Introduction
Primary mediastinal sarcomas are rare (1). Similar to other primary sarcomas,
achieving long-term survival often requires multimodal therapy
including complete local resection, chemotherapy, and radiation
(2). Accomplishing complete
resection of anterior mediastinal sarcomas poses challenges given
proximity to critical mediastinal structures such as the phrenic
nerve, heart, and great vessels. Accurate tumor identification and
margin assessment during mediastinal dissection is further
complicated when occurring after neoadjvant chemotherapy, an
approach commonly implemented for sarcomas.
Our group has previously reported successful
utilization of near-infrared (NIR) fluorescent, real-time
intraoperative imaging to improve pulmonary metastasectomy
(3). In this report, we detail
successful utilization of this approach to enhance accurate
identification of malignancy during resection of a thymic
carcinosarcoma in a patient that previously underwent neoadjuvant
chemotherapy. This report highlights how this approach can improve
a surgeon's ability to identify disease and safely obtain adequate
margins during resection of primary mediastinal sarcomas and,
perhaps more broadly, other solid tumors located near critical
structures.
Case report
A 59-year-old male was seen in our
multi-disciplinary thoracic oncology institute for management of an
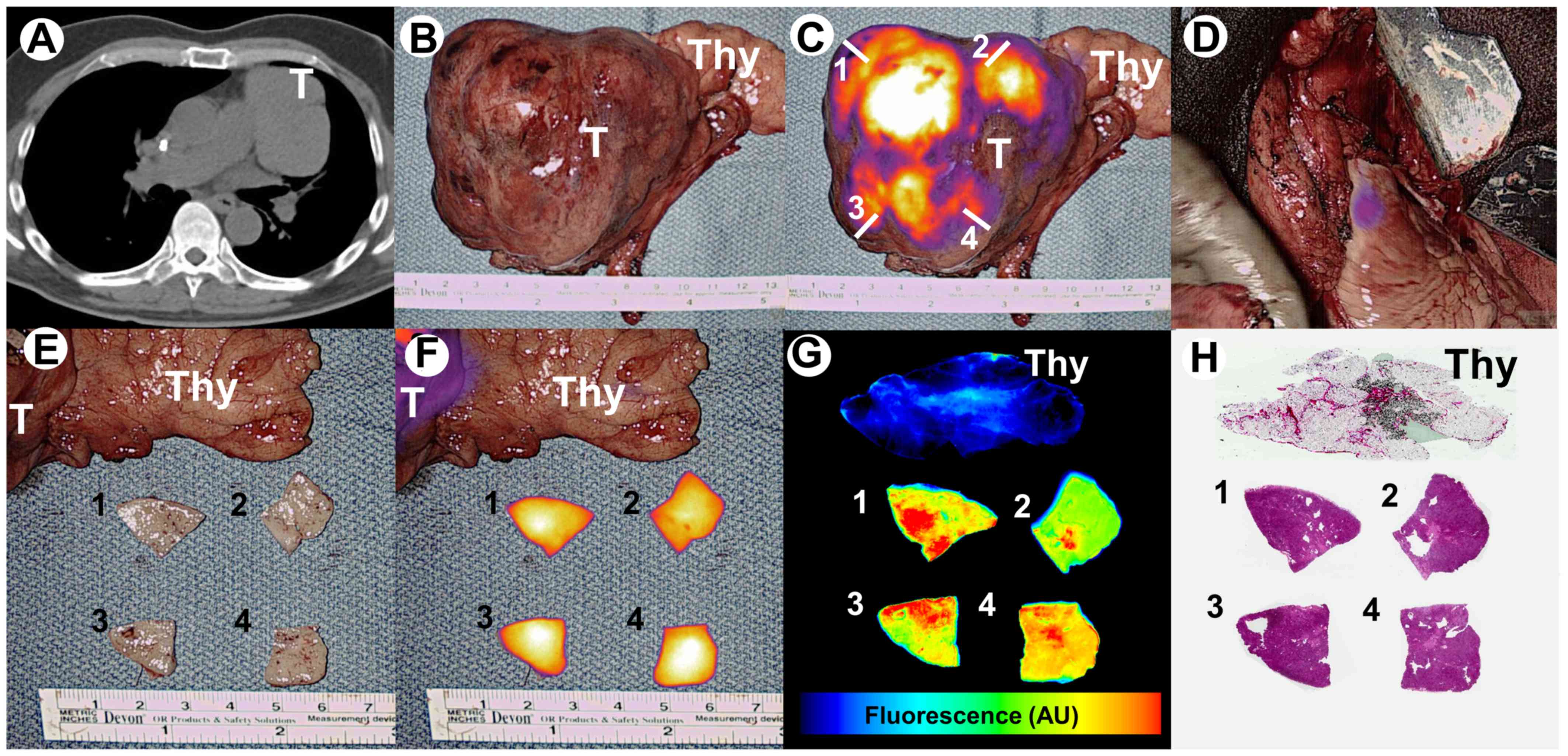
incidentally identified 5.7×7.5×8.4 cm anterior mediastinal mass
(Fig. 1A). This mass was abutting
the left pulmonary artery without obvious vascular invasion.
Metastatic work-up revealed no suspicious metastases. A
transthoracic needle biopsy was suspicious for a thymic
carcinosarcoma with rhabdomyosarcomatous elements. Six rounds of
neoadjuvant chemotherapy with Adriamycin, Ifosfosfamide and
Vinicristine. After completing neoadjuvant treatment, the patient
was consented for resection via left thoracosternotomy with NIR
intraoperative imaging.
Twenty-four hours before resection, intravenous
indocyanine-green (ICG) was delivered (5 mg/kg). During resection,
the tumor displayed high levels of fluorescence
(tumor-to-background signal ratio of 3.6) (Fig. 1B and C). Real-time fluorescent
feedback aided the surgeon when during dissection from critical
mediastinal structures including the phrenic nerve, pulmonary
artery and aorta. After resection, negligible residual fluorescence
(tumor-to-background signal ratio of 1.2) was appreciated within
the operating field which increased the surgeon's confidence of
complete resection (Fig. 1D).
On backtable evaluation, tumor samples were obtained
from 4 quadrants (Fig. 1C and E) and
fluorescence was evaluated using a macroscopic NIR fluorescence
system (Visionsense®, Philadelphia, PA) (Fig. 1F). Specimen were then evaluated using
a microscopic NIR fluorescent imaging system (LiCor Odyssey,
Lincoln, NE) and by H&E staining (Fig. 1G and H). Margins were clear thus
confirming findings of intraoperative fluorescent imaging. Of note,
utilization of intraoperative imaging added 11 min to the case
duration.
Histopathologic analysis revealed a biphasic tumor,
composed of spindle and rhabdoid morphology cells along with
epithelioid cells in other regions. Upon immunohistochemical
analysis, spindle and rhabdoid cells stained positive for desmin,
myogenin, smooth muscle actin, HHF-35, and INI-1; patchy weak
positivity for S100 and MelanA was also noted. Epithelioid cells
were noted to be positive for AE1/3, CAM5.2, CK5/6, and p40; weak
positivity for PAX8 was also found. Overall, this morphology and
immunohistochemical staining pattern was consistent with thymic
carcinosarcoma with rhabdomyosarcomatous elements.
The subject recovered uneventfully without drug
toxicity and remains disease free after 1 year of follow-up.
Discussion
In this report, we provide preliminary evidence
suggesting safety and feasibility of NIR intraoperative imaging
with ICG for anterior mediastinal sarcomas after neoadjuvant
therapy. Real-time fluorescent feedback provides additional visual
information that can help the surgeon during tumor localization,
margin assessment and dissection from nearby vital structures.
ICG has traditionally been utilized by clinicians to
assess perfusion and determine vascularity (4). Our group has recently described ICG as
a tumor mapping agent (3). For tumor
mapping, ICG is delivered at a dose of 5 mg/kg with imaging
occurring 24 h later. Under these dosing parameters, ICG functions
by exploiting abnormally leaky capillaries and increased pressure
gradients (the Enhanced Permeability and Retention Effect) which
are found in most solid malignancies, including sarcomas (5). In this report, we found optical
properties of ICG to be excellent for intrathoracic imaging. One
reconcilable drawback, however, was persistent low levels of
background fluorescence from the aorta, which is likely due to low
levels of circulating albumin bound ICG. Nevertheless, accurate
tumor identification was feasible given significantly higher levels
of tumor-specific signal.
Although additional studies are needed, these
initial findings remain encouraging. First, delivery of this dosage
of ICG was well tolerated, which is consistent with our previous
experiences with ICG (3). Second, we
found that the addition of intraoperative imaging was efficient and
addended only several minutes to the case duration. Third, reliable
real-time fluorescent feedback (100% accuracy) assisted the surgeon
during dissection from critical mediastinal structures and
suggested complete resection. Lastly, we found high levels of
fluorescence after neoadjuvant chemotherapy, an approach that is
applied to a variety of solid tumors, including sarcomas. Further
investigation of this approach in patients with primary sarcomas
and other mediastinal neoplasms, such as ectopic parathyroid
adenomas and thymoma, is ongoing (NCT02280954). In the future, this
technology may be a reliable tool to enhance a surgeon's ability to
perform a variety of oncologic procedures including tumor
localization, margin assessment and intraoperative staging.
Acknowledgements
JDP was supported by a grant from the American
Philosophical Society, the NIH (F32 CA210409) and the Association
for Academic Surgery Research Grant. SS was supported by the NIH
(R01 CA193556).
References
|
1
|
Gladish GW, Sabloff BM, Munden RF, Truong
MT, Erasmus JJ and Chasen MH: Primary thoracic sarcomas.
Radiographics. 22:621–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gutowski CJ, Basu-Mallick A and Abraham
JA: Management of bone sarcoma. Surg Clin North Am. 96:1077–1106.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keating J, Newton A, Venegas O, Nims S,
Zeh R, Predina J, Deshpande C, Kucharczuk J, Nie S, Delikatny EJ
and Singhal S: Near-infrared intraoperative molecular imaging can
locate metastases to the lung. Ann Thorac Surg. 103:390–398. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boni L, David G, Mangano A, Dionigi G,
Rausei S, Spampatti S, Cassinotti E and Fingerhut A: Clinical
applications of indocyanine green (ICG) enhanced fluorescence in
laparoscopic surgery. Surg Endosc. 29:2046–2055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang JX, Keating JJ, Jesus EM, Judy RP,
Madajewski B, Venegas O, Okusanya OT and Singhal S: Optimization of
the enhanced permeability and retention effect for near-infrared
imaging of solid tumors with indocyanine green. Am J Nucl Med Mol
Imaging. 5:390–400. 2015.PubMed/NCBI
|