sauty A and Herbort CP:
Uveitis with occult choroiditis due to Mycobacterium kansasii:
Limitations of interferon-gamma release assay (IGRA) tests (case
report and mini-review on ocular non-tuberculous mycobacteria and
IGRA cross-reactivity). Int Ophthalmol. 32:499–506. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Laisaar T, Viiklepp P and Hollo V:
Long-term follow-up after thoracoscopic resection of solitary
pulmonary tuberculoma. Indian J Tuberc. 61:51–56. 2014.PubMed/NCBI
|
|
28
|
Abe M, Kobashi Y, Mouri K, Obase Y,
Miyashita N, Nakata M and Oka M: Solitary pulmonary nodule due to
Mycobacterium kansasii. Intern Med. 50:775–778. 2011. View Article : Google Scholar : PubMed/NCBI
|