Introduction
Colorectal cancer (CRC) is one of the most common
human malignant tumors worldwide and morbidity associated with CRC
is increasing annually (1). It has
been reported that CRC was the third most commonly diagnosed cancer
in males and the second in females, with ~1.4 million cases and
693,900 mortalities occurring in 2015 (2). To date, 5-fluorouracil (5-FU) is widely
used as a primary chemotherapeutic agent and constitutes the
fundamental basis of chemotherapy treatment for patients with CRC
since it was introduced in 1957 (3–6).
Although targeted epidermal growth factor receptor monoclonal
antibodies, namely cetuximab and panitumumab, have been introduced
and have been used for many patients with CRC and benefits are
achieved, 5-FU is still a basic chemotherapy for CRC clinical
treatment (7). Furthermore, several
agents, such as 5-FU plus leucovorin (LV) or infusional 5-FU plus
LV and oxaliplatin, have been established as the generalized
regimen for the treatment of patients with CRC (8). For example, according to the guidelines
of the National Comprehensive Cancer Network, FOLFOX6 chemotherapy
including continuous infusion of 5-FU combined with oxaliplatin and
calcium folinate has become the standard chemotherapy regimen for
postoperative patients with CRC (9,10). In
addition, oral forms of 5-FU-related drugs, such as capecitabine or
tegafur plus uracil and doxifluridine (5′-DFUR), have also been
developed for convenient administration and have been widely used
in patients with CRC (11–13). However, the clinical effectiveness of
5-FU-based chemotherapy differs among patients (14). It is important to select 5-FU-based
chemotherapy so that each patient may benefit and experience the
least harmful side effects. To predict the clinical efficacy of
5-FU-based chemotherapy in CRC patients, it is essential to define
a predictive biomarker associated with 5-FU treatment.
Thymidine phosphorylase (TP) is an important
metabolizing enzyme that catalyzes the conversion of 5-FU to its
more active nucleoside form, 5-fluoro-2′-deoxyuridine, representing
one of the main pathways through which this drug exerts its
cytotoxic effect (15). One of the
roles of TP is controlling the intracellular levels of thymidine,
which at higher concentrations becomes toxic to cells and causes
replication errors in DNA (16).
Research has demonstrated that the levels of TP are higher in
tumors compared with normal tissues in a wide range of solid tumors
(15,17–20). The
expression of TP may be correlated with the efficacy of 5-FU-based
chemotherapy (21). However, in
cancer development, it has been reported that TP functions as the
molecule platelet-derived endothelial cell growth factor in cells
and exhibits angiogenic properties in tumors (22,23). In
2009, a study by Bronckaers et al (15) reported that TP had a dual role in
cancer development. TP may prevent apoptosis and induce
angiogenesis to promote tumor growth and metastasis, which is the
targeted function of TP inhibitors; however, TP is also
indispensable for the activation of the extensively used 5-FU
prodrugs, such as doxifluridine and capecitabine (15). As it has been demonstrated that TP
has a complicated role in CRC development and 5-FU treatment,
whether TP may predict the prognosis of patients with CRC treated
with 5-FU-based chemotherapy remains uncertain. Various studies
(4,5,24,25) have
investigated the association between the levels of TP and survival
in CRC patients; however, a certain conclusion regarding this has
not been drawn. Although the majority have reported poorer overall
survival (OS) and progression-free survival (PFS) in patients with
tumors expressing high TP levels, there are also reports that have
demonstrated no association between them, resulting in greatly
different estimates of the prognostic value of TP expression
between studies (26–29). The ability to use TP expression to
predict the response of patients with CRC to 5-FU-based
chemotherapy thus remains controversial.
The aim of the present study was to evaluate the
scientific evidence for the effect of TP expression in patients
with CRC treated with 5-FU-based chemotherapy by using a standard
meta-analysis of data from published studies.
Materials and methods
Search strategy
Searches were conducted on Wiley Online Library
(onlinelibrary.wiley.com), Scopus
(scopus.com/home.uri), PubMed (ncbi.nlm.nih.gov/pubmed), the Web of Science
(webofknowledge.com), the Cochrane
library (cochranelibrary.com), Ovid MEDLINE
(hsl.lib.umn.edu/biomed/help/ovid-medline), SinoMed
(sinomed.ac.cn) and China National Knowledge
Infrastructure (CNKI; cnki.net) without language
limitation. The last search update was April 28, 2015. The search
strategy predominantly included terms suggestive of four factors:
i) TP (i.e., ‘thymidine phosphorylase’, ‘platelet-derived
endothelial cell growth factor’ and ‘PD-ECGF’); ii) 5-FU (i.e.,
‘5-fluorouracil’, ‘adrucil’, ‘carac’, ‘efudex’, ‘efudix’,
‘5-fluoro-1H, 3H-pyrimidine-2, 4-dione’ and ‘5-fluoropyrimidines’);
iii) colorectal (i.e., ‘colon’, ‘rectal’, ‘colorectal’ and
‘rectum’); and iv) cancer (i.e., ‘cancer’, ‘carcinoma’, ‘neoplasm’,
‘tumor’ and ‘malignant’). Article types were restricted to clinical
trials or randomized controlled trials in humans. The reference
lists of primary studies and previous meta-analyses were
scrutinized for additional publications.
Inclusion and exclusion criteria
The potential trials were screened according to the
following criteria: i) Patients had a diagnosis of CRC; ii) all
patients received 5-FU-based chemotherapy; iii) the studies
reported one or more indicators, including objective response rate
(ORR), PFS, disease-free survival (DFS), relapse-free survival
(RFS) and OS, to compare the prognosis of patients stratified by TP
expression. Studies providing information on survival were
included, while studies without survival analysis, investigating
response rates only were excluded; iv) the results were part of the
original analysis; v) when results reported by the same author were
acquired from the same patient population in more than one
publication, only the study involving the highest number of
patients was included; and vi) retrospective, prospective or
randomized controlled trials were included. Trials evaluating
progression with time to tumor progression, which was defined as
time from the initiation date of 5-FU-based chemotherapy to the
first radiographic evidence of disease progression or mortality,
were also included. Trials lacking complete data that were still in
progress and without full text articles online were excluded. The
present study attempted to obtain the data with the longest
follow-up when reports overlapped or were repeated.
Data extraction and definitions
Data extracted included the first author,
publication year, study type, chemotherapy regimen, lesions tested,
TP evaluation method, study size, high TP level and percentage of
patients with high TP expression. For clinical outcome, the number
of responders were collected for calculating odds ratio (OR) and
95% estimation intervals for ORR. Hazard ratios (HRs) and 95%
confidence intervals (CI) were also extracted for OS, PFS, DFS and
RFS. If a separate HR was not provided, the HR and its variance
were estimated from the published survival curves using previously
described methods and models (30,31).
Adjusted HRs and estimation intervals were also collected when
reported. Objective response included complete response and partial
response, and non-response consisted of stable disease and
progressive disease, according to the Response Evaluation Criteria
in Solid Tumors (32) or World
Health Organization criteria (33).
PFS was defined as the time from the initiation date of 5-FU-based
therapy to the first evidence of disease progression or mortality
from any cause. OS was defined as the time from the initiation date
of 5-FU-based therapy to mortality from any cause. DFS was defined
as the length of time after being treated with 5-FU-based therapy
during which no disease was found. RFS was defined as the length of
time after being treated with 5-FU-based therapy during which
patients survived without any signs or symptoms of CRC. All data
were extracted by two independent investigators. Discussions were
used to reach an agreement if discrepancies existed.
Statistical analysis
The required information was extracted by two
independent reviewers using pre-determined data extraction forms.
The data were analyzed using Stata 12.0 software (StataCorp LP,
College Station, TX, USA). Heterogeneity across studies was
evaluated by the Q test and were quantified by the I2
test. If the tests of heterogeneity were significant (P<0.05),
the effect sizes were calculated with the random effects model
using the DerSimonian-Laird method. Otherwise, the fixed-effect
model with inverse variance weights was used. A funnel plot and an
Egger test were used to assess publication bias. Subgroup analysis
was conducted in the different treatment settings and TP detection
methods. Sensitivity analysis was used to test the stability when
large heterogeneity was presented. All P-values reported were
two-sided. Publication biases were assessed by the Egger's test
(P<0.05 indicated an existing publication bias) and were
reflected by the symmetry of the funnel plot on the natural
logarithm of RRs or HRs (34).
Results
Study selection and
characteristics
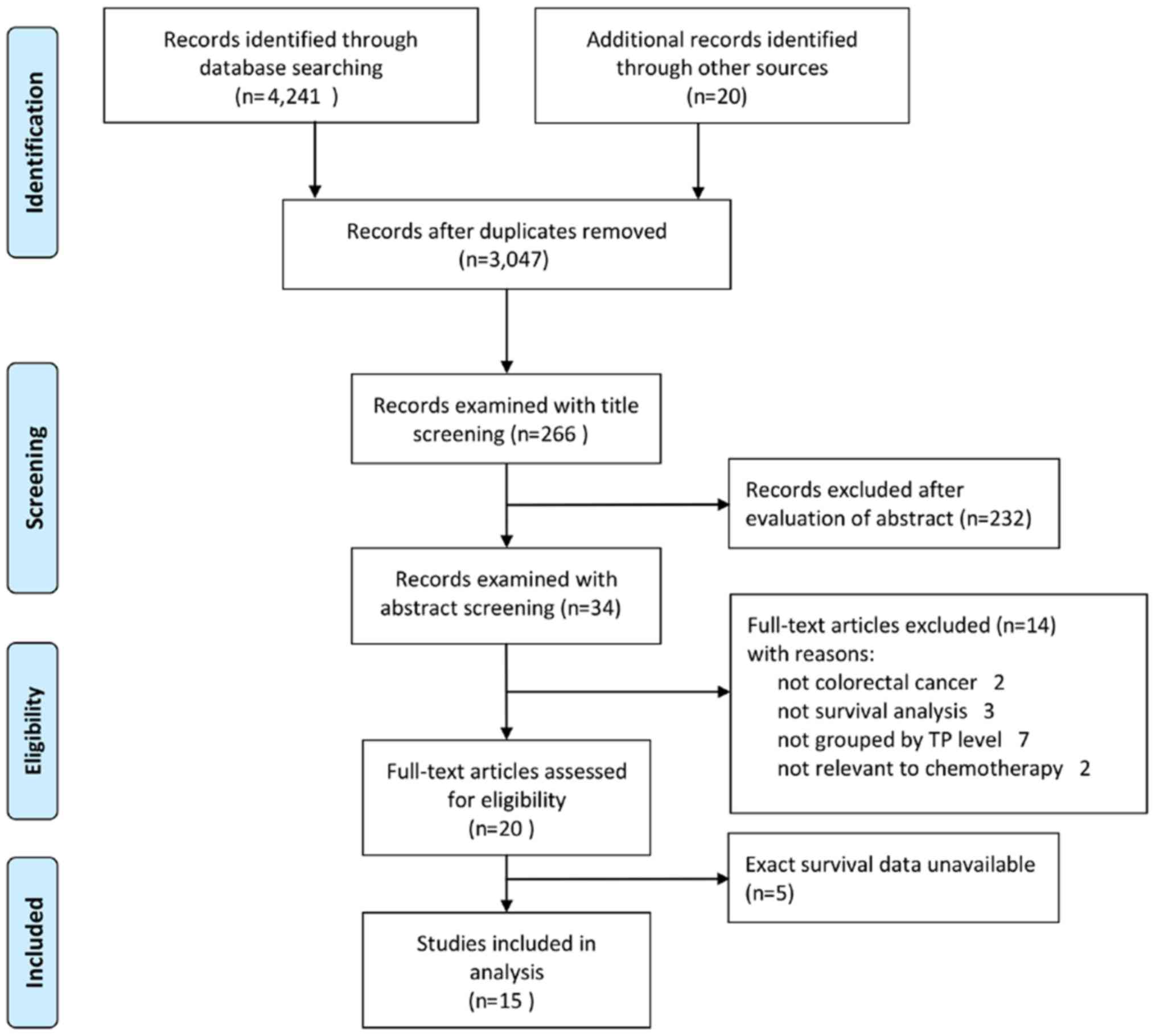
The search strategy identified 3,047 potentially
relevant articles (398 from the Web of Science, 1,609 from the
Wiley Online Library, 669 from Scopus, 237 from PubMed, 3 from Ovid
MEDLINE, 17 from the Cochrane Library, 22 from SinoMed and 73 from
CNKI). Following review of the titles, 266 of these studies were
included. Subsequently, a total of 232 studies were excluded
following abstract review. Among the 34 studies remaining, two were
not relevant to CRC (studying TP in gastric cancer and
hepatocellular carcinoma) and three were not survival analyses
(comparing the TP level in different tissue or other research).
There were seven studies in which the outcomes were not compared at
different TP levels and two studies that were not relevant to 5-FU
chemotherapy. Thus, 14 articles were excluded and 20 studies were
eligible for data extraction. However, five of these 20 studies did
not offer eligible data and it was no possible to obtain exact
survival information from these five articles. After completing the
selection process, data from a total of 15 studies (4,5,24–29,35–41)
involving 1225 patients (Fig. 1)
were systematically analyzed.
Main characteristics for individual studies were
summarized in Table I, including
nationality, study type, 5-FU-based drugs and chemotherapy
regimens. Of the 15 studies, 9 (4,24,25,27,36–39,41)
were conducted in Asia and seven were from Japan. A total of 10
(4,5,25,26,28,35,37–39,41)
were retrospective studies, one (29) was a prospective study and the other
four (24,27,35,40) were
not stated. Although the chemotherapy regimen varied in all 15
articles, they all used 5-FU-based drugs. Additionally, TP
expression for individual studies were summarized in Table II. Treatment setting was separated
into two kinds, primary and metastatic tumors, according to their
different chemotherapy regimens. Of the studies, six articles
studied primary tumors, seven studied metastatic tumors and two
studied both. In terms of follow-up period, 10 articles reported
the median follow-up period while another five articles did not.
There were 13 studies (4,5,24–29,35–38,41)
that stated the lesion tested. Among them, one study was tested on
metastatic cancer tissue, 10 on primary tissue and two on both.
There were two TP evaluation methods used among these studies,
immunohistochemistry (IHC) and quantitative polymerase chain
reaction (qPCR).
 | Table I.Summary of main characteristics for
individual studies. |
Table I.
Summary of main characteristics for
individual studies.
| Author/(Refs.),
year | Nationality | Study type | 5-FU-based
drugs | Chemotherapy
regimen | Quality score |
|---|
| Ahn et al
(24), 2005 | Korea | NS | 5-FU | FOLFIRI or
FOLFOX | 7 |
| Kataoka et
al (25), 2015 | Japan | Retrospective | 5-FU | FOLFOX +
bevacizumab or FOLFOX + cetuximab | 6 |
| Shigeta et
al (4), 2014 | Japan | Retrospective | 5-FU | 5-FU + LV or UFT +
LV | 6 |
| Ogawa et al
(35), 2014 | Japan | NS | 5-FU | S-1 | 7 |
| Donada et al
(36), 2011 | Italy | Retrospective | 5-FU | 5-FU + LV | 6 |
| Petrioli et
al (26), 2010 | Italy | Retrospective | 5-FU | 5-FU or CAP | 5 |
| Lindskog et
al (5), 2014 | Swiss | Retrospective | 5-FU | 5-FU + LV or 5-FU +
OX or MIFL or CAP or CAP + OX or CAP + IRI | 6 |
| Yamada et al
(27), 2008 | Japan | NS | 5-FU | UFT or UFT +
LV | 6 |
| Jensen et al
(28), 2008 | Denmark | Retrospective | 5-FU | Mayo | 7 |
| Yanagisawa et
al (37), 2007 | Japan | Retrospective | 5-FU | MIFL | 6 |
| Meropol et
al (29), 2006 | USA | Prospective | 5-FU | CAP + IRI | 6 |
| Ichikawa et
al (38), 2003 | Japan | Retrospective | 5-FU | UFT + LV | 7 |
| Tokunaga et
al (39), 2002 | Japan | Retrospective | 5-FU | UFT | 7 |
| Metzger et
al (40), 1998 | USA | NS | 5-FU | 5-FU/LV | 7 |
| Soong et al
(41), 2008 | Singapore | Retrospective | 5-FU | 5-FU/LV | 6 |
 | Table II.Summary of TP expression for
individual studies. |
Table II.
Summary of TP expression for
individual studies.
| Author/(Refs.),
year | Treatment
setting | Median follow-up
period, months | Association with
prognosis | Lesion tested | TP evaluation
method | Study size (no. of
patients) | Patients with high
TP level, n (%) |
|---|
| Ahn et al
(24), 2005 | Metastatic | NS | None | Primary | IHC | 45 | 22 (49) |
| Kataoka et
al (25), 2015 | Metastatic | 42.6 | None | Both | qPCR | 36 | 18 (50) |
| Shigeta et
al (4), 2014 | Primary | 66 | Good | Primary | qPCR | 101 | 71 (70) |
| Ogawa et al
(35), 2014 | Primary | 12 | Good | Primary | RT-qPCR | 54 | 27 (50) |
| Donada et al
(36), 2011 | Primary | 91.2 | None | Primary | qPCR | 55 | 27 (49) |
| Petrioli et
al (26), 2010 | Metastatic | 20.4 | Good | Metastatic | IHC | 41 | 21 (51) |
| Lindskog et
al (5), 2014 | Metastatic | 29 | Poor | Primary | qPCR | 125 | 62 (50) |
| Yamada et al
(27), 2008 | Primary | 30 | None | Primary | qPCR | 103 | 51 (50) |
| Jensen et al
(28), 2008 | Both | NS | None | Primary | IHC | 300 | 150 (50) |
| Yanagisawa et
al (37), 2007 | Primary | 15.7 | None | Primary | IHC | 13 | 5 (39) |
| Meropol et
al (29), 2006 | Metastatic | NS | Good | Both | IHC | 67 | 24 (36) |
| Ichikawa et
al (38), 2003 | Metastatic | 14 | None | Primary | qPCR | 37 | 18 (49) |
| Tokunaga et
al (39), 2002 | Both | NS | Poor | NS | IHC | 80 | 54 (68) |
| Metzger et
al (40), 1998 | Metastatic | NS | Poor | NS | RT-qPCR | 38 | 10 (26) |
| Soong et al
(41), 2008 | Primary | 52.4 | Poor | Primary | IHC | 130 | 86 (66) |
Assessment of study quality
To conduct the quality assessments for the 15
studies, the Newcastle-Ottawa Scale (NOS) was used. The NOS is
composed of eight items that assess patient selection, study,
comparability and outcome (42). A
summary of the quality assessment results is demonstrated in
Table I.
Data analysis
OS
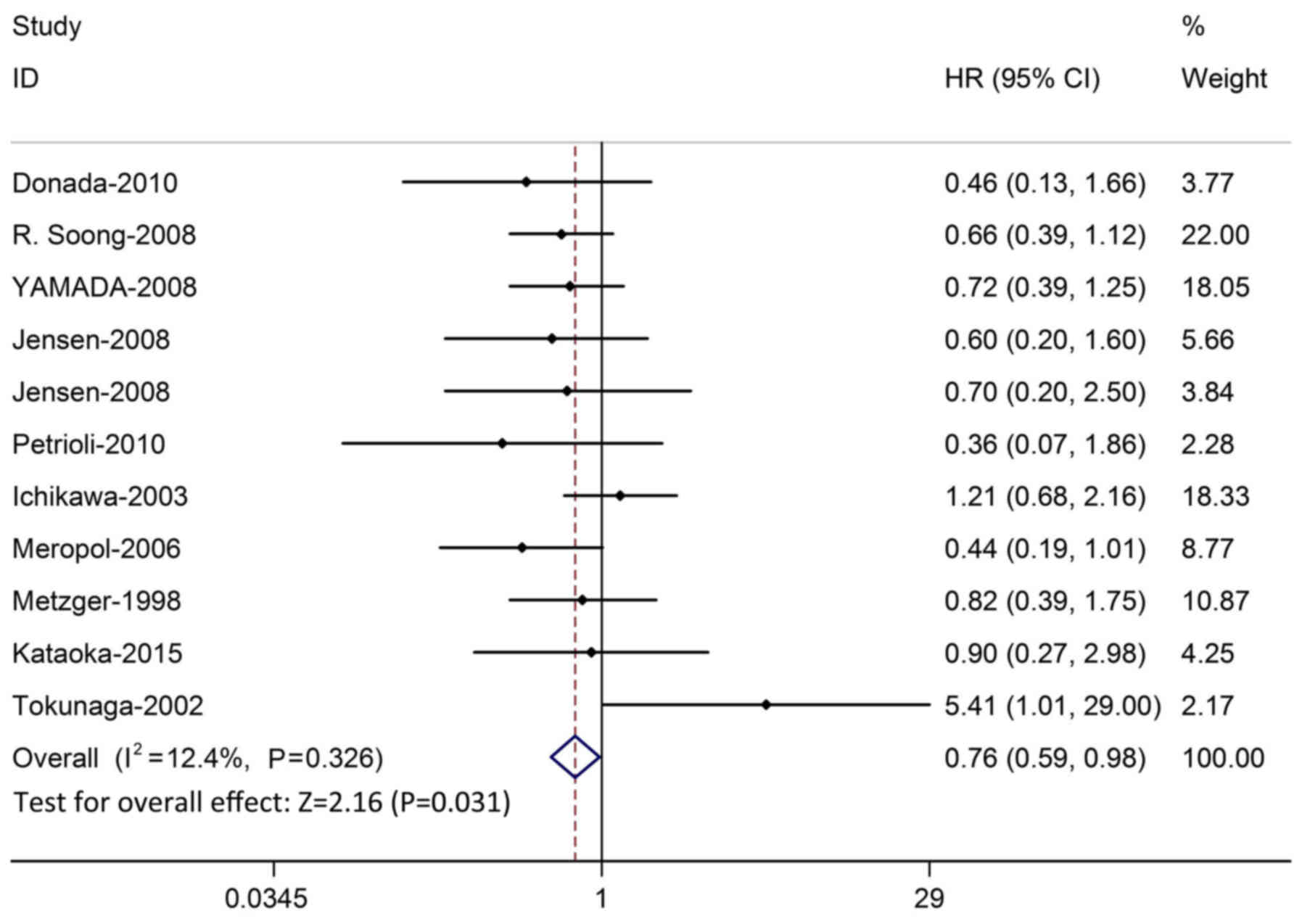
The meta-analysis was performed on 10 studies (887
patients) investigating the association between TP and OS. As the
heterogeneity test was not significant (χ2=11.42;
P=0.326; I2=12.4%), the fixed-effects model was used to
calculate the HR. The pooled HR from the 10 studies was 0.76
(P=0.031; 95% CI, 0.59–0.98; Fig.
2), which indicated that there was significant correlation
between the OS and TP in CRC patients treated with 5-FU-based
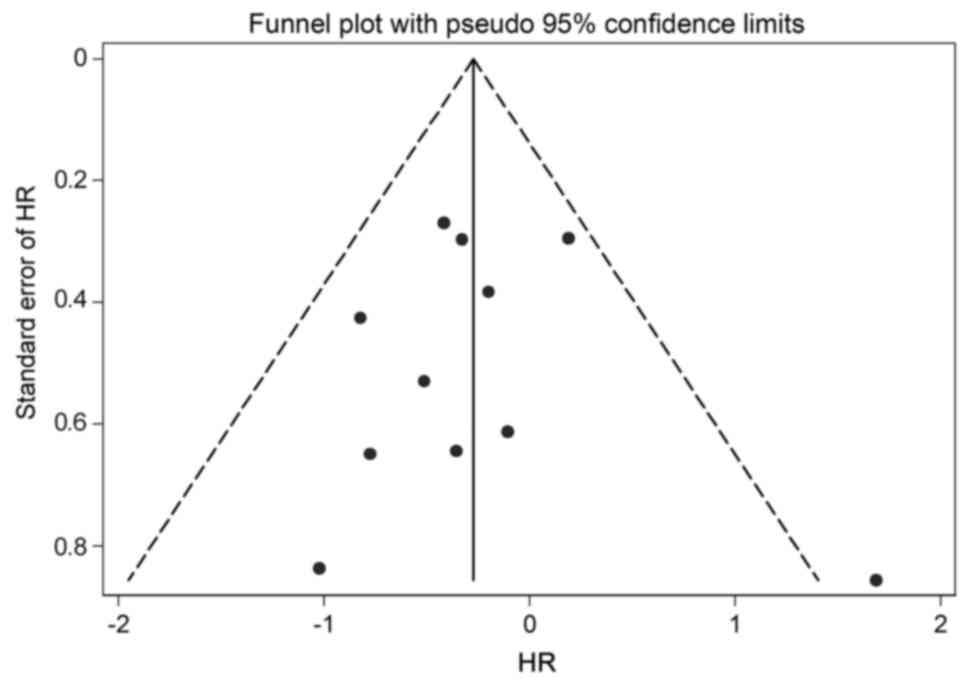
chemotherapy. The funnel plot and Egger's test demonstrated that no
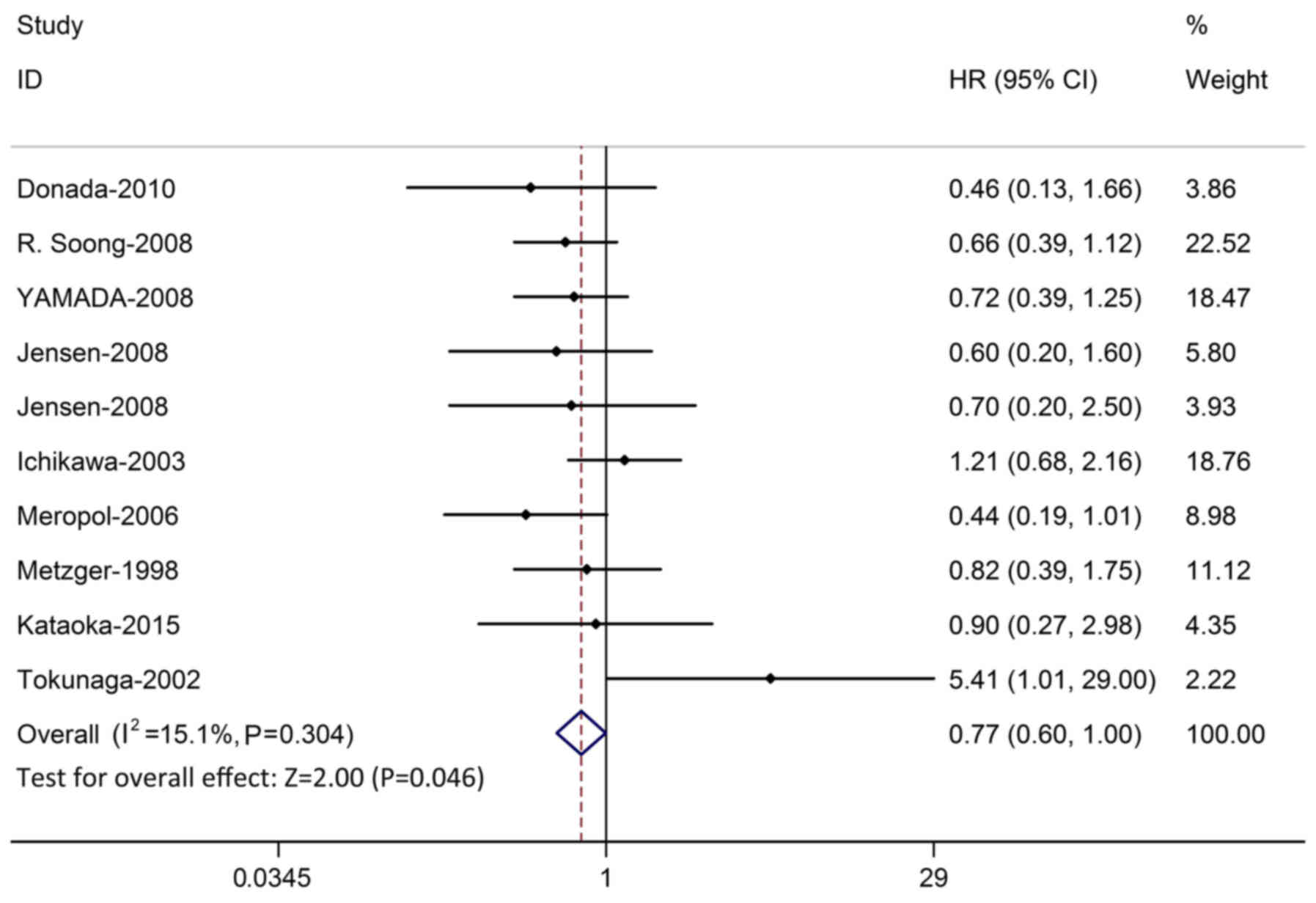
significant publication bias was detected (P=0.963; Fig. 3). In order to test the stability of
the result, the studies whose quality score was below five and was
displayed as significant were excluded. Sensitivity analysis
demonstrated that the result of OS was stable (Fig. 4).
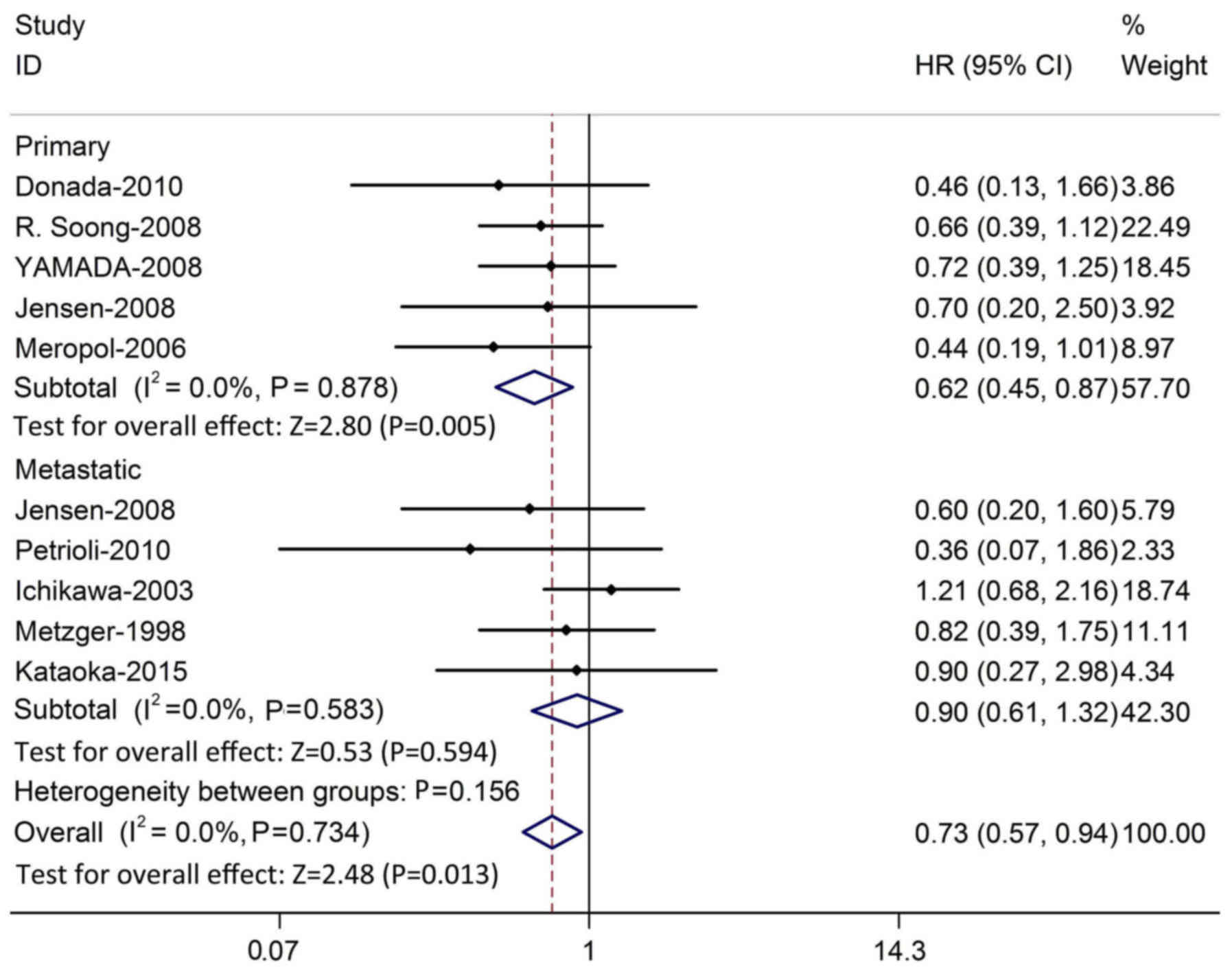
Following this, analysis was restricted to the five
studies assessing TP expression in primary tumors. The pooled HR
was 0.62 (P=0.005; 95% CI, 0.45–0.87) without evidence of study
heterogeneity (χ2=1.20; P=0.878; I2=0.0%).
Five studies assessed TP expression in metastatic tumors, and the
pooled HR was 0.90 (P=0.594; 95% CI, 0.61–1.32), without evidence
of heterogeneity (χ2=2.85; P=0.583; I2=0.0%;
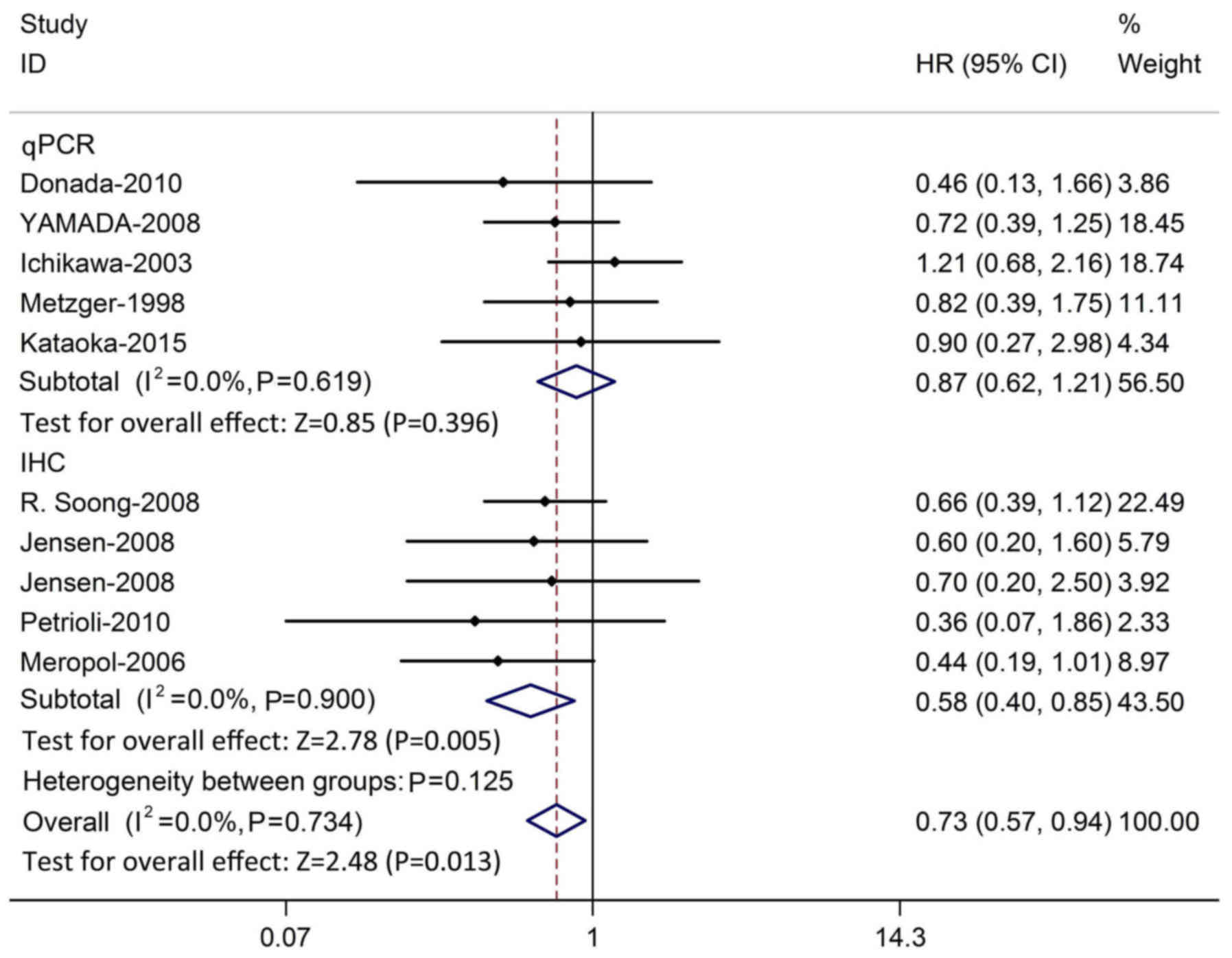
Fig. 5). To assess the effect of the
method used to evaluate TP expression, subgroup analysis was
performed based on IHC or qPCR. HR was pooled from all 10 studies
using either qPCR or IHC. A larger pooled HR was demonstrated in
studies using the qPCR method (HR=0.87; P=0.396; 95% CI,
0.62–1.21), compared with that from studies using the IHC method
(HR=0.58; P=0.022; 95% CI, 0.40–0.85). There was no evidence of
heterogeneity in qPCR-based studies (χ2=2.65; P=0.619;
I2=0.0%) or IHC-based studies (χ2=7.50;
P=0.900; I2=0.0%; Fig.
6).
ORR, PFS, DFS and RFS
Table III detailed
the meta-analysis results of ORR, PFS, DFS and RFS. Of the 15
eligible studies, five (25,32–34,36) (200
patients) reported data available for ORR, and the pooled OR was
0.822 (P=0.628; 95% CI, 0.373–1.812) with evidence of heterogeneity
(χ2=10.56; P=0.031; I2=62.4%). The Egger's
test demonstrated that no publication bias was detected (P=0.096).
There were three studies (4,29,33) for
PFS, three (26,27,30) for
DFS and two (3,31) for RFS. The pooled HRs for PFS, DFS
and RFS were 0.752 (P=0.511; 95% CI, 0.321–1.760), 1.415 (P=0.579;
95% CI, 0.416–4.816) and 0.711 (P=0.022; 95% CI, 0.531–0.951)
respectively, all without evidence of heterogeneity. The number of
studies used here is not large enough to reach a conclusion.
Therefore, more trials regarding this should be performed to fully
determine the association between TP and survival in patients with
CRC treated with 5-FU-based chemotherapy.
 | Table III.Results of meta-analysis for ORR,
PFS, DFS and RFS. |
Table III.
Results of meta-analysis for ORR,
PFS, DFS and RFS.
|
|
|
|
|
| Heterogeneity |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Index | No. of
articles | Pooled odds
ratio/hazard ratio | 95% confidence
interval | P-value | χ2 | I2 | P1 | P2 |
|---|
| ORR | 5 | 0.822 | 0.373–1.812 | 0.628 | 10.56 | 62.4% | 0.031 | 0.096 |
| PFS | 3 | 0.752 | 0.321–1.760 | 0.511 | 13.49 | 85.2% | 0.0001 | 0.361 |
| DFS | 3 | 1.415 | 0.416–4.816 | 0.579 | 5.14 | 61.1% | 0.077 | 0.586 |
| RFS | 2 | 0.711 | 0.531–0.951 | 0.022 | 0.10 | 0.00% | 0.754 | – |
Discussion
The present meta-analysis reviewed 15 eligible
articles to determine the association between TP expression and the
prognosis of patients with CRC treated with 5-FU-based
chemotherapy. The results demonstrated that higher TP expression is
correlated with better prognosis, as evaluated by OS, and may serve
as a predictor of prognosis in 5-FU-based chemotherapy for CRC.
However, this is not the case for ORR, PFS, DFS and RFS.
Furthermore, this seems to be the case for patients with primary
tumors and patients whose TP evaluation method is IHC, according to
subgroup analysis of OS. In patients with metastatic tumors and
patients whose TP evaluation method is qPCR, TP expression does not
appear to predict prognosis.
The value of high TP expression in predicting good
OS appears to be stronger in studies conducted in a primary
treatment setting than those conducted in a metastatic treatment
setting. For studies that were all in a metastatic treatment
setting and reported ORR and PFS, there was no significant
difference between TP expression and prognosis of CRC. This may
partly result from different 5-FU-based chemotherapy regimens that
primary and metastatic tumors are usually treated with. Different
drugs, such as oxaplatin, irinotecan and capecitabine, accompanied
with 5-FU regimens may cause various effects (43). For DFS and RFS, which were both
conducted in primary treatment settings, the opposite conclusion
was reached. TP expression does not appear to predict prognosis of
CRC treated with 5-FU-based chemotherapy in DFS; however, it does
predict a good prognosis in RFS, although, there were only three
studies dealing with DFS and two dealing with RFS. Therefore, these
results should be interpreted with caution considering the small
number of contributing studies.
Furthermore, it was observed that higher TP
expression may predict better prognosis in studies using IHC but
not qPCR. This may be due to the different cut-off values used to
assign TP status in the qPCR studies. Dichotomization in some of
the qPCR studies depended on median value, while others depended on
likely response. This was not the case for the IHC studies. TP
expression in the IHC studies was quantified by a visual grading
system based on the intensity of staining and classified into four
grades, from 0 (undetectable staining) to 3 (very high intensity of
staining).
As TP is an enzyme that not only participates in
5-FU metabolism, but also converts 5′-DFUR to 5-FU (21), it was hypothesized to be a potential
predictor of response. However, experimental studies also reported
that high TP expression is associated with the decreased
sensitivity of CRC to 5-FU (44) and
some clinical trials demonstrated no clinically useful correlation
between TP expression and the response to post-operative adjuvant
chemotherapy with agents such as 5-FU/leucovorin and 5′-DFUR
(28). The earlier results were
always controversial, while the results of the present
meta-analysis are consistent with the previous three articles by
Ogawa et al (35), Petrioli
et al (26) and Meropol et
al (29), which indicated a
positive correlation between high TP expression and positive
outcomes in CRC treated with 5-FU-based chemotherapy.
The results of the association between TP expression
and the prognosis of 5-FU-based chemotherapy in CRC varied among
the 15 articles chosen for analysis. Six articles by Ahn et
al (24), Yamada et al
(27), Jensen et al (28), Donada et al (36) and Ichikawa et al (38) indicated that there was no association
between the expression of TP and the prognosis of 5-FU-based
chemotherapy. Two articles by Kataoka et al (25) and Yanagisawa et al (37) identified the association but did not
express it in detail. However, the trial conducted by Shigeta et
al (4) indicated that high TP
expression was associated with a trend for improved prognosis in
RFS. There were also two articles by Lindskog et al
(5) and Tokunaga et al
(39) that indicated that high or
low TP expression was an independent poor prognostic factor, in
contrast to the present result. The apparent discrepancy may be
explained in several ways. First, sample size may be insufficient
to achieve adequate statistical power for specific biomarker end
points. The number of CRC patients participating in the trials
should be higher, so that the discrepancy between results may be
minimized. Second, the inverse association between TP expression
and the DFS and response to 5-FU may be a consequence of the role
of TP as an angiogenetic factor. TP, which is identical to
platelet-derived endothelial cell growth factor (45), and the degeneration products, thymine
and 2-deoxy-D-ribose, have angiogenic and anti-apoptotic effects
(46). Although the role of TP in
tumor proliferation is yet to be fully elucidated, TP has
angiogenetic activity and its enzymatic activity is required for
angiogenesis (47). A previous study
demonstrated that TP prevents hypoxia-induced apoptosis and that
the degradation products of thymidine are involved in this response
(48). Thus, TP expression may
provide an advantage for tumor growth in CRC by not only increasing
the intratumoral microvessel density, but also by attenuating
apoptosis (46), which suggests that
the suppression of TP may result in the inhibition of growth of
TP-positive tumors in patients with CRC.
Furthermore, the present results suggested that the
association between TP expression and prognosis of CRC is different
between primary CRC tumors and metastatic CRC tumors. It was
hypothesized that TP may be correlated with metastasis and advanced
CRC, and it was also considered that TP may induce angiogenesis in
tumor tissues. Perhaps one of the dual roles of TP, that it
participates in the metabolism of 5-FU in CRC cancer cells to
defend cancer cells, is stronger than the role of it inducing
angiogenesis. However, the mechanisms need to be further studied,
as the interactions between TP expression and other factors of
angiogenesis are not known.
The present meta-analysis has several notable
limitations. First, the cut-off line of high and low TP expression
was different across each trial, and it was not defined with a
standardized value in the present review. Second, only six trials
reported HRs and variances, and so HRs and variances had to be
calculated or converted for other trials from the reported survival
curves, which may introduce unavoidable bias. Third, the majority
of trials were retrospective trials, which may cause selective
bias. Fourth, the lesions tested were predominantly from primary
tumors, which may result in bias. Finally, 5-FU, utilized as a
first line treatment for CRC, is used as an intramuscular injection
agent, or in combination with oral drugs, such as capecitabine and
tegafur, which may influence the efficacy of 5-FU.
In spite of the above limitations, the present
meta-analysis demonstrated that higher TP expression is correlated
with better prognosis in CRC treated with 5-FU-based chemotherapy.
Additional investigation is necessary to provide more specific
information about the association between TP expression and
5-FU-based treatment for patients with CRC.
Acknowledgements
The present sudy was supported by Guangdong
Provincial Department of Science and Technology (grant no.
2014B020212016), Guangdong Innovative Research Team Program (grant
no. 2009010058), National Key Clinical Discipline and Overseas
Excellent Professor Project, Ministry of Education, China.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shigeta K, Ishii Y, Hasegawa H, Okabayashi
K and Kitagawa Y: Evaluation of 5-fluorouracil metabolic enzymes as
predictors of response to adjuvant chemotherapy outcomes in
patients with stage II/III colorectal cancer: A decision-curve
analysis. World J Surg. 38:3248–3256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lindskog EB, Derwinger K, Gustavsson B,
Falk P and Wettergren Y: Thymidine phosphorylase expression is
associated with time to progression in patients with metastatic
colorectal cancer. BMC Clin Pathol doi. 14:252014. View Article : Google Scholar
|
|
6
|
Mayer RJ: Moving beyond fluorouracil for
colorectal cancer. N Engl J Med. 343:963–964. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JJ, Beumer JH and Chu E: Therapeutic
drug monitoring of 5-fluorouracil. Cancer Chemother Pharmacol.
78:447–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maindrault-Goebel F, Louvet C, André T,
Carola E, Lotz JP, Molitor JL, Garcia ML, Gilles-Amar V, Izrael V,
Krulik M and de Gramont A: Oxaliplatin added to the simplified
bimonthly leucovorin and 5-fluorouracil regimen as second-line
therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur J
Cancer. 35:1338–1342. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Quasar Collaborative Group, . Gray R,
Barnwell J, McConkey C, Hills RK, Williams NS and Kerr DJ: Adjuvant
chemotherapy versus observation in patients with colorectal cancer:
A randomized study. Lancet. 370:2020–2029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Twelves C, Wong A, Nowacki MP, Abt M,
Burris H III, Carrato A, Cassidy J, Cervantes A, Fagerberg J,
Georgoulias V, et al: Capecitabine as adjuvant treatment for stage
III colon cancer. N Engl J Med. 352:2696–2704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lembersky BC, Wieand HS, Petrelli NJ,
O'Connell MJ, Colangelo LH, Smith RE, Seay TE, Giguere JK, Marshall
ME, Jacobs AD, et al: Oral uracil and tegafur plus leucovorin
compared with intravenous fluorouracil and leucovorin in stage II
and III carcinoma of the colon: Results from National Surgical
Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol.
24:2059–2064. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Labianca R, Nordlinger B, Beretta GD,
Brouquet A and Cervantes A: ESMO Guidelines Working Group: Primary
colon cancer: ESMO Clinical Practice Guidelines for diagnosis,
adjuvant treatment and follow-up. Ann Oncol. 21 Suppl 5:v70–v77.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deboever G, Hiltrop N, Cool M and
Lambrecht G: Alternative treatment options in colorectal cancer
patients with 5-fluorouracil- or capecitabine-induced
cardiotoxicity. Clin Colorectal Cancer. 12:8–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bronckaers A, Gago F, Balzarini J and
Liekens S: The dual role of thymidine phosphorylase in cancer
development and chemotherapy. Med Res Rev. 29:903–953. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Brien TS, Fox SB, Dickinson AJ, Turley
H, Westwood M, Moghaddam A, Gatter KC, Bicknell R and Harris AL:
Expression of the angiogenic factor thymidine
phosphorylase/platelet-derived endothelial cell growth factor in
primary bladder cancers. Cancer Res. 56:4799–4804. 1996.PubMed/NCBI
|
|
17
|
Miwa M, Ura M, Nishida M, Sawada N,
Ishikawa T, Mori K, Shimma N, Umeda I and Ishitsuka H: Design of a
novel oral fluoropyrimidine carbamate, capecitabine, which
generates 5-fluorouracil selectively in tumours by enzymes
concentrated in human liver and cancer tissue. Eur J Cancer.
34:1274–1281. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takebayashi Y, Yamada K, Miyadera K,
Sumizawa T, Furukawa T, Kinoshita F, Aoki D, Okumura H, Yamada Y,
Akiyama S and Aikou T: The activity and expression of thymidine
phosphorylase in human solid tumours. Eur J Cancer. 32A:1227–1232.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nozawa T, Enomoto T, Koshida Y, Sato Y and
Kuranami M: Specific enhanced expression of platelet-derived
endothelial cell growth factor in submucosa of human colorectal
cancer. Dis Colon Rectum. 47:2093–2100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amatori F, Di Paolo A, Del Tacca M,
Fontanini G, Vannozzi F, Boldrini L, Bocci G, Lastella M and Danesi
R: Thymidylate synthase, dihydropyrimidine dehydrogenase and
thymidine phosphorylase expression in colorectal cancer and normal
mucosa in patients. Pharmacogenet Genomics. 16:809–816. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Bruin M, van Capel T, Van der Born K,
Kruyt FA, Fukushima M, Hoekman K, Pinedo HM and Peters GJ: Role of
platelet-derived endothelial cell growth factor/thymidine
phosphorylase in fluoropyrimidine sensitivity. Br J Cancer.
88:957–64. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moghaddam A, Zhang HT, Fan TP, Hu DE, Lees
VC, Turley H, Fox SB, Gatter KC, Harris AL and Bicknell R:
Thymidine phosphorylase is angiogenic and promotes tumor growth.
Proc Natl Acad Sci USA. 92:pp. 998–1002. 1995, View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takebayashi Y, Akiyama S, Akiba S, Yamada
K, Miyadera K, Sumizawa T, Yamada Y, Murata F and Aikou T:
Clinicopathologic and prognostic significance of an angiogenic
factor, thymidine phosphorylase, in human colorectal carcinoma. J
Natl Cancer Inst. 88:1110–1117. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahn MJ, Choi JH, Oh HS, Lee YY, Kim IS,
Choi IY, Lee KH, Song KW and Park CK: Thymidylate synthase,
thymidine phosphorylase, VEGF and p53 protein expression in primary
colorectal cancer for predicting response to 5-fluorouracil-based
chemotherapy. Cancer Res Treat. 37:216–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kataoka K, Kanazawa A, Nakajima A,
Yamaguchi A and Arimoto A: Prognostic value of biomarkers in
metastatic colorectal cancer patients. J Surg Res. 194:343–350.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Petrioli R, Bargagli G, Lazzi S, Pascucci
A, Francini E, Bellan C, Conca R, Martellucci I, Fiaschi AI,
Lorenzi B, et al: Thymidine phosphorylase expression in metastatic
sites is predictive for response in patients with colorectal cancer
treated with continuous oral capecitabine and biweekly oxaliplatin.
Anticancer Drugs. 21:313–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamada H, Iinuma H and Watanabe T:
Prognostic value of 5-fluorouracil metabolic enzyme genes in Dukes'
stage B and C colorectal cancer patients treated with oral
5-fluorouracil-based adjuvant chemotherapy. Oncol Rep. 19:729–735.
2008.PubMed/NCBI
|
|
28
|
Jensen SA, Vainer B, Witton CJ, Jørgensen
JT and Sørensen JB: Prognostic significance of numeric aberrations
of genes for thymidylate synthase, thymidine phosphorylase and
dihydrofolate reductase in colorectal cancer. Acta Oncol.
47:1054–1061. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meropol NJ, Gold PJ, Diasio RB, Andria M,
Dhami M, Godfrey T, Kovatich AJ, Lund KA, Mitchell E and Schwarting
R: Thymidine phosphorylase expression is associated with response
to capecitabine plus irinotecan in patients with metastatic
colorectal cancer. J Clin Oncol. 24:4069–4077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duffaud F and Therasse P: New guidelines
to evaluate the response to treatment in solid tumors. Bull Cancer.
87:881–886. 2000.(In French). PubMed/NCBI
|
|
33
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Egger M, Smith Davey G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ogawa M, Watanabe M, Mitsuyama Y, Anan T,
Ohkuma M, Kobayashi T, Eto K and Yanaga K: Thymidine phosphorylase
mRNA expression may be a predictor of response to post-operative
adjuvant chemotherapy with S-1 in patients with stage III
colorectal cancer. Oncol Lett. 8:2463–2468. 2014.PubMed/NCBI
|
|
36
|
Donada M, Bonin S, Nardon E, De Pellegrin
A, Decorti G and Stanta G: Thymidilate synthase expression predicts
longer survival in patients with stage II colon cancer treated with
5-flurouracil independently of microsatellite instability. J Cancer
Res Clin Oncol. 137:201–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yanagisawa Y, Maruta F, Iinuma N, Ishizone
S, Koide N, Nakayama J and Miyagawa S: Modified
Irinotecan/5FU/Leucovorin therapy in advanced colorectal cancer and
predicting therapeutic efficacy by expression of tumor-related
enzymes. Scand J Gastroenterol. 42:477–484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ichikawa W, Uetake H, Shirota Y, Yamada H,
Takahashi T, Nihei Z, Sugihara K, Sasaki Y and Hirayama R: Both
gene expression for orotate phosphoribosyltransferase and its ratio
to dihydropyrimidine dehydrogenase influence outcome following
fluoropyrimidine-based chemotherapy for metastatic colorectal
cancer. Br J Cancer. 89:1486–1492. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tokunaga Y, Hosogi H, Hoppou T, Nakagami
M, Tokuka A and Ohsumi K: Prognostic value of thymidine
phosphorylase/platelet-derived endothelial cell growth factor in
advanced colorectal cancer after surgery: Evaluation with a new
monoclonal antibody. Surgery. 131:541–547. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Metzger R, Danenberg K, Leichman CG,
Salonga D, Schwartz EL, Wadler S, Lenz HJ, Groshen S, Leichman L
and Danenberg PV: High basal level gene expression of thymidine
phosphorylase (platelet-derived endothelial cell growth factor) in
colorectal tumors is associated with nonresponse to 5-fluorouracil.
Clin Cancer Res. 4:2371–2376. 1998.PubMed/NCBI
|
|
41
|
Soong R, Shah N, Salto-Tellez M, Tai BC,
Soo RA, Han HC, Ng SS, Tan WL, Zeps N, Joseph D, et al: Prognostic
significance of thymidylate synthase, dihydropyrimidine
dehydrogenase and thymidine phosphorylase protein expression in
colorectal cancer patients treated with or without
5-fluorouracil-based chemotherapy. Ann Oncol. 19:915–919. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Salonga D, Danenberg KD, Johnson M,
Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman
L, Diasio RB and Danenberg PV: Colorectal tumors responding to
5-fluorouracil have low gene expression levels of dihydropyrimidine
dehydrogenase, thymidylate synthase, and thymidine phosphorylase.
Clin Cancer Res. 6:1322–1327. 2000.PubMed/NCBI
|
|
45
|
Nishimura G, Terada I, Kobayashi T,
Ninomiya I, Kitagawa H, Fushida S, Fujimura T, Kayahara M, Shimizu
K, Ohta T and Miwa K: Thymidine phosphorylase and dihydropyrimidine
dehydrogenase levels in primary colorectal cancer show a
relationship to clinical effects of 5′-deoxy-5-fluorouridine as
adjuvant chemotherapy. Oncol Rep. 9:479–482. 2002.PubMed/NCBI
|
|
46
|
Matsuura T, Kuratate I, Teramachi K, Osaki
M, Fukuda Y and Ito H: Thymidine phosphorylase expression is
associated with both increase of intratumoral microvessels and
decrease of apoptosis in human colorectal carcinomas. Cancer Res.
59:5037–5040. 1999.PubMed/NCBI
|
|
47
|
Elamin YY, Rafee S, Osman NO, Byrne KJ and
Gately K: Thymidine phosphorylase in cancer; enemy or friend?
Cancer Microenviron. 9:33–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bijnsdorp IV, Azijli K, Jansen EE,
Wamelink MM, Jakobs C, Struys EA, Fukushima M, Kruyt FA and Peters
GJ: Accumulation of thymidine-derived sugars in thymidine
phosphorylase overexpressing cells. Biochem Pharmacol. 80:786–792.
2010. View Article : Google Scholar : PubMed/NCBI
|