Introduction
The median post-progression survival (PPS) for
ovarian cancer patients is ~2 years (1). Ovarian cancer is a well-known
chemosensitive malignancy. However, once the cancer relapses, it
may be difficult to treat (1).
Although a proportion of the patients achieve long-term PPS, the
determining clinicopathological factors have yet to be fully
elucidated.
It is considered that longer progression-free
survival (PFS) may prolong overall survival (OS) based on the
theory of reducing the number of cells available for subsequent
mutation (2). Another study reported
that the time-to-relapse is the most important prognostic factor in
ovarian cancer, as subsequent chemotherapy regimens and the
response to subsequent chemotherapy are determined based on this
time interval (3). It was previously
reported that secondary debulking surgery (SDS) was an effective
treatment for recurrent ovarian carcinoma; however, the criteria
for selecting SDS candidates remain unclear (4).
In the present study, the clinicopathological
characteristics of patients achieving a longer PPS were analyzed in
comparison with those of patients who succumbed to the disease
earlier.
Patients and methods
Patient selection
Among patients with ovarian cancer who underwent
first-line therapy between 1995 and 2006, those who developed
relapse until the end of 2012 at the National Defense Medical
College Hospital (Tokorozawa, Japan) were enrolled in the present
study. The patients who achieved a 5-year PPS after the first
recurrence or progression were defined as long-term survivors (LS),
whereas those who succumbed to relapsed ovarian cancer within 5
years after the first recurrence or progression were defined as
short-term survivors (SS). The patients who succumbed to other
diseases and those with insufficient follow-up data were excluded
(Fig. 1). The aim of this study was
to identify differences in the clinicopathological characteristics
between LS and SS based on the data acquired until the end of
2012.
Survival
In this study, OS was defined as the time from
initial diagnosis of the disease to death, and PFS was defined as
the time from initial diagnosis of the disease to diagnosis of
clinical relapse, progression, or death. PPS was measured from the
time of the first recurrence or progression to death. Platinum-free
interval (PFI) was defined as the time between the end of the
platinum-based chemotherapy and the first occurrence of
relapse.
Treatment and follow-up
The patients were classified based on the
International Federation of Gynecology and Obstetrics (FIGO) 1988
stage classification. Primary therapy included primary debulking
surgery + adjuvant chemotherapy, neoadjuvant chemotherapy +
interval debulking surgery, and first-line chemotherapy alone for
inoperable patients. All the patients received platinum agents as
first-line or adjuvant chemotherapy. SDS was only considered for
patients whose identified recurrent tumors were expected to be
completely resectable based on the clinician's discretion. For
follow-up, physical and sonography examinations with carbohydrate
antigen 125 (CA125) levels were evaluated every 3 months for 2
years. After 2 years, these examinations were performed every 3–6
months for at least 5 years. Computed tomography images were
routinely checked every 6–12 months, or when the examinations
raised the suspicion of recurrence. The Response Evaluation
Criteria in Solid Tumors (RECIST), version 1.1 (5) was used to evaluate the effectiveness of
the treatment. The Gynecologic Cancer Intergroup CA125 criteria
were not used for diagnosis of recurrence (6). Performance status (PS) was evaluated
based on the Eastern Cooperative Oncology Group (ECOG) PS
system.
The clinical variables evaluated were as follows:
Age at primary therapy, PS at primary therapy, FIGO stage,
histology, residual tumor (RT) after primary therapy, PFI, age at
recurrence, PS at recurrence, recurrent site, SDS, second-line
chemotherapy regimens, response to second-line chemotherapy and
number of chemotherapy regimens after the first recurrence.
Statistical analysis
Statistical analysis was performed using the JMP
10.0.0 software package (SAS Institute, Inc., Tokyo, Japan). The
χ2 test and Mann-Whitney U test were used to evaluate
differences in patient characteristics. Multivariable analyses were
based on the logistic regression method and Kaplan-Meier survival
curves of OS, PFS and PPS were compared using the log-rank test.
Statistical significance was defined as P<0.05.
This study was approved by the Institutional Review
Board of the National Defense Medical College.
Results
Patient characteristics
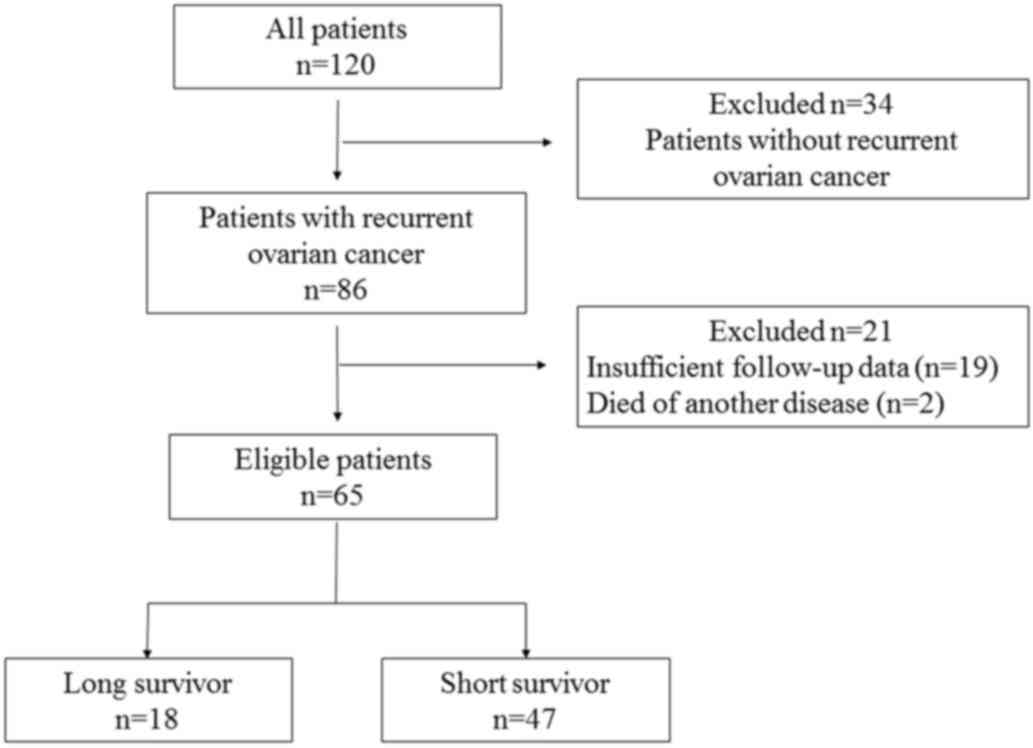
Among a total of 120 patients with ovarian cancer,
86 developed relapse, of whom 21 patients were excluded from the
present study (2 patients succumbed to another disease and data
were insufficient for 19 patients). Finally, 65 patients met the
inclusion criteria and were enrolled in the study: 18 patients
(28%) were classified as LS and 47 patients (72%) as SS (Fig. 1).
Patient survival
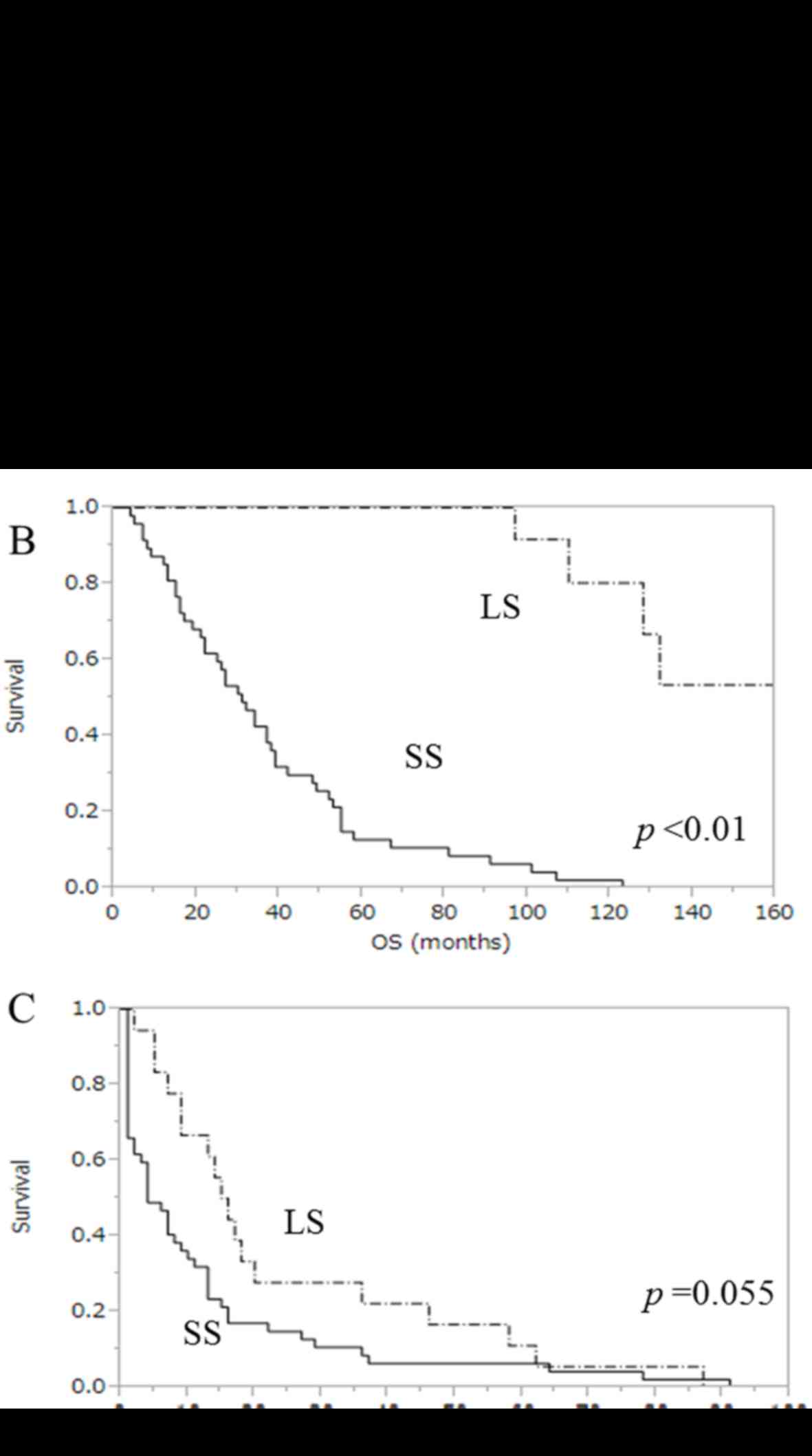
Kaplan-Meier survival curves for OS, PFS and PPS are
presented in Fig. 2. The median PPS
in LS and SS was 82.5 months (range, 60–122 months) and 15 months
(range, 3–59 months), respectively (P<0.01, Fig. 2A). The median OS was 102.5 months in
LS (range, 71–162 months) and 31 months in SS (range, 4–123 months)
(P<0.01, Fig. 2B). In addition,
the median PFS after primary therapy in LS and SS was 15.5 months
(range, 71–162 months) and 4 months (range, 1–91 months),
respectively (P=0.055, Fig. 2C).
Patient characteristics
The clinicopathological characteristics of the
patients are summarized in Table I.
Significant differences between the two groups included RT after
primary therapy (P<0.01), PFI (P=0.01), PS at recurrence
(P<0.01), SDS (P=0.03) and favourable response to second-line
chemotherapy (P<0.01).
 | Table I.Characteristics of the patients
(n=65). |
Table I.
Characteristics of the patients
(n=65).
|
| Long-term survivors,
n (%) | Short-term survivors,
n (%) |
|
|---|
|
|
|
|
|
|---|
| Characteristics | (n=18) | (n=47) | P-value |
|---|
| Age at primary
therapy (years) |
|
|
|
| ≤54 | 12 (67) | 20 (43) | 0.08 |
|
>54 | 6
(33) | 27 (57) |
|
| PS at primary
therapy |
|
|
|
| 0 | 12 (67) | 30 (64) | 0.83 |
| ≥1 | 6
(33) | 17 (36) |
|
| FIGO stage |
|
|
|
| I | 5
(28) | 7
(15) | 0.23 |
|
II–IV | 13 (72) | 40 (85) |
|
| Histology |
|
|
|
|
Serous | 6
(33) | 18 (38) | 0.14 |
|
Others | 12 (67) | 29 (62) |
|
| RT after primary
therapy |
|
|
|
| Yes | 1
(6) | 20 (43) | <0.01 |
| No | 17 (94) | 27 (57) |
|
| PFI at first
recurrence (months) |
|
|
|
|
<6 | 3
(17) | 24 (51) | 0.01 |
| ≥6 | 15 (83) | 23 (49) |
|
| Age at first
recurrence (years) |
|
|
|
| ≤57 | 11 (61) | 21 (45) | 0.23 |
|
>57 | 7
(39) | 26 (55) |
|
| PS at first
recurrence |
|
|
|
| 0 | 17 (94) | 27 (57) | <0.01 |
| ≥1 | 1
(6) | 20 (43) |
|
| Recurrent site |
|
|
|
| Distant
metastasis | 4
(22) | 7
(15) | 0.48 |
|
Others | 14 (78) | 40 (85) |
|
| Secondary debulking
surgery |
|
|
|
| Yes | 6
(33) | 5
(11) | 0.03 |
| No | 12 (67) | 42 (89) |
|
| Second-line
chemotherapy |
|
|
|
|
Platinum-based | 16 (89) | 35 (74) | 0.18 |
|
Others | 2
(11) | 12 (26) |
|
| Response to
second-line chemotherapy |
|
|
|
|
CR/PR | 8
(44) | 5
(11) | <0.01 |
|
SD/PD | 10 (56) | 42 (89) |
|
| Number of regimens
after the first recurrence |
|
|
|
| ≤2 | 8
(44) | 31 (66) | 0.11 |
| ≥3 | 10 (56) | 16 (34) |
|
By multivariate analysis, SDS (OR=13.3; 95% CI:
1.39–226.7), favourable response to second-line chemotherapy
(OR=16.5; 95% CI: 1.84–313.4) and receiving ≥3 regimens after the
first recurrence (OR=9.01; 95% CI: 1.28–117.7) were identified as
factors associated with long-term PPS (Table II).
 | Table II.Multivariate analysis for predictive
factors of long-term survivors. |
Table II.
Multivariate analysis for predictive
factors of long-term survivors.
| Variables | OR | 95% CI | P-value |
|---|
| Age at primary
therapy (years) |
|
|
|
|
≤54 | 2.44 | 0.18–45.5 | 0.50 |
|
>54 | 1 |
|
|
| PS at primary
therapy |
|
|
|
| 0 | 1.30 | 0.19–11.0 | 0.79 |
| ≥1 | 1 |
|
|
| FIGO stage |
|
|
|
| I | 9.69 | 0.87–168.6 | 0.07 |
|
II–IV | 1 |
|
|
| Histology |
|
|
|
|
Serous | 8.11 | 0.99–105.8 | 0.06 |
|
Others | 1 |
|
|
| RT after primary
therapy |
|
|
|
|
Yes | 0.53 | 0.02–6.98 | 0.64 |
| No | 1 |
|
|
| PFI at first
recurrence (months) |
|
|
|
|
<6 | 0.32 | 0.03–2.45 | 0.33 |
| ≥6 | 1 |
|
|
| Age at first
recurrence (years) |
|
|
|
|
≤57 | 1.05 | 0.05–17.6 | 0.97 |
|
>57 | 1 |
|
|
| PS at first
recurrence |
|
|
|
| 0 | 2.36 | 0.23–58.6 | 0.49 |
| ≥1 | 1 |
|
|
| Recurrent site |
|
|
|
| Distant
metastasis | 2.85 | 0.31–33.7 | 0.35 |
|
Others | 1 |
|
|
| Secondary debulking
surgery |
|
|
|
|
Yes | 13.3 | 1.39–226.7 | 0.03 |
| No | 1 |
|
|
| Second-line
chemotherapy |
|
|
|
|
Platinum-based | 3.68 | 0.40–57.2 | 0.26 |
|
Others | 1 |
|
|
| Response to
second-line chemotherapy |
|
|
|
|
CR/PR | 16.5 | 1.84–313.4 | 0.01 |
|
SD/PD | 1 |
|
|
| Number of regimens
after the first recurrence |
|
|
|
| ≥3 | 9.01 | 1.28–117.7 | 0.03 |
| ≤2 | 1 |
|
|
Discussion
Recent studies reported that PFS is not associated
with OS, particularly in patients with advanced ovarian cancer
(2,7,8).
Shimokawa et al suggested that PPS rather than PFS was more
significantly associated with OS (7). Additionally, another study reported
that percentage gains in PFS are not associated with percentage
gains in PPS (2). Our study also
suggested that PFS was not associated with PPS. For this reason,
although primary therapy for ovarian cancer is important,
appropriate treatment after relapse may be more important for
improving OS, in addition to prolonging PPS of ovarian cancer
patients.
In the present study, SDS was significantly
associated with LS after recurrence. The effectiveness of SDS has
been long discussed, not only for platinum-sensitive, but also
platinum-resistant recurrence (9–12). Some
studies achieved long median OS (>50 months) after recurrence
(11,12). However, the criteria for SDS
candidacy have not fully established; therefore, the benefit of
surgery for patients with relapsed ovarian cancer may be limited
(13). Recently, selection criteria
for operable patients with recurrent ovarian cancer were suggested
by the DESKTOP trial: i) Good PS, ii) no or small volume of ascites
at recurrence, and iii) no gross residual disease after primary
surgery (14). Another study
suggested that the predictive factors for complete resection at SDS
included FIGO stage, complete primary surgery, PFI, PS, CA125 value
at recurrence and ascites at recurrence (15). In the present study, none of the
patients who underwent SDS had ascites, but some patients who had
not met these criteria still achieved long-term survival. It is
suggested that the selection of candidates for SDS requires further
discussion.
The response to chemotherapy after recurrence also
affected OS after recurrence. The response rates to second-line
chemotherapy are generally different according to the
platinum-sensitivity status: 20–25% for platinum-sensitive cases,
and 10–20% for platinum-resistant cases (16,17). Our
results suggested that the patients who exhibited complete or
partial response to second-line regimens may become LS.
Additionally, multiple lines of chemotherapy after recurrence also
affected the PPS. Previous studies suggested the effectiveness of
multiple lines of chemotherapy in ovarian cancer (18,19).
This fact may be associated with homologous recombinant deficiency
(HRD), as HRD has been suggested to be associated with
chemosensitivity and longer OS (20). Generally, the duration of response to
second-line regimens is shorter compared with that of primary
chemotherapy (1). However, patients
with recurrent disease may achieve longer OS by receiving several
lines of chemotherapy. Hoskins et al reported that some
selected patients based on PS and interval of chemotherapy may
benefit from successive chemotherapy (18). Further studies are required to
confirm the effectiveness of multiple lines of chemotherapy.
In conclusion, three factors favouring long PPS in
ovarian cancer were identified, namely SDS, favourable response to
second-line chemotherapy and ≥3 chemotherapy regimens after the
first recurrence. Appropriate post-recurrence treatment is crucial
for longer PPS. Although further analyses are required to evaluate
the clinical significance of these factors, our study revealed
potential clinicopathological markers favouring long-term PPS in
recurrent ovarian cancer.
References
|
1
|
Ozols RF: Systemic therapy for ovarian
cancer: Current status and new treatment. Semin Oncol. 33 2 Suppl
6:S3–S11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sundar S, Wu J, Hillaby K, Yap J and
Lilford R: A systematic review evaluating the relationship between
progression free survival and post progression survival in advanced
ovarian cancer. Gynecol Oncol. 125:493–499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Burg ME, De Wit R, van Putten WL,
Logmans A, Kruit WH, Stoter G and Verweij J: Weekly cisplatin and
daily oral etoposide is highly effective in platinum pretreated
ovarian cancer. Br J Cancer. 86:19–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zang RY, Zhand ZY, Li ZT, Cai SM, Tang MQ,
Chen J and Liu Q: Impact of secondary cytoreductive surgery on
survival of patients with advanced epithelial ovarian cancer. Eur J
Surg Oncol. 26:798–804. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rustin GJ, Vergote I, Eisenhauer E,
Pujade-Lauraine E, Quinn M, Thigpen T, du Bois A, Kristensen G,
Jakobsen A, Sagae S, et al: Definitions for response and
progression in ovarian cancer clinical trials incorporating RECIST
1.1 and CA 125 agreed by the Gynecological Cancer Intergroup
(GCIG). Int J Gynecol Cancer. 21:419–423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimokawa M, Ohki M and Kaku T:
Correlation of progression-free and post-progression survival with
overall survival in phase III trials of first-line chemotherapy for
advanced epithelial ovarian cancer. Eur J Gynaecol Oncol.
36:370–375. 2015.PubMed/NCBI
|
|
8
|
Vidal F, Guerby P, Luyckx M, Haddad P,
Stoeckle E, Morice P, Leblanc E, Lecuru F, Daraï E, Classe JM, et
al: Are early relapses in advanced-stage ovarian cancer doomed to a
poor prognosis? PLoS One. 11:e01477872016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petrillo M, Anchora L Pedone, Tortorella
L, Fanfani F, Gallotta V, Pacciani M, Scambia G and Fagotti A:
Secondary cytoreductive surgery in patients with isolated
platinum-resistant recurrent ovarian cancer: A retrospective
analysis. Gynecol Oncol. 134:257–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bristow RE, Puri I and Chi DS:
Cytoreductive surgery for recurrent ovarian cancer: A
meta-analysis. Gynecol Oncol. 112:265–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salani R, Santillan A, Zahurak ML,
Giuntoli RL II, Gardner GJ, Armstrong DK and Bristow RE: Secondary
cytoreductive surgery for localized, recurrent epithelial ovarian
cancer: Analysis of prognostic factors and survival outcome.
Cancer. 109:685–691. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laas E, Luyckx M, De Cuypere M, Selle F,
Daraï E, Querleu D, Rouzier R and Chéreau E: Secondary complete
cytoreduction in recurrent ovarian cancer: Benefit of optimal
patient selection using scoring system. Int J Gynecol Cancer.
24:238–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van de Laar R, Massuger LF, van Gorp T,
IntHout J, Zusterzeel PL and Kruitwagen RF: External validation of
two prediction models of complete secondary cytoreductive surgery
in patients with recurrent epithelial ovarian cancer. Gynecol
Oncol. 137:210–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harter P, du Bois A, Hahmann M, Hasenburg
A, Burges A, Loibl S, Gropp M, Huober J, Fink D, Schröder W, et al:
Surgery in recurrent ovarian cancer: The arbeitsgemeinchaft
gynaekologische onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol.
13:1702–1710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian WJ, Chi DS, Sehouli J, Tropé CG,
Jiang R, Ayhan A, Cormio G, Xing Y, Breitbach GP, Braicu EI, et al:
A risk model for secondary cytoreductive surgery in recurrent
ovarian cancer: An evidence-based proposal for patient selection.
Ann Surg Oncol. 19:597–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vergote IB, Garcia A, Micha J, Pippitt C,
Bendell J, Spitz D, Reed N, Dark G, Fracasso PM, Ibrahim EN, et al:
Randomized multicenter phase II trial comparing two schedules of
etirinotecan pegol (NKTR-102) in women with recurrent
platinum-resistant/refractory epithelial ovarian cancer. J Clin
Oncol. 31:4060–4066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Markman M, Markman J, Webster K, Zanotti
K, Kulp B, Peterson G and Belinson J: Duration of response to
second-line, platinum-based chemotherapy for ovarian cancer:
Implications for patient management and clinical trial design. J
Clin Oncol. 22:3120–3125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoskins PJ and Le N: Identifying patients
unlikely to benefit from further chemotherapy: A descriptive study
of outcome at each relapse in ovarian cancer. Gynecol Oncol.
97:862–869. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Griffiths RW, Zee YK, Evans S, Mitchell
CL, Kumaran GC, Welch RS, Jayson GC, Clamp AR and Hasan J: Outcomes
after multiple lines of chemotherapy for platinum-resistant
epithelial cancers of the ovary, peritoneum, and fallopian tube.
Int J Gynecol Cancer. 21:58–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun C, Li N, Ding D, Weng D, Meng L, Chen
G and Ma D: The role of BRCA status on the prognosis of patients
with epithelial ovarian cancer: A systematic review of the
literature with a meta-analysis. PLoS One. 9:e952852014. View Article : Google Scholar : PubMed/NCBI
|