Introduction
In patients with cancer, a systemic inflammatory
response leads to increased protein breakdown and progressive
nutritional decline by a direct catabolic effect on skeletal muscle
and other host tissue (1–4). Such progressive nutritional decline
leads to poorer survival (1–4). The measurement of the systemic
inflammatory response has been subsequently refined using a
selective combination of serum albumin and C-reactive protein (CRP)
(termed the modified Glasgow Prognostic Score, mGPS) and has been
revealed to have prognostic value, independent of tumor stage, in
non-small cell lung, gastrointestinal and renal cancer (5,6).
Lung cancer remains one of the most common and fatal
malignant diseases. The overall survival in patients with lung
cancer, particularly those with small cell lung cancer (SCLC),
remains poor and has not improved to a satisfactory level, despite
the progress made in various therapeutic modalities (7). The treatment for patients with SCLC
with a systemic inflammatory response may be complex due to a high
level of post-therapeutic pulmonary complications and mortality.
However, the clinic pathological features of patients with SCLC
with a systemic inflammatory response have not been clarified, and
the influence of the existence of the systemic inflammatory
response on survival in these patients has rarely been examined
(8). In the present study, the
prognostic significance of coexistent systemic inflammatory
response has been examined in patients with SCLC.
Patients and methods
All the patients with pathologically or
cytologically proven SCLC who were admitted to Tsukuba University
Hospital, Tsukuba Medical Center Hospital ((Tsukuba, Japan) and
Mito Medical Center, University of Tsukuba (Mito, Japan) between
April 1999 and July 2016 were analyzed retrospectively. Patients
with SCLC were divided into two groups according to the staging
system of the Veterans Administration Lung Cancer Study Groups:
Limited stage (LD) or extensive stage (ED). Patients with LD-SCLC
have involvement of the ipsilateral hemithorax within a single
radiation port. ED-SCLC is defined as the presence of apparent
metastatic disease. This classification has an important role in
the indication of treatment and outcome. The present study was
approved by the institutional review committee of Mito Medical
Center, University of Tsukuba (Mito, Japan) (no. 16–20). Informed
consent was obtained from all the patients for use of their
data.
Serum albumin and CRP were those measured at the
time of the diagnosis of SCLC. The mGPS was calculated according to
the method by Forrest et al (9): Patients with a normal albumin level
(3.5 g/dl) and CRP (1.0 mg/dl) were allocated a score of 0, those
with both low albumin (<3.5 g/dl) and high CRP (>1.0 mg/dl)
were scored 2. Patients with only an elevated CRP (>1.0 mg/dl)
were assigned a score of 1.
The Mann-Whitney U test and the χ2 test
were used to determine statistically significant differences
between the two groups. To assess survival curves, the Kaplan-Meier
method was used. To evaluate the statistical significance of
differences, the log-rank test was used. The length of survival was
defined as the interval in months from the date of the initial
therapy or supportive care until the date of last follow-up or the
date of mortality. For multivariate analysis of the effect of
clinicopathological factors on survival, the Cox proportional
hazards model was used. All statistical analysis was conducted
using SPSS 10.1 for Windows (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
During the study period, 319 patients were diagnosed
pathologically or cytologically as having SCLC. Table I presents the characteristics of
these patients. They were 273 (85.6%) males and 46 females. The
median patient age was 71 (range, 49–94) years. In total, 109
(34.1%) patients were ≥75 years old. There were 192 (78.3%)
patients with good performance status (PS) (Eastern Cooperative
Oncology Group 0–1) (10), and 103
(32.3%) patients with LD-SCLC. Among all the patients, 97 (30.4%)
had serum albumin <3.5 g/dl, and 127 (39.8%) of them had serum
C-reactive protein (CRP) >1.0 mg/dl. In total, 192 (60.2%)
patients had mGPS=0, 54 (16.9%) had mGPS=1 and 73 (22.9%) had
mGPS=2. Table II presents the
association between mGPS and various patient characteristics. The
results indicated that aged patients (≥75 years), those with poor
PS (PS, 2–4), extensive disease and those receiving supportive care
only had a higher mGPS. For male patients, smokers did not have a
higher mGPS.
 | Table I.Characteristics of 319 patients with
lung cancer. |
Table I.
Characteristics of 319 patients with
lung cancer.
| Variables | N (%) |
|---|
| Age (years) | Median, 71 |
|
| Range, 49–94 |
| ≥75 | 109 (34.1) |
| Sex |
|
| Male | 273 (85.6) |
|
Female | 46 (14.4) |
| Smoking habit |
|
| Ex- or
current smoker | 304 (95.3) |
| Never
smoker | 15 (4.7) |
| Performance
status |
|
| 0–2 | 192 (78.3) |
| 3–4 | 127 (21.7) |
| Clinical stage |
|
| Limited
disease | 103 (32.3) |
| Extensive
disease | 216 (67.7) |
| Initial
treatment |
|
|
Chemotherapy | 276 (86.5) |
|
Supportive care | 43 (13.5) |
 | Table II.Patient characteristics and mGPS. |
Table II.
Patient characteristics and mGPS.
|
| mGPS score, N
(%) |
|---|
|
|
|
|---|
| Variable | 0 | 1 | 2 | P-value |
|---|
| No. of patients | 192 | 54 | 73 |
|
| Age (≥75 years) | 54 (28.1) | 19 (35.2) | 36 (49.3) | 0.005 |
| Sex (male) | 161 (83.9) | 49 (90.4) | 63 (86.3) | 0.741 |
| Smoking habit
(present) | 182 (94.8) | 52 (96.3) | 70 (95.9) | 0.866 |
| PS (2–4) | 56 (29.2) | 21 (38.9) | 50 (68.5) | 0.001 |
| Disease (extensive
disease) | 119 (62.5) | 35 (64.8) | 62 (84.9) | 0.002 |
| Treatment (supportive
care) | 18 (9.4) | 5 (9.3) | 20 (27.4) | 0.004 |
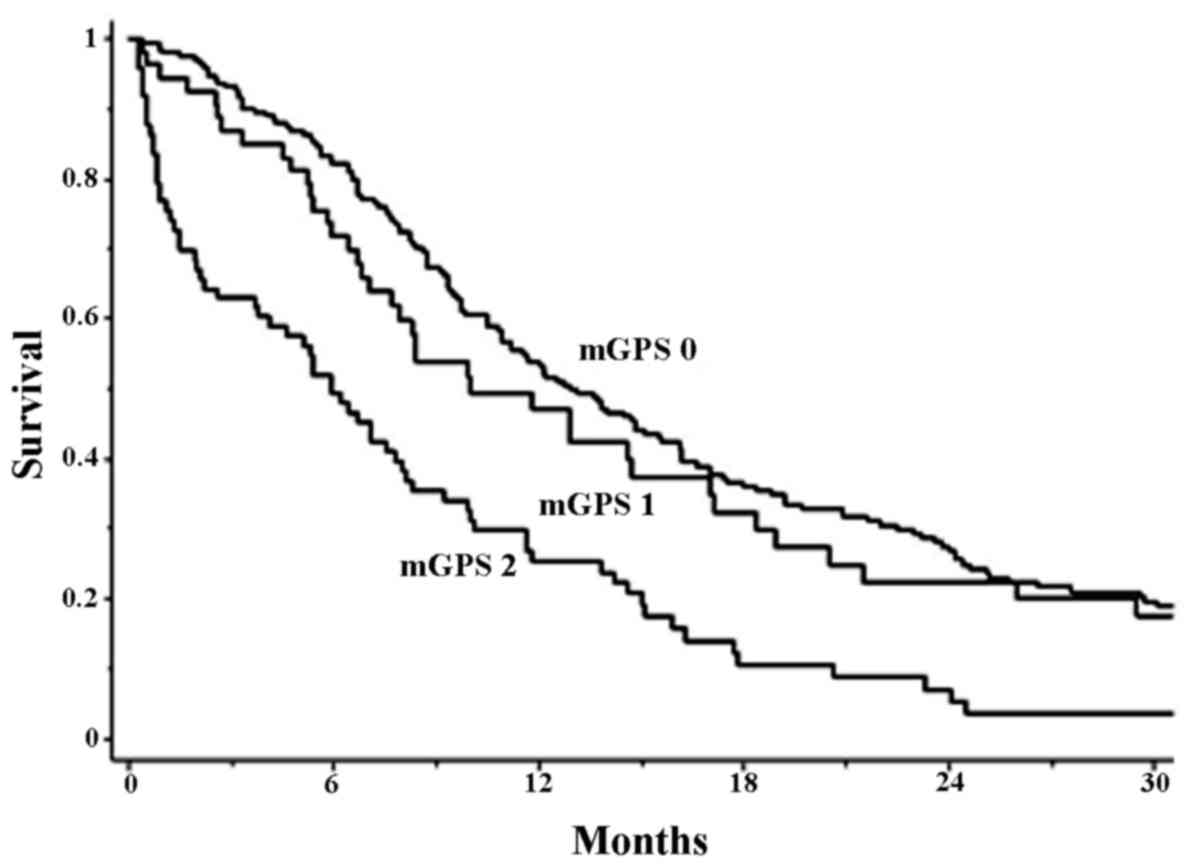
Fig. 1 reveals the
survival curves of the patients with mGPS=0, 1 and 2. The median
survival time of patients was 13.1, 10.0 and 5.9 month,
respectively. There was a statistically significant difference in
survival among these patient groups (P=0.0001). Table II presents the results of uni- and
multivariate analysis of prognostic factors in these patients. In
univariate analysis, aged 75 years old [median survival time (MST)
in patients ≥75 years, 8.7 months; MST in those ≤74 years, 12.7
months), poor PS (MST in patients with PS 2–4, 6.2 months; MST in
those with PS 0–1, 14.6 months), extensive disease (MST in patients
with ED, 8.0 months; MST in those with LD, 19.2 months), supportive
care only (MST in patients with supportive care only, 1.5 months;
MST in those with chemotherapy, 12.7 months) and higher mGPS (MST
in patients with mGPS=2, 5.9 months; MST in those with mGPS=1, 10.0
months; MST in those with mGPS=2, 13.1 months) were unfavorable
prognostic factors. In the multivariate analysis, extensive disease
and supportive care were unfavorable prognostic factors. In
addition, mGPS=2 was confirmed as an unfavorable prognostic factor
in the multivariate analysis (Table
III).
 | Table III.Univariate and multivariate analyses
of unfavorable prognostic factors in 319 patients with small cell
lung cancer. |
Table III.
Univariate and multivariate analyses
of unfavorable prognostic factors in 319 patients with small cell
lung cancer.
|
|
|
| Multivariate
analysisb |
|---|
|
|
|
|
|
|---|
| Factors | Univariate
analysisa MST | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age (>75 vs.
<75) | 8.7 vs. 12.7 | 0.001 | 1.25 | 0.95–1.63 | 0.111 |
| Sex (male vs.
female) | 10.5 vs. 13.8 | 0.346 |
|
|
|
| Smoking (present vs.
absent) | 10.8 vs. 12.5 | 0.571 |
|
|
|
| PS (2–4 vs. 0–1) | 6.2 vs. 14.6 | 0.001 | 1.34 | 0.99–1.81 | 0.055 |
| Disease extent (ED
vs. LD) | 8.0 vs. 19.2 | 0.001 | 2.43 | 1.82–3.24 | 0.001 |
| 1st-line Tx (SC vs.
chemo) | 1.5 vs. 12.7 | 0.001 | 5.95 | 3.76–9.11 | 0.001 |
| mGPS (2 vs. 1 vs.
0) | 5.9 vs. 13.1 | 0.001 |
|
|
|
| mGPS (mGPS=1) |
|
| 1.23 | 0.86–1.74 | 0.247 |
| mGPS (mGPS=2) |
|
| 2.04 | 1.51–2.78 | 0.001 |
Discussion
McMillan et al (5) proposed a prognostic score as a measure
of systemic inflammation and nutritional status determined by serum
albumin values and the CRP value (5). This prognostic score is now believed to
reflect the condition of cachexia in malignant disease. In various
cancer types such as gastrointestinal cancer, it is evaluated as an
independent prognostic factor (11,12). Its
clinical significance has been also evaluated in NSCLC (13,14), but
has scarcely evaluated in patients with SCLC (8). At present, there is only one previous
study that evaluates the clinical significance of mGPS as a
prognostic factor (8). Due to the
lack of data on CRP and albumin in this previous study, mGPS in
359/460 consecutive patients was evaluated. There were only 18
(5.0%) patients with PS 2, 11 (3.1%) patients with mGPS=2, and 20
(5.6%) patients with albumin <3.5 g/dl. These data revealed that
the proportion of patients with prognostic values, independent of
tumor stage, was too low to evaluate. In addition, median age of
the patients was 60 years. Therefore, the results from this
previous study may differ from those of a typical population of
patients with SCLC. The present study was performed to evaluate the
prognostic significance of the presence of a systemic inflammatory
response in patients with SCLC. The majority of patients with lung
cancer are aged and have comorbid wasting diseases such as chronic
obstructive pulmonary disease and chronic heart disease; therefore,
they potentially have a high risk of developing cachexia. For
patients with NSCLC, the clinical usefulness of anamorelin, an
anti-cachexia drug in global phase III and domestic phase II
studies in Japan have recently been reported (15,16). For
patients with SCLC, early intervention such as prescription of
anti-cachexia and nutritional support may improve prognosis.
In the present study, the prognostic significance of
a systemic inflammatory response was evaluated using mGPS in
unselected patients with SCLC. As has been reported in a previous
study (8), it was demonstrated that
male sex, extensive disease, poor PS and no chemotherapy were
unfavorable prognostic factors. In addition to these factors, the
present study revealed that systemic inflammatory response (mGPS=2)
was unfavorable prognostic factor. These results suggested that
patients with SCLC with a systemic inflammatory response may have a
poor prognosis.
Many clinical trials have not shown the outcome of
treatment of patients with SCLC patients with a systemic
inflammatory response, as these studies excluded patients with
impairment of organ function and active inflammation. Therefore,
there is scarce published information with regard to the results of
unfavorable prognostic factors in unselected patients with SCLC.
Therefore, the present study included those with systemic
inflammatory response, and revealed that a systemic inflammatory
response (mGPS=2) was unfavorable prognostic factor. The results
suggested that patients with SCLC with a systemic inflammatory
response may have a poor prognosis. Based on the results, it is
suggested that clinicians must take the patient's medical
condition, including systemic inflammatory response, into
consideration when deciding whether to offer a standard therapy
that may increase treatment-associated mortality.
Due to the design of clinical trials in which
eligibility criteria preclude the involvement of patients with
impairment of organ function, numerous published studies have not
revealed the outcome of treatment of patients with SCLC with a
systemic inflammatory response. As a result, there is little
published information regarding the results of treatment and
prognostic factors in unselected groups of patients with SCLC,
including those with SCLC and a systemic inflammatory response.
Therefore, the results of treatment and prognostic factors in
unselected patients with SCLC who were admitted to the three
hospitals were evaluated. In the present series of patients, female
sex, LD-SCLC and good PS were favorable prognostic factors for
SCLC, as has been reported in a previous study (8). Additionally, it was revealed that
patients with a systemic inflammatory response had poorer overall
survival than those without, and the existence of a systemic
inflammatory response was one of the unfavorable prognostic factors
for survival in patients with SCLC. It is very important to
understand why a systemic inflammatory response worsened the
prognosis of SCLC in these patients. Therefore, standard therapy or
adequate palliative care may be essential to provide prolonged
quality survival, which is the primary goal of therapy for patients
with SCLC with systemic inflammatory responses.
In conclusion, the prognostic significance of the
presence of a systemic inflammatory response, as determined using
mGPS, in SCLC was demonstrated in the current study. A well-planned
large sized prospective study is required to support these
results.
References
|
1
|
Fearon KC, Barber MD, Falconer JS,
McMillan DC, Ross JA and Preston T: Pancreatic cancer as a model:
Inflammatory mediators, acute-phase response, and cancer cachexia.
World J Surg. 23:584–588. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McMillan DC, Scott HR, Watson WS, Preston
T, Milroy R and McArdle CS: Longitudinal study of body cell mass
depletion and the inflammatory response in cancer patients. Nutr
Cancer. 31:101–105. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McMillan DC, Watson WS, O'Gorman P,
Preston T, Scott HR and McArdle CS: Albumin concentrations are
primarily determined by the body cell mass and the systemic
inflammatory response in cancer patients with weight loss. Nutr
Cancer. 39:210–213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meek CL, Wallace AM, Forrest LM and
McMillan DC: The relationship between the insulin-like growth
factor-1 axis, weight loss, an inflammation-based score and
survival in patients with inoperable non-small cell lung cancer.
Clin Nutr. 29:206–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McMillan DC: An inflammation-based
prognostic score and its role in the nutrition-based management of
patients with cancer. Proc Nutr Soc. 67:pp. 257–262. 2008;
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McMillan DC: Systemic inflammation,
nutritional status and survival in patients with cancer. Curr Opin
Clin Nutr Metab Care. 12:223–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dowell JE: Small cell lung cancer: Are we
making progress? Am J Med Sci. 339:68–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou T, Hong S, Hu Z, Hou X, Huang Y, Zhao
H, Liang W, Zhao Y, Fang W, Wu X, et al: A systemic
inflammation-based prognostic scores (mGPS) predicts overall
survival of patients with small-cell lung cancer. Tumour Biol.
36:337–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Evaluation of cumulative prognostic
scores based on the systemic inflammatory response in patients with
inoperable non-small-cell lung cancer. Br J Cancer. 89:1028–1030.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crumley AB, Stuart RC, McKernan M, Going
JJ, Shearer CJ and McMillan DC: Comparison of pre-treatment
clinical prognostic factors in patients with gastro-oesophageal
cancer and proposal of a new staging system. J Gastrointest Surg.
14:781–787. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roxburgh CS, Platt JJ, Leitch EF, Kinsella
J, Horgan PG and McMillan DC: Relationship between preoperative
comorbidity, systemic inflammatory response, and survival in
patients undergoing curative resection for colorectal cancer. Ann
Surg Oncol. 18:997–1005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan H, Shao ZY, Xiao YY, Xie ZH, Chen W,
Xie H, Qin GY and Zhao NQ: Comparison of the glasgow prognostic
score (GPS) and the modified glasgow prognostic score (mGPS) in
evaluating the prognosis of patients with operable and inoperable
non-small cell lung cancer. J Cancer Res Clin Oncol. 142:1285–1297.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu L, Chen S, Ma S and Zhang S: Glasgow
prognostic score predicts prognosis of non-small cell lung cancer:
A meta-analysis. Springerplus. 5:4392016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Temel JS, Abernethy AP, Currow DC, Friend
J, Duus EM, Yan Y and Fearon KC: Anamorelin in patients with
non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2):
Results from two randomised, double-blind, phase 3 trials. Lancet
Oncol. 17:519–531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takayama K, Katakami N, Yokoyama T, Atagi
S, Yoshimori K, Kagamu H, Saito H, Takiguchi Y, Aoe K, Koyama A, et
al: Anamorelin (ONO-7643) in Japanese patients with non-small cell
lung cancer and cachexia: Results of a randomized phase 2 trial.
Support Care Cancer. 24:3495–3505. 2016. View Article : Google Scholar : PubMed/NCBI
|