Introduction
Non-small-cell lung cancer (NSCLC) is the most
common malignancy in industrialized countries, in incidence as well
as mortality (1,2). The probability of survival at 5 years,
considering all-stage disease, remains low, at ~15% Approximately
60–70% of the patients have advanced-stage disease at diagnosis,
and the treatment options are limited (3,4). The
main cause of NSCLC is cigarette smoking, and the risk is directly
proportional to the duration of exposure to smoking and the number
of cigarettes smoked (5). Squamous
cell carcinoma is the second most common type of NSCLC, and is
closely associated with cigarette smoking. From a biological point
of view, smoking patients have different characteristics from
non-smokers. Non-smokers develop a disease in which genetic
abnormalities are more relevant, with tumor cell proliferation
largely depending on epidermal growth factor receptor (EGFR). In
smokers, however, EGFR is wild-type, whereas a mutation of the
K-ras oncogene is very common. We herein present the case of a
72-year-old male smoker, with stage IV (T3N1M1) squamous cell
carcinoma of the lung, who was unresponsive to chemotherapy but
responsive to tyrosine kinase inhibitor (TKI) treatment.
Case report
In July 2008, a 72-year-old male patient was
diagnosed via CT-guided fine-needle aspiration biopsy with poorly
differentiated squamous cell carcinoma of the inferior lobe of the
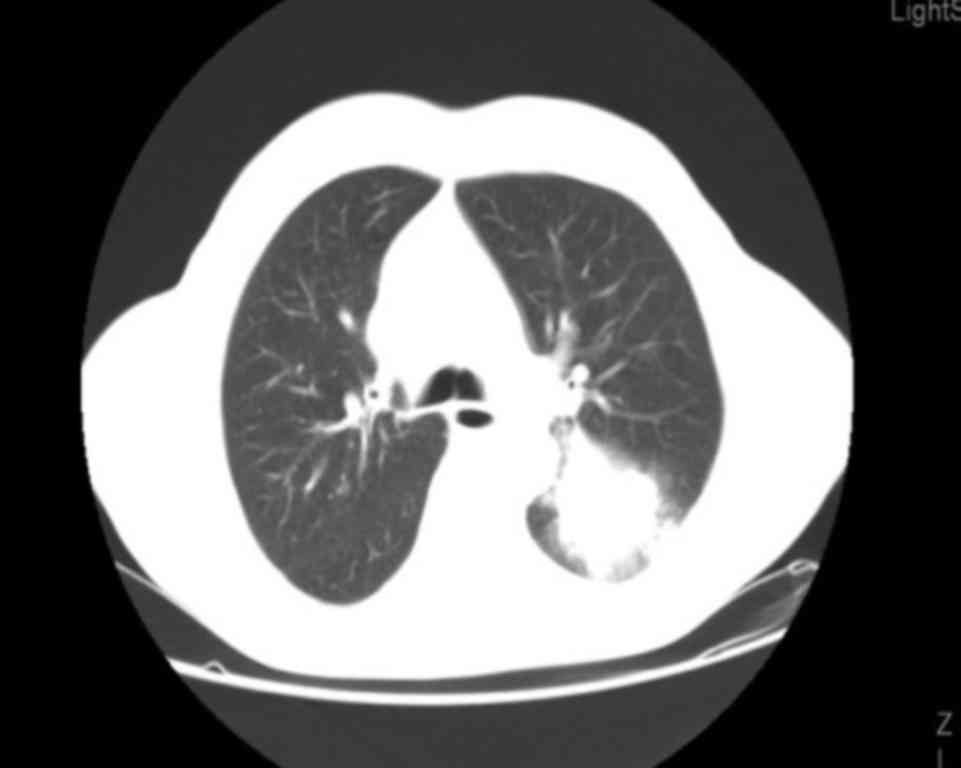
left lung, with a maximum diameter of 53 mm. The patient also
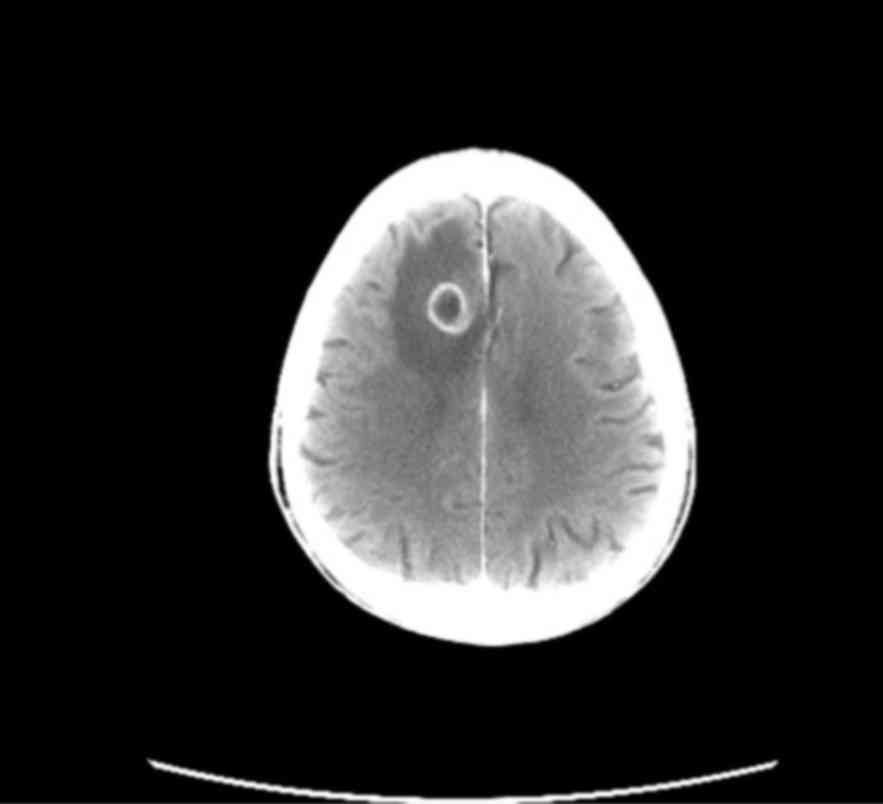
presented with ipsilateral hilar lymphadenopathy (Fig. 1) and a 30-mm solitary brain
metastasis of the right frontal lobe (Fig. 2). The conclusive diagnosis was stage
IV squamous lung cell carcinoma (cT3cN1cM1). In September 2008,
stereotactic radiotherapy was performed on the brain metastasis, at
a dose of 25 Gy. In October 2008, chemotherapy was initiated, with
carboplatin (area under the curve 5) and paclitaxel 175
mg/m2 on day 1, every 21 days.
The patient received 2 cycles of chemotherapy, with
considerable toxic effects, including National Cancer Institute
(NCI) grade 3 peripheral neuropathy, grade 3 leukopenia, and grade
3 asthenia. The treatment was discontinued due to unacceptable
levels of toxicity. In December 2008, second-line chemotherapy was
initiated, with pemetrexed 500 mg/m2 on day 1, every 21
days. Two cycles were administered prior to treatment
discontinuation, due to disease progression (the lung lesion
increased to a maximum diameter of 80 mm). In February 2009,
third-line treatment with erlotinib 150 mg/day was initiated. After
2 weeks of treatment, a grade 3 skin rash developed. The treatment
was discontinued for 10 days, during which time the rash was
managed with sulfosalicylate-based moisturizing ointments and
creams, and oral minocycline at a dose of 100 mg/day (improvement
of skin rash to grade 1). The skin rash regressed to grade 1–2 and
erlotinib is readministered at a reduced dose of 100 mg/day. During
the first 3 months of treatment, the patient suffered repeated
exacerbations of the skin rash, reaching grade 3 in intensity,
leading to periods of erlotinib discontinuation for 2 weeks. Three
months later (May 2009), a whole-body computed tomography (CT) scan
revealed a reduction in the size of the lung lesion from 80 to 42
mm, with stability of the brain metastasis and no new disease
locations, which was classed as a partial response according to the
Response Evaluation Criteria In Solid Tumors (https://ctep.cancer.gov/protocoldevelopment/docs/recist_guideline.pdf).
The patient continued treatment with erlotinib 100 mg/day,
undergoing a whole-body CT scan every 3 months. The predominant
toxic effect was skin rash, which varied between grade 1 and 2 over
the years. For a total of 78 months (February 2009-September 2015),
the patient was treated with erlotinib 100 mg/day and was planned
to receive this treatment unless disease progression occurred. At
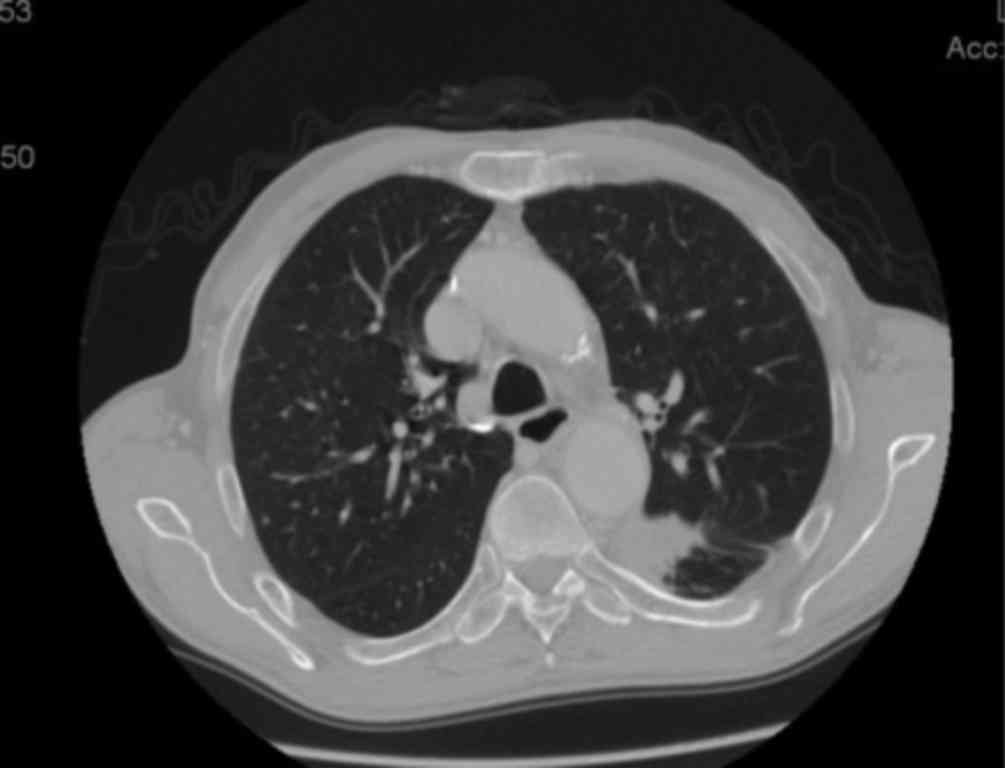
the last whole-body CT scan, the size of the lung lesion had been
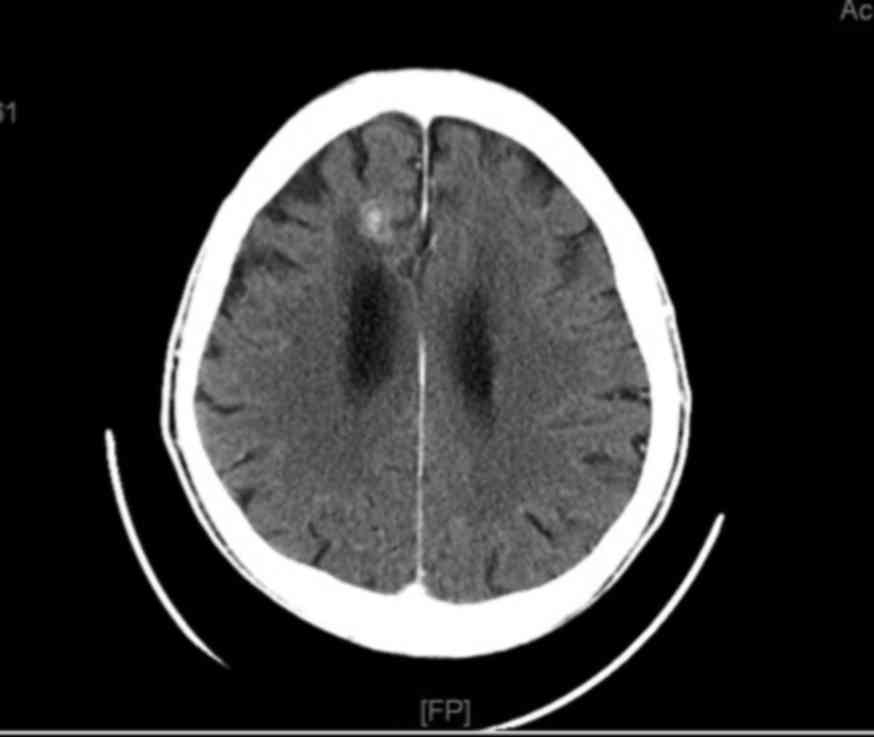
reduced to 32 mm (Fig. 3) and the
brain metastasis had been reduced to 20 mm (Fig. 4). The patient maintained an Eastern
Cooperative Oncology Group performance status of 1 and a good
quality of life. It should be noted that, in the present case, the
EGFR mutation status was unknown, as an examination was never
performed due to insufficient cytological material. In October 2015
the patient died from causes unrelated to cancer (road traffic
accident).
The patient provided informed consent to the
publication of the case details and associated images.
Discussion
Squamous cell carcinoma of the lung, which comprises
25% of all NSCLC cases, is closely associated with cigarette
smoking. The probability of finding activating mutations (codons
19–21 of the EGFR gene) in squamous cell carcinoma is relatively
low (<3 vs. 15% in adenocarcinomas). The presence of EGFR
mutations is a predictor of response to TKI treatment (gefitinib
and erlotinib). Erlotinib is a TKI of EGFR and is currently
approved for the treatment of metastatic NSCLC after failure of at
least one prior chemotherapy regimen, as maintenance therapy in
patients with stable disease after 4 cycles of standard first line
platinum-based chemotherapy, and as a first-line treatment in the
presence of activating EGFR mutations. The indication for second-
and third-line treatment is based on data from the BR.21 (6) study, which compared erlotinib vs. best
supportive care in an unselected population, showing an increase in
overall survival (OS) with erlotinib (6.7 vs. 4.7 months).
The factors predictive of response to erlotinib are
as follows: Adenocarcinoma histology, female gender, non-smoker
status and Asian ethnicity. These factors were not found to be
predictive of survival, however, as nearly all patients benefit
from treatment with this drug, including smokers (6), although it should be remembered that
cigarette smoking reduces exposure to erlotinib by 50–60%. Another
predictive factor investigated is the intensity of the rash: In
exploratory analyses conducted in the BR.21 (6), SATURN (7), TITAN (8)
and EURTAC (9) studies, it was
observed that a higher grade of rash is associated with greater
benefit in terms of progression-free survival (PFS) or OS. This
assertion, however, required further confirmation by prospective
studies designed ad hoc. In tumours characterized by the
presence of mutations in codons 19–21 of the tyrosine kinase domain
of EGFR (10), EGFR TKIs are
associated with a high rate of objective response, PFS and OS
(11). Erlotinib, in particular, has
two positive phase III trials in this setting, one on Chinese and
one on Caucasian patients (9,12). The
clinical case under consideration is characterized by the fact that
the patient did not have the clinical characteristics considered
predictive of response to the drug, as he was male, a smoker and
had squamous cell carcinoma histology. Our patient was ‘refractory’
to chemotherapy, as he exhibited rapid progression of the disease;
however, the selection of erlotinib as second line treatment has
resulted in an excellent response in the lung and a reduction of
the brain lesion after 3 months of treatment (13). The effectiveness of the drug was
accompanied in the first 3 months by an intense skin rash located
on the face, in the upper lip and periorbital region, which,
however, subsided after 2–3 weeks and stabilized at grade 1. Skin
rash is a frequent adverse event with erlotinib; it is, however,
manageable by dose reduction and topical application of
moisturizing creams, or administration of antibiotics in the case
of bacterial superinfection. Correctly informing and monitoring the
patient is a crucial element of therapy with erlotinib.
The results of the clinical case under consideration
are encouraging, not only in terms of the objective response, but
also in terms of quality of life and survival. The patient remained
alive 72 months after initiation of treatment with erlotinib, and
maintained a PS ECOG of 1. Although his EGFR mutation status was
unknown, it is hypothesized to be positive, associated with other
biomolecular factors predictive of positive response. The PFS with
erlotinib in the EURTAC (9), SLCG
(14), CALGB30406 (15) and OPTIMAL (12) studies ranges from 9.7 to 15.7 months.
In the present case, at 72 months of therapy with erlotinib, there
has been no evidence of disease progression. Our patient, while not
having factors predicting response to TKIs (adenocarcinoma,
non-smoker, female, Asian) exhibited a atypical, excellent response
and long-term survival. In conclusion, it may be stated that the
survival data reported in the clinical case examined herein are
surprising and go beyond the current statistics.
References
|
1
|
Jemal A, Bray E, Center M, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sant M, Allemani C, Santaquilani M, Knijn
A, Marchesi F and Capocaccia R: EUROCARE Working Group. EUROCARE-4:
Survival of cancer patients diagnosed in 1995–1999. Results and
commentary. Eur J Cancer. 45:931–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spiro SG and Silvestri GA: Oive hundred
years of lung cancer. Am J Respir Crit Care Med. 172:523–529. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Youlden DR, Cramb SM and Baade PD: The
International epidemiology of lung cancer: Geographical
distribution and secural trends. J Thorac Oncol. 3:818–831. 2008.
View Article : Google Scholar
|
|
5
|
Alberg AJ and Samet JM: Epidemiology of
lung cancer. Chest. 123 (1 Suppl):S21–S49. 2003. View Article : Google Scholar
|
|
6
|
Shepherd FA, Pereira Rodrigues J, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neal JW: The SATURN trial: The value of
maintenance erlotinib in patients with non-small-cell lung cancer.
Future Oncol. 6:1827–1832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ciuleanu T, Stelmakh L, Cicenas S,
Miliauskas S, Grigorescu AC, Hillenbach C, Johannsdottir HK,
Klughammer B and Gonzalez EE: Efficacy and safety of erlotinib
versus chemotherapy in second-line treatment of patients with
advanced, non-small-cell lung cancer with poor prognosis (TITAN): A
randomized multicentre, open-label, phase 3 study. Lancet Oncol.
13:300–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (EURTAC): A
multicentre, open-label, randomized phase 3 study. Lancet Oncol.
13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson BE and Jänne PA: Epidermal growth
factor receptor mutations in patients with non-small cell lung
cancer. Cancer Res. 65:7525–7529. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jackman DM, Yeap BY, Sequist LV, Lindeman
N, Holmes AJ, Joshi VA, Bell DW, Huberman MS, Halmos B, Rabin MS,
et al: Exon 19 deletion mutations of epidermal growth factor
receptor are associated with prolonged survival in non-small cell
lung cancer patients treated with gefitinib or erlotinib. Clin
Cancer Res. 12:3908–3914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Popat S, Hughes S, Papadopoulos P, Wilkins
A, Moore S, Priest K, Meehan L, Norton A and O'Brien M: Recurrent
responses to non-small cell lung cancer brain metastases with
erlotinib. Lung Cancer. 56:135–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grace Li: TLCR is endorsed by the Spanish
Lung Cancer Group (SLCG): New horizons for strong academic
collaboration in lung cancer. J Thorac Dis. Dec 5;E217–E218.
2013.PubMed/NCBI
|
|
15
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (EURTAC): A
multicentre, open-label, randomized phase 3 study. Lancet Oncol.
13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|