Introduction
Spindle cell carcinomas (SpCCs) are biphasic tumors
that typically exhibit a mesenchymal appearance and consist of
squamous cell carcinoma (SCC) and malignant spindle cell components
(1). SpCC preferentially occurs in
the head and neck, particularly in the oral and laryngeal mucosal
tissues. Macroscopically, these tumors are characterized by an
ulcerated surface and polypoid appearance (1–6).
Histological features associated with the transition area between
spindle cells and SCC components/surface epithelium suggest an
epithelial origin of the sarcomatoid component, which typically
resembles fibrosarcoma or undifferentiated pleomorphic sarcoma
(malignant fibrous histiocytoma) (1,7,8). The development of SpCC is often
associated with radiotherapy, and radiation-induced SpCC tends to
include foci of osteosarcomatous, chondrosarcomatous, or
rhabdomyosarcomatous differentiation (1,9). In the
present report, the authors discuss a patient with recurrent SpCC
following glossectomy for a primary SCC. The tumor was spherical
and located in the deep lingual layer of the tongue, and the
patient had not undergone postoperative radiotherapy for the
initial tumor, in contrast to patterns observed for typical cases
of SpCC. As the mean interval between treatment of the primary
tumor and local recurrence in cases of early-stage SCC of the
tongue is ~15 months (10), the
present case is also unusual in that recurrence was observed 4
years following initial glossectomy. The current report focuses on
the peculiar features and the differential diagnosis of the present
case.
Case report
In 2011, a 62-year-old woman with a history of
sarcoidosis, psychotic depression and hypertension presented to the
Department of Clinical Oral Oncology at Nagasaki University
Hospital (Nagasaki, Japan) with reports of continuous stomatitis
and pain on the left side of her tongue. Although the patient had
smoked ~10 cigarettes per day for 20 years, she had been
tobacco-free for 10 years. She also reported an alcohol intake of
two glasses of shochu water per day. Intraoral examination revealed
a presumably malignant tumor of the tongue, measuring ~30×28 mm,
which was immediately evaluated via imaging and incisional biopsy.
Metastasis to two cervical lymph nodes on the affected side was
suspected based on contrast-enhanced computed tomography (CE-CT)
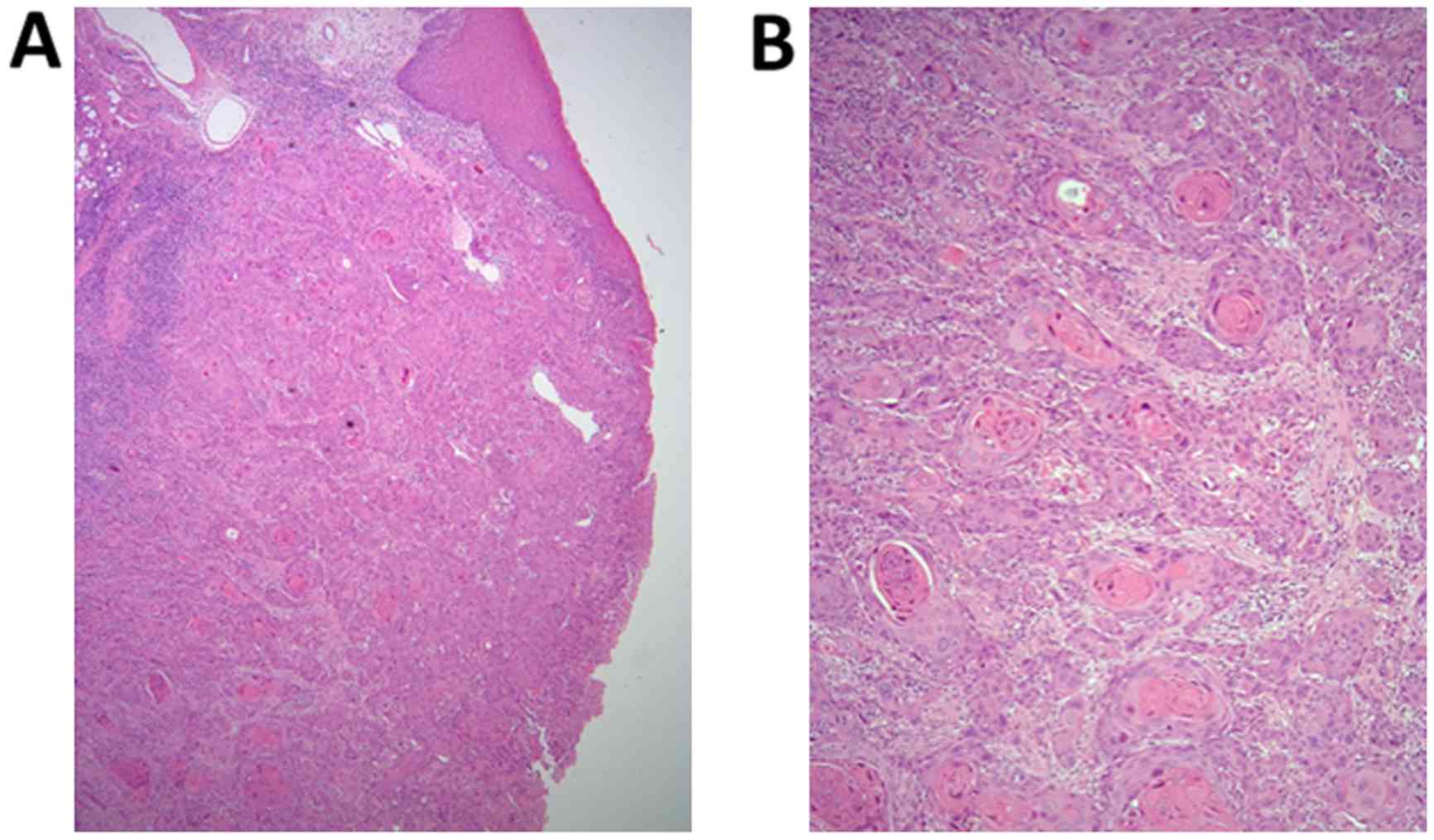
findings. The biopsy specimen exhibited signs of
well-differentiated SCC invading the submucosal tissue and lingual
muscle from the mucosal epithelium, with apparent cancer pearls
(Fig. 1A and B), following which the
diagnosis of well-differentiated SCC of the left tongue (T2N2bM0,
stage IV) was confirmed. Under general anesthesia, the patient
underwent partial glossectomy with adequate tumor-free margins,
left neck dissection, and soft tissue reconstruction using a
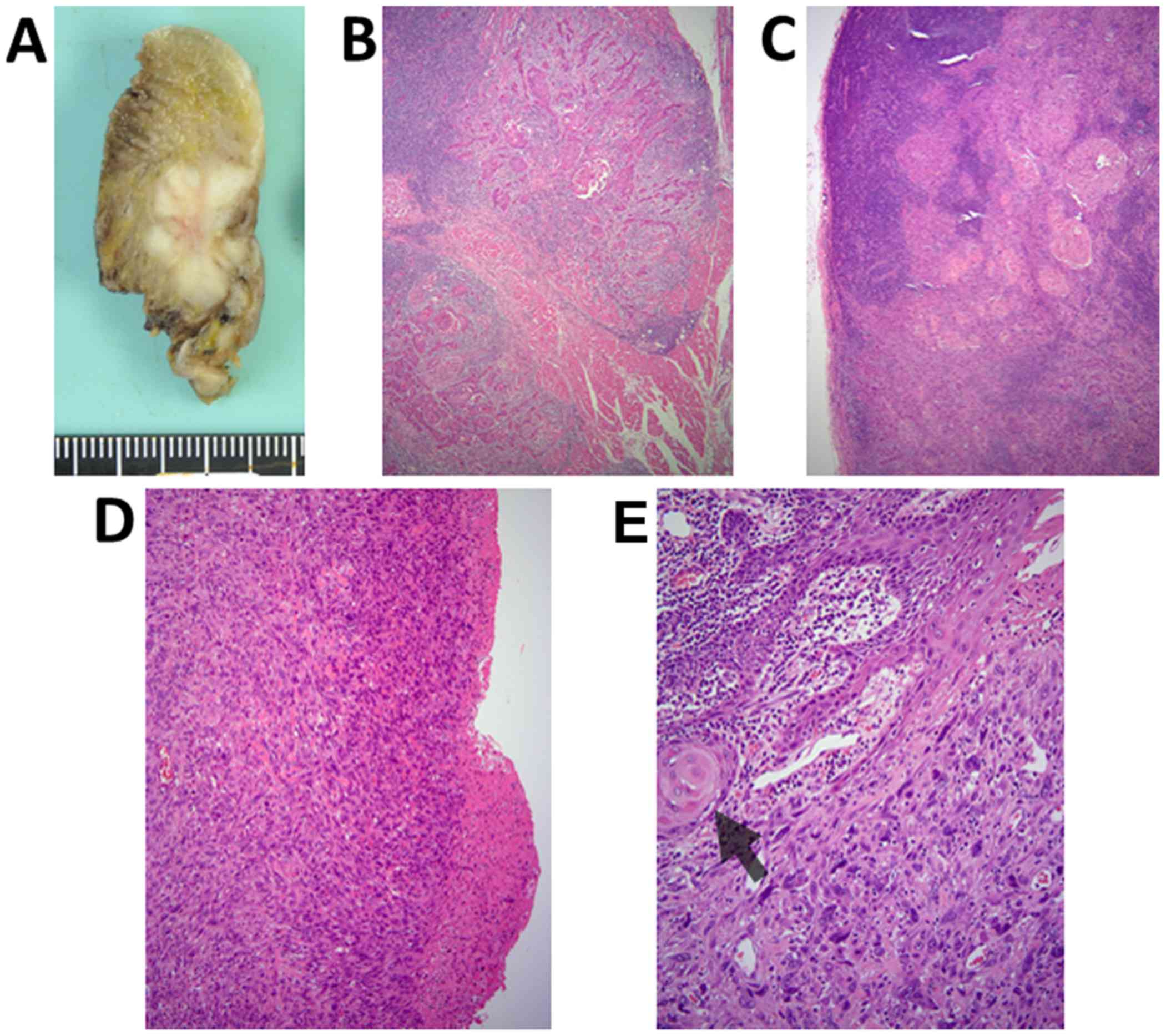
vascularized forearm flap. As observed for the biopsy specimen, the
tissue obtained during surgery was primarily indicative of SCC
invading the lingual muscle (Fig.
2A-C). Notably, proliferation of atypical spindle cells with
large hyperchromatic nuclei was observed beneath the ulcerative
region of the tongue. These spindle cells transitioned out of the
dysplastic mucosal epithelium at the periphery of the ulcer
(Fig. 2D and E). Metastasis of SCC
without extranodal infiltration was noted in one level III lymph
node (left cervical). Atypical spindle cells were not detected in
the metastatic focus. A histological diagnosis of
well-differentiated SCC was determined due to the relatively small
number of spindle cells around the ulcerative region of the
tongue.
As the tumor was <1 mm from the margin of the
surgical specimen, additional tissue was resected 1 month after the
first surgery. The residual SCC was detected in the resected
specimen, and there was no evidence of residual tumor around the
resection margin. As the lymph node metastasis did not involve
extracapsular spreading, postoperative adjuvant chemoradiotherapy
was not performed.
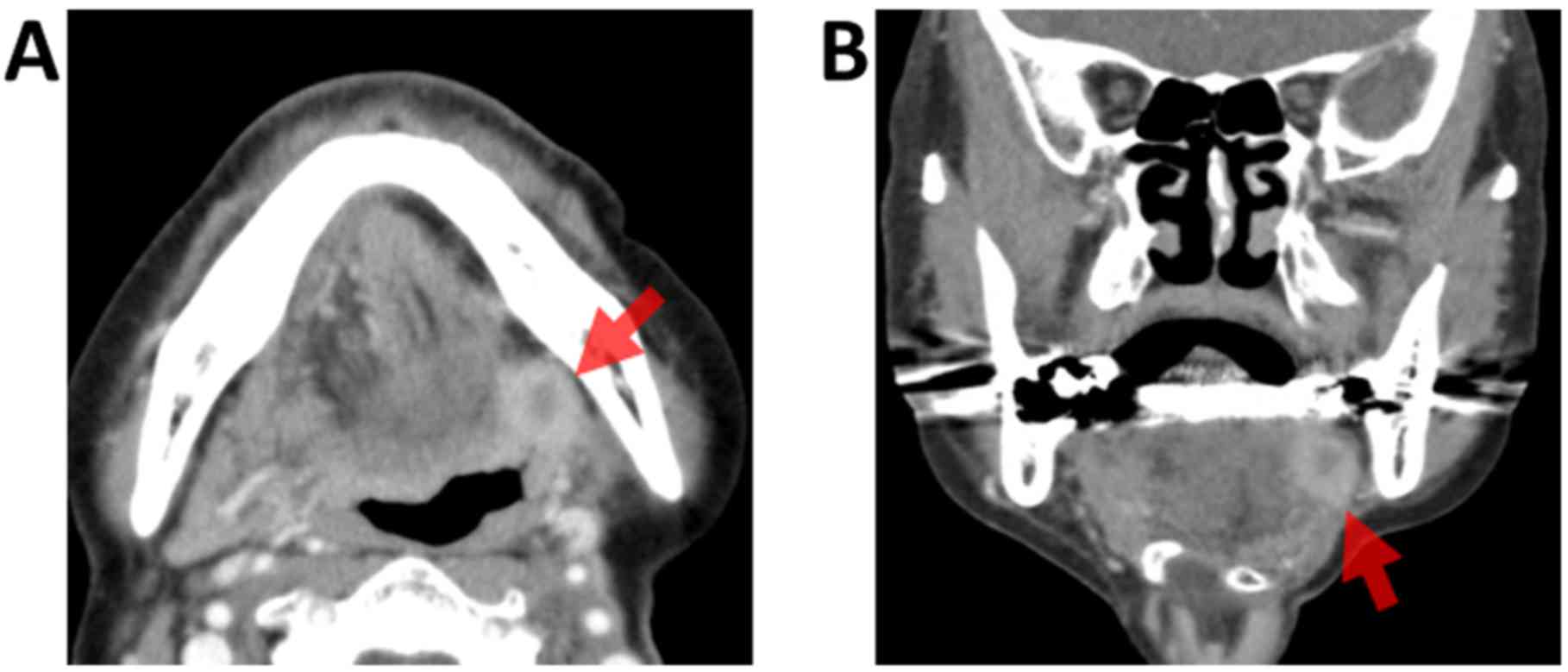
During routine follow-up 4 years after the initial
surgery, CE-CT revealed recurrence of the tumor (16×13×13 mm) at
the root of the left tongue behind the grafted forearm flap, which
exhibited heterogeneous enhancement. An incisional biopsy was
performed under general anesthesia to confirm whether the tumor
involved recurrent SCC or other independent malignancies, due to
the location of the tumor deep within the tongue (Fig. 3A and B). Biopsy results revealed
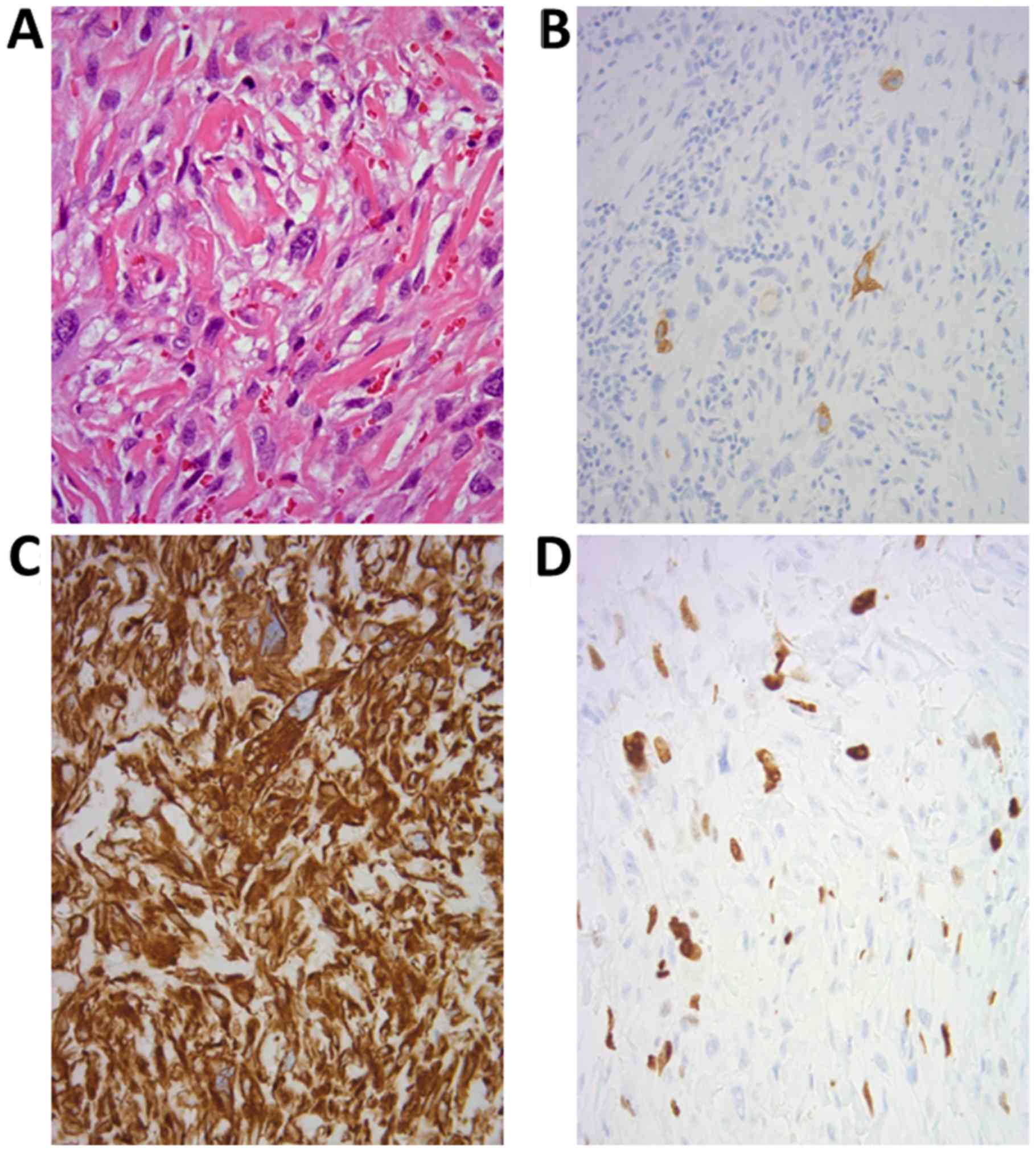
atypical spindle or oval cells with hyperchromatic abnormal nuclei
accompanied by fibrous tissue, suggestive of sarcoma. No epithelial
components were detected in the tumor (Fig. 4A and B). Immunohistochemically,
cytokeratin AE1/AE3 was expressed in the sparse spindle cells,
vimentin was strongly positive, and the MIB-1 (Ki-67) labeling
index was 22.1% (Fig. 4C-E), whereas
the results for S100 and leukocyte common antigen (CD45) were
negative. A preliminary diagnosis of sarcomatoid tumor was
determined based on the biopsy results, as the possibility of a
malignant epithelial tumor could not be ruled out due to the
patient's history of primary SCC.
Under general anesthesia, left hemi-glossectomy,
right neck dissection, and soft tissue reconstruction using a
vascularized free rectus abdominis flap were performed using a
mandibular swing approach due to the deep and posterior position of
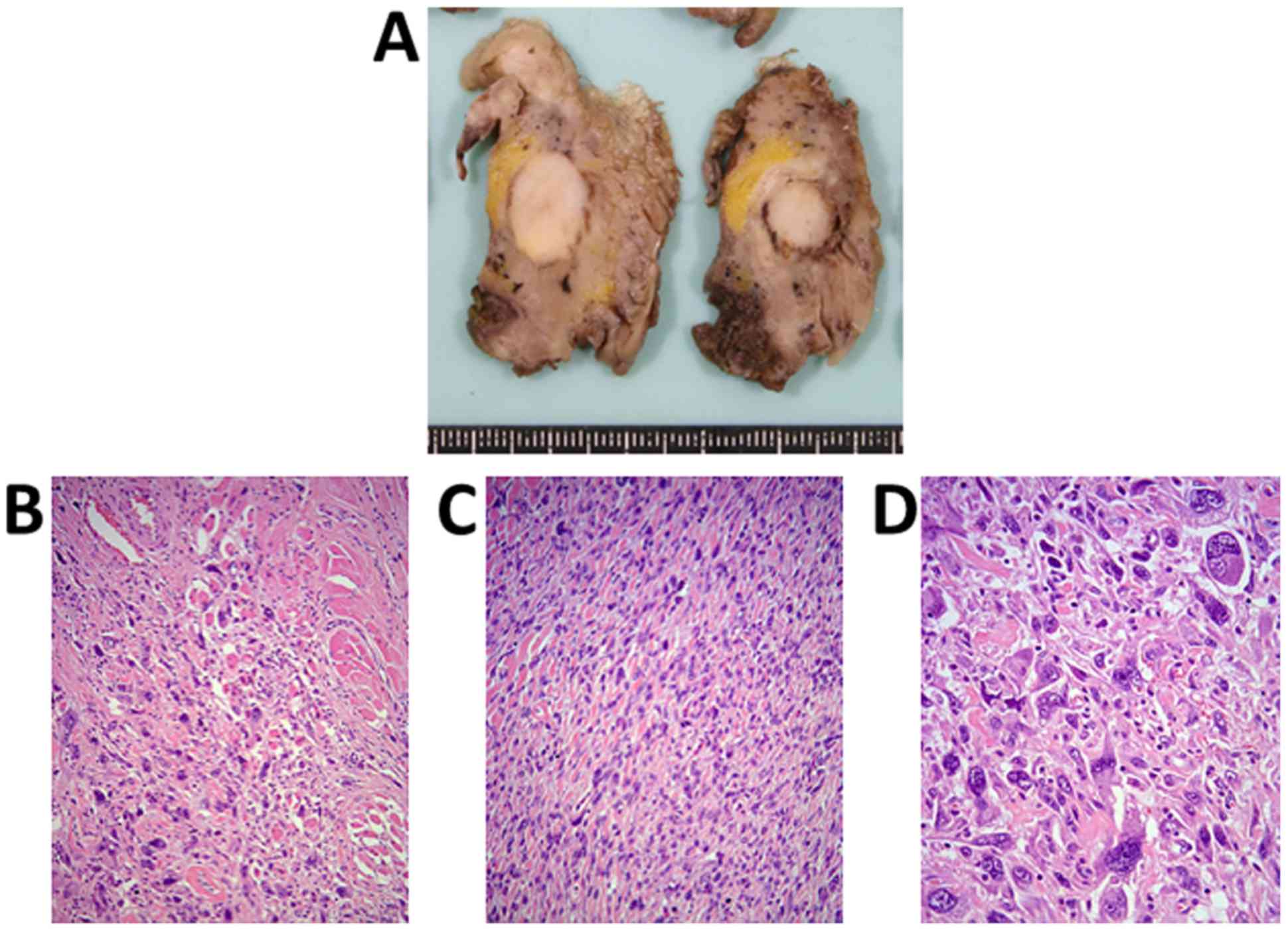
the tumor. The maximum diameter of the spherical tumor was 18 mm,
and it did not extend to the tongue mucosa (Fig. 5A). Microscopically, the tumor
exhibited a sarcomatoid appearance without capsulation, and there
was no extension to the mucosal epithelium; however, atypical
spindle cells had infiltrated the adjacent striated muscle and
adipose tissue (Fig. 5B). The tumor
exhibited several sarcomatoid features: Monotonous atypical spindle
cell proliferation in an ordered fashion (Fig. 5C); and intense anaplastic appearance
involving cellular pleomorphism with large, bizarrely shaped nuclei
or multinucleated cells (Fig. 5D).
Furthermore, there was a malignant fibrous histiocytoma-like
pattern, including storiform arrangement of collagen bundles
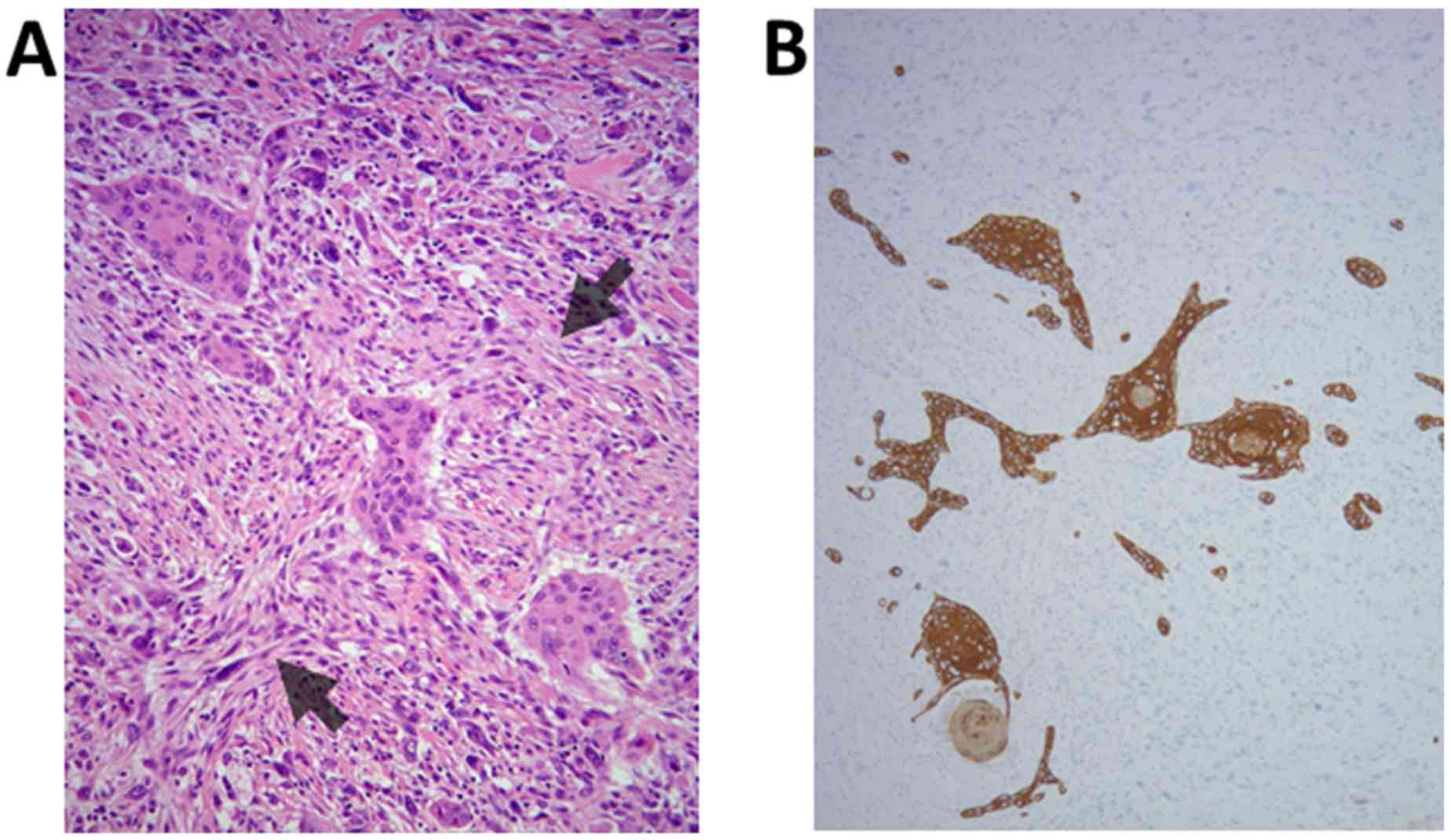
(Fig. 6A). Finally, accurate
examination using numerous tumor sections revealed the presence of
tiny SCC components within the tumor (Fig. 6A). Morphological transition from SCC
to sarcomatoid cells was not apparent. Immunohistochemically, the
sarcomatoid cells were positive for vimentin and α-smooth muscle
actin, while AE1/AE3 was expressed in the SCC components and in
sparse sarcomatoid cells (Fig. 6B).
The two components were negative for S100, HMB-45, CD34, myoglobin
and desmin. Therefore, we speculated that the sarcomatoid cells
were derived from SCC due to their patchy immunoreactivity with
AE1/AE3 and the patient's history of deeply invading SCC. Thus, a
final diagnosis of SpCC was made.
The patient's postoperative course was event-free,
with no evidence of tumor recurrence or metastasis at the 1-year
follow-up.
The present study was conducted in accordance with
the Declaration of Helsinki, and was approved by the Ethical Review
Board of Nagasaki University. Appropriate consents, permissions,
and releases were also obtained from the patient.
Discussion
SpCCs are biphasic, malignant epithelial tumors that
consist of both SCC and sarcomatoid components. The SCC component
may be scant or even inapparent on light microscopy (1). Therefore, histological diagnosis of
SpCC is extremely difficult when SCC components are not detected
(3). In such cases, there is a
possibility for the tumor to be misdiagnosed as a fibrosarcoma or
undifferentiated pleomorphic sarcoma (malignant fibrous
histiocytoma). If sarcomatoid tissue includes features suggestive
of differentiation, diagnoses of osteosarcoma, chondrosarcoma and
rhabdomyosarcoma are also conceivable. Viswanathan et al
(6) developed a diagnostic
immunohistochemical algorithm for identifying spindle cell
neoplasms in biopsied mucosa of the head and neck when the tumor
lacks epithelial components. Nevertheless, diagnosis of SpCC
remains difficult, as spindle cells of the SpCC demonstrate
variable immunoreactivities with cytokeratin antibodies (2–4,7). Takata et al (11) suggested that the absence of staining
for keratin in sarcomatoid tumor cells does not always exclude
SpCC. In the present case, the biopsy specimen obtained from the
secondary tumor lacked SCC components. However, SpCC was not
eliminated as a possibility, based on the clinical course, small
focus of spindle cell proliferation associated with mucosal
epithelium in the primary carcinoma, and relatively low number of
AE1/AE3-positive spindle cells. Once the preliminary diagnosis of
sarcomatoid tumor was determined based upon the biopsy results,
detailed examination of the resected tumor demonstrated a mixture
of spindle cells and small SCC components. Thus, a final diagnosis
of SpCC was determined. These findings suggest that SpCC should be
considered during the differential diagnosis of recurrent
sarcomatoid tumors occurring at the site of surgical resection of
SCC.
SpCC typically exhibits exophytic or polypoid
nodules and mucosal ulceration (1,3–6). However, in the present case, spherical
nodules were observed within the deep muscle and adipose tissue of
the tongue (Figs. 3 and 5A). Thus, we speculate that the present
tumor developed from SCC persisting in the deep-infiltrating region
following the patient's initial partial glossectomy. The
sarcomatoid components may therefore have derived not from mucosal
squamous epithelium but from the small number of SCC cells at the
epithelial-mesenchymal transition (EMT).
Another peculiar feature of the present case is that
the patient had not received radiotherapy following the partial
glossectomy. The occurrence of SpCC is associated with smoking,
alcohol consumption, and radiation exposure (1,5,12), and instances of SpCC recurrence have
been observed following radiotherapy for various primary
malignancies, including SCC (9,11,13).
Radiation is thought to induce EMT in various normal and neoplastic
tissues (14–17). Although SpCC in the present case was
not induced by radiotherapy, the small focus of spindle cell
elements transitioning from the dysplastic squamous epithelium in
the primary tumor suggested that the neoplastic squamous cells
exhibited induced characteristics of EMT. A number of reports have
also suggested that various forms of sarcoma, such as malignant
fibrous histiocytoma (undifferentiated pleomorphic sarcoma), can
manifest as locoregional recurrence of SCC following radiotherapy
of the head and neck (18–21). We speculate that some sarcomas may
emerge as sarcomatoid components of SpCC. However, if the number of
SCC components is extremely small, the tumor may be misdiagnosed as
SpCC. Thus, the identification of epithelial components is required
for the differential diagnosis of SpCC in cases of recurrent
radiation-induced sarcoma at the site of previous surgical
resection of a malignant epithelial tumor.
In conclusion, SpCC with marked anaplasia remains
difficult to diagnose, as noted in the present case. The present
findings further suggest that, during histological diagnosis of
recurrent sarcomatoid tumors associated with primary epithelial
malignancies, immunohistochemical examination and identification of
SCC components are essential for ensuring the accuracy of the
diagnosis.
Glossary
Abbreviations
Abbreviations:
|
SpCC
|
spindle cell carcinoma
|
|
SCC
|
squamous cell carcinoma
|
|
CE-CT
|
contrast-enhanced computed
tomography
|
References
|
1
|
Cardesa A and Zidar N: Spindle cell
carcinomaWorld Health Organization Classification of Tumours,
Pathology & Genetics, Head and Neck Tumours. Barnes L, Eveson
J, Reichart P and Sidransky D: IARC Press; Lyon: pp. 127–128.
2005
|
|
2
|
Biradar MV, Dantkale SS, Abhange RS, Kamra
HT and Birla K: Spindle cell carcinoma of the tongue: A rare
variant of squamous cell carcinoma. Ecancermedicalscience.
8:4472014.PubMed/NCBI
|
|
3
|
Rizzardi C, Frezzini C, Maglione M,
Tirelli G and Melato M: A look at the biology of spindle cell
squamous carcinoma of the oral cavity: Report of a case. J Oral
Maxillofac Surg. 61:264–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Romañach MJ, Azevedo RS, Carlos R, de
Almeida OP and Pires FR: Clinicopathological and
immunohistochemical features of oral spindle cell carcinoma. J Oral
Pathol Med. 39:335–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson LD, Wieneke JA, Miettinen M and
Heffner DK: Spindle cell (sarcomatoid) carcinomas of the larynx: A
clinicopathologic study of 187 cases. Am J Surg Pathol. 26:153–170.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Viswanathan S, Rahman K, Pallavi S, Sachin
J, Patil A, Chaturvedi P, D'Cruz A, Agarwal J and Kane SV:
Sarcomatoid (spindle cell) carcinoma of the head and neck mucosal
region: A clinicopathologic review of 103 cases from a tertiary
referral cancer centre. Head Neck Pathol. 4:265–275. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bavle RM, Govinda G, Venkataramanaiah PG,
Muniswamappa S and Venugopal R: Fallacious carcinoma- Spindle cell
variant of squamous cell carcinoma. J Clin Diagn Res. 10:ZD05–ZD08.
2016.PubMed/NCBI
|
|
8
|
Watson RF, Chernock RD, Wang X, Liu W, Ma
XJ, Luo Y, Wang H and El-Mofty SK and Lewis JS Jr: Spindle cell
carcinomas of the head and neck rarely harbor
transcriptionally-active human papillomavirus. Head Neck Pathol.
7:250–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leifer C, Miller AS, Putong PB and Min BH:
Spindle-cell carcinoma of the oral mucosa. A light and electron
microscopic study of apparent sarcomatous metastasis to cervical
lymph nodes. Cancer. 34:597–605. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pinsolle V, Truilhé Y, Majoufre C,
Michelet V and Pinsolle J: Posterior marginal mandibulectomy for
cancer of the oral cavity and otopharynx. Experience with 14
clinical cases. Ann Chir Plast Esthet. 42:223–227. 1997.PubMed/NCBI
|
|
11
|
Takata T, Ito H, Ogawa I, Miyauchi M,
Ijuhin N and Nikai H: Spindle cell squamous carcinoma of the oral
region. An immunohistochemical and ultrastructural study on the
histogenesis and differential diagnosis with a clinicopathological
analysis of six cases. Virchows Arch A Pathol Anat Histopathol.
419:177–282. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramamurti A, Venkataraman M, Narasimhan M
and Rao SR: Spindle cell carcinoma of the gingiva: A rare
occurrence. Contemp Clin Dent. 4:500–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lichtiger B, Mackay B and Tessmer CF:
Spindle-cell variant of squamous carcinoma. A light and electron
microscopic study of 13 cases. Cancer. 26:1311–1320. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung JW, Hwang SY, Hwang JS, Oh ES, Park S
and Han IO: Ionising radiation induces changes associated with
epithelial-mesenchymal transdifferentiation and increased cell
motility of A549 lung epithelial cells. Eur J Cancer. 43:1214–1224.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan Y, Zhou C, Yuan D, Zhang J and Shao C:
Radiation exposure promotes hepatocarcinoma cell invasion through
epithelial mesenchymal transition mediation by H2S/CSE pathway.
Radiat Res. 185:96–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Luo H, Jiang Z, Yue J, Hou Q, Xie
R and Wu S: Fractionated irradiation-induced EMT-like phenotype
conferred radioresistance in esophageal squamous cell carcinoma. J
Radiat Res. 57:370–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou YC, Liu JY, Li J, Zhang J, Xu YQ,
Zhang HW, Qiu LB, Ding GR, Su XM Mei-Shi and Guo GZ: Ionizing
radiation promotes migration and invasion of cancer cells through
transforming growth factor-beta-mediated epithelial-mesenchymal
transition. Int J Radiat Oncol Biol Phys. 81:1530–1537. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Makimoto Y, Yamamoto S, Takano H, Motoori
K, Ueda T, Kazama T, Kaneoya K, Shimofusa R, Uno T, Ito H, et al:
Imaging findings of radiation-induced sarcoma of the head and neck.
Br J Radiol. 80:790–797. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marchitto G, Marci V, Berrone M and
Pentenero M: Early arising sarcoma after adjuvant radiotherapy for
oral squamous cell carcinoma. J Oral Maxillofac Surg. 74:862.e1–e8.
2016. View Article : Google Scholar
|
|
20
|
Sadati KS, Haber M and Sataloff RT:
Malignant fibrous histiocytoma of the head and neck after radiation
for squamous cell carcinoma. Ear Nose Throat J. 83:278, 280–281.
2004.
|
|
21
|
Satomi T, Watanabe M, Kaneko T,
Matsubayashi J, Nagao T and Chiba H: Radiation-induced malignant
fibrous histiocytoma of the maxilla. Odontology. 99:203–208. 2011.
View Article : Google Scholar : PubMed/NCBI
|