Introduction
Recurrence rates for cervical cancer are 11–22% in
FIGO stage IB-IIA and 28–64% in FIGO stage IIB-IVA (1). Treatment for patients who suffer
recurrent cervical cancer is designed to offer the patient and her
family medical, emotional, and spiritual care near the end of life.
Predicting these patients' life expectancy is thus important for
clinicians and patients. Realistic survival estimates help
clinicians decide on appropriate medical interventions, discharge
planning and timing of referral to palliative care services.
Systemic inflammatory responses (SIRs), such as
relative differences in neutrophil, platelet, lymphocyte counts,
and albumin, neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte
ratio (PLR), and prognostic nutritional index (PNI), have been
shown to promote various cancer characteristics (2,3) and to
affect survival (4–7). PLR has been shown to predict prognosis
in patients with recurrent cervical cancer after undergoing CCRT
(8). Although some data on survival
outcomes for patients whose cervical cancers recur after undergoing
CCRT has been published (8),
patients treated only with surgery or surgery plus adjuvant
treatment have not been sufficiently investigated. In this study,
we investigated the correlation between inflammatory markers (NLR,
PLR and PNI) of patients with recurrent cervical cancers.
Patients and methods
Patients
The study population consisted of 79 patients whose
cervical cancer recurred after undergoing concurrent chemoradiation
therapy (CCRT), or radical hysterectomy with or without CCRT in the
Department of Obstetrics and Gynecology of our hospital between
April 2004 and December 2015. A total of 79 women underwent
positron emission tomography/computed tomography (PET/CT) or CT
within the 2 weeks prior to any oncologic treatment. The images
were evaluated by 2 diagnostic radiology physicians informed about
the clinical data of the patient at the time of the scan.
The study protocol was approved by our hospital's
institutional review board [Systemic inflammatory responses were
examined prognostic predictor of survival in patients with
recurrent cervical cancer (Number: 1605-514)]. Informed consent was
obtained from all patients.
Laboratory analysis
Differential white blood cell (WBC) counts and
albumin levels were measured within 1 week before their treatments;
WBC, neutrophil, lymphocyte, and platelet counts were measured in
automated blood cell counters (Bayer HealthCare, Diagnostics
Division, Tarrytown, NY, USA). Levels of serum albumin were
measured by latex nephelometry (LT Auto Wako, Osaka, Japan). NLR
was defined as the absolute neutrophil count (µl) divided by the
absolute lymphocyte count (µl); PLR was defined as the absolute
platelet count (µl) divided by the lymphocyte count (µl). PNI was
calculated as described previously (9); briefly, PNI=[10× albumin
(g/dl)]+[0.005× total lymphocyte count (µl)].
Treatment
Patients with recurrent cervical cancer had several
possible treatments. In general, radiotherapy is the main option
for patients with pelvic recurrence, or with solitary localized
recurrence outside the radiation field, after previous
radiotherapy. Chemotherapy is the main option for patients with
recurrence within the radiation field, or metastases to multiple
organs, following previous radiotherapy. Conventional TC was also
administered as second-line chemotherapy to patients that developed
evidence of clinical or radiographic relapse within the 6 months
subsequent to completing adjuvant and/or neo-adjuvant chemotherapy.
Chemotherapy for the treatment of recurrent disease was continued
until complete response (CR) or progressive disease (PD) was
identified. Patients with PD received regimens of chemotherapy that
were different from the adjuvant and second-line combinations. We
used second-line chemotherapy, as weekly TC-paclitaxel; 80
mg/m2 and carboplatin (area under the
plasma-concentration curve [AUC]: 2). Third-line chemotherapy
consisted of single-agent irinotecan (CPT-11; 70 mg/m2
weekly for 3 weeks followed by 1 week off); fourth-line
chemotherapy was single-agent gemcitabine (GEM; 700
mg/m2 weekly for 3 weeks followed by 1 week off).
Surgery was considered for solitary distant metastases or local
recurrences. Palliative treatment was considered in some cases
after comprehensive assessment of the effectiveness of radiotherapy
and/or chemotherapy, patients' performance status, and degree of
cancer spread. We evaluated 13 patients treated with surgery, 14
with radiation, 48 with chemotherapy, and 4 with palliative care.
Patients had follow-up examinations approximately every 1–2
months.
Statistical analysis
Statistical analyses used the Mann-Whitney U-test
for comparisons with controls (10).
Receiver operating characteristic (ROC) curves were generated for
pre-treatment NLR, PLR, and PNI to determine cut-off values that
predicted 12-month, 24-months and overall survival that yielded
optimal sensitivity and specificity; patients were then grouped by
these cut-off values. 12-months, 24-months and overall survival of
the groups were analyzed using the Kaplan-Meier method (11). We performed univariate and
multivariate analyses using Cox's proportional hazards model to
determine which factors predicted 12-months, 24-months and OS after
adjusting for effects of known prognostic factors (12,13).
Analyses were performed using SPSS Software, version 20.0 (SPSS
Inc., Chicago, IL, USA). P<0.05 was considered significant.
Results
Patients' ages, histology, treatment-free interval,
number of metastases, distant metastasis and treatment are listed
in Table I. Their median SIR values
were NLR: 3.52 (range: 1.28–14.30); PLR: 271.52 (range:
114.30–885.99); and PNI: 45.30 (range: 26.26–56.55; Table II). NLR (P=0.003) and PLR
(P<0.001) were significantly associated with treatment type. PNI
was significantly associated with number of metastasis (P=0.022),
hematogenous metastasis (P=0.027) and treatment type (P<0.001;
Mann-Whitney U-test).
 | Table I.Patient and tumor characteristics. |
Table I.
Patient and tumor characteristics.
| Baseline
characteristics Age at diagnosis, years | Numbers | All patients Mean,
52.4; range, 25–78 (%) |
|---|
| Histology |
|
|
| SCC | 50 | 63.3 |
| AD | 18 | 22.7 |
| ADSQ | 7 | 8.9 |
|
Others | 4 | 5.1 |
| Treatment free
intervial |
|
|
| ≤6
months | 33 | 41.8 |
| 7-12
months | 24 | 30.4 |
| 13-24
months | 11 | 13.9 |
| >25
months | 11 | 13.9 |
| Number of
metastasis |
|
|
|
Simple | 44 | 55.7 |
|
Multiple | 35 | 44.3 |
| Distant
metastasis |
|
|
|
Hematogenous metastasis | 29 | 36.7 |
|
Lymphogenous metastasis | 34 | 43 |
| Treatment |
|
|
|
Operation | 13 | 16.4 |
|
Radiation | 14 | 17.7 |
|
Chemotherapy | 48 | 60.8 |
|
Palliative care | 4 | 5.1 |
 | Table II.Associations of NLR, PLR, and PNI with
clinical factors in recurrent cervical cancer. |
Table II.
Associations of NLR, PLR, and PNI with
clinical factors in recurrent cervical cancer.
| Variable | Numbers | NLR | P-value | PLR | P-value | PNI | P-value |
|---|
| Histology |
|
| 0.564 |
| 0.408 |
| 0.076 |
| SCC | 50 | 3.86±2.76 |
| 283.05±124.66 |
| 44.85±6.29 |
|
|
Non-SCC | 29 | 3.45±3.46 |
| 247.45±209.18 |
| 47.27±4.7.53 |
|
| Treatment free
interval |
|
| 0.541 |
| 0.422 |
| 0.124 |
| ≤6
months | 34 | 3.88±3.29 |
| 293.28±154.56 |
| 44.98±6.95 |
|
| >7
months | 45 | 3.46±2.79 |
| 263.97±164.01 |
| 47.35±6.53 |
|
| Number of
metastasis |
|
| 0.472 |
| 0.706 |
| 0.022a |
|
Simple | 44 | 3.46±1.98 |
| 267.39±130.4 |
| 47.13±6.34 |
|
|
Multiple | 35 | 3.97±3.77 |
| 281.45±186.92 |
| 43.61±7.11 |
|
| Hematogenous
metastasis |
|
| 0.564 |
| 0.915 |
| 0.027a |
|
Absent | 50 | 3.49±2.97 |
| 273.37±147.79 |
| 46.98±6.70 |
|
|
Present | 29 | 3.90±3.14 |
| 277.37±181.31 |
| 43.43±6.93 |
|
| Lymphogenous
metastasis |
|
| 0.282 |
| 0.634 |
| 0.34 |
|
Absent | 45 | 3.90±2.87 |
| 287.451±169.91 |
| 46.76±6.86 |
|
|
Present | 34 | 3.15±3.28 |
| 270.01±147.18 |
| 45.28±6.69 |
|
| Treatment |
|
| 0.003a |
|
<0.001a |
|
<0.001a |
| Operation
or radiation | 27 | 2.53±1.90 |
| 196.34±114.55 |
| 48.61±4.98 |
|
|
Chemotherapy or palliative
cares | 52 | 4.28±3.20 |
| 319.25±167.62 |
| 42.45±6.98 |
|
Patients with recurrent cervical cancer had median
OS of 15.0 months over follow-up periods of 2–93 months. At the
last follow-up point, 54 had died of their disease, and 25 were
alive with disease. The time between diagnosis and mortality was ≤6
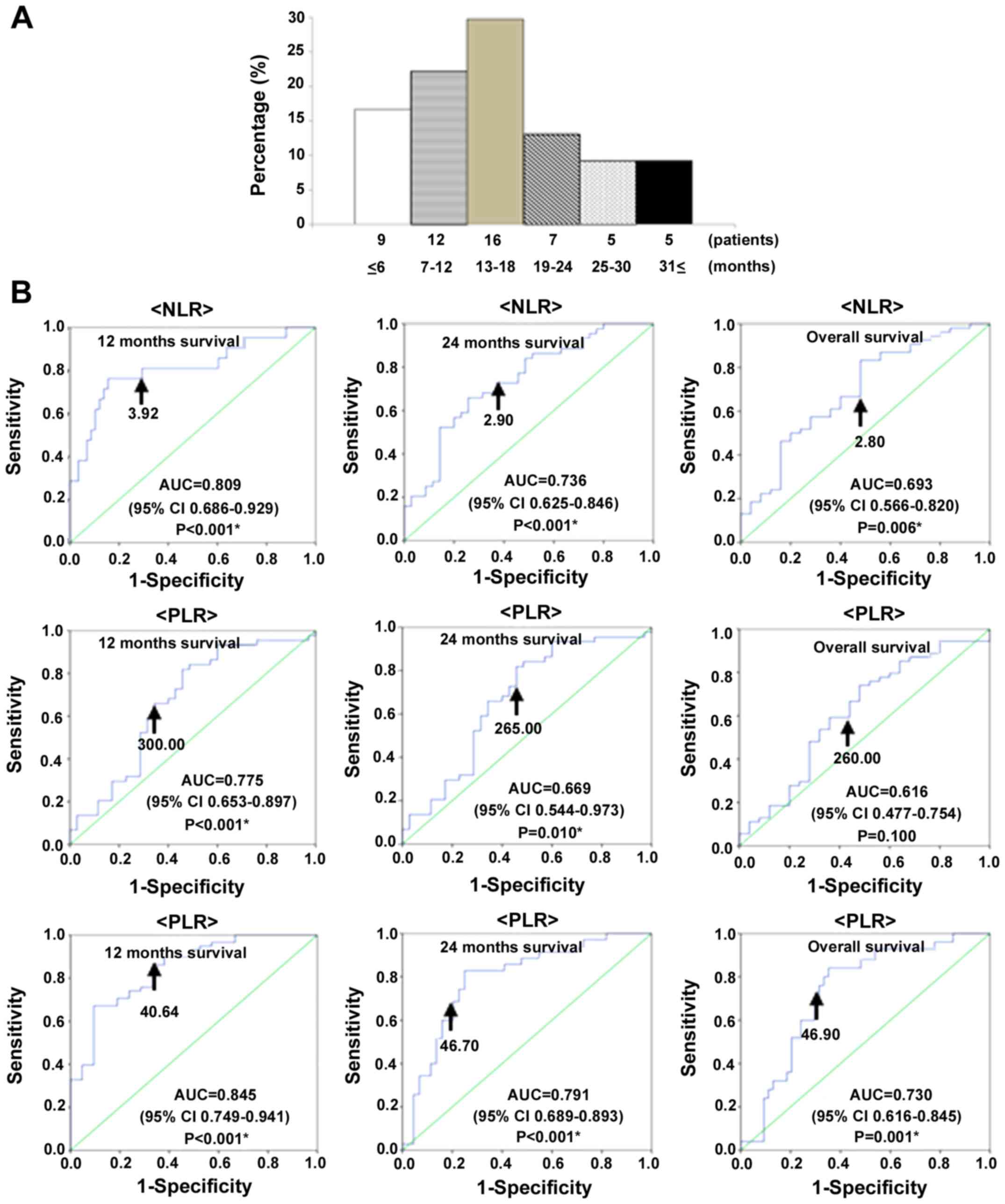
months in 9 patients (16.7%), 7–12 months in 12 patients (22.2%),
13–18 months in 16 patients (29.7%), 19–24 months in 7 patients
(13.0%), 19–24 months in 5 patients (9.2%), and ≥31 months in 5
patients (9.2%; Fig. 1A).
We used receiver operating characteristic (ROC)
curve analyses to determine optimal cut-off values of NLR, PLR, and
PNI to predict 12-month, 24-month and overall survival. The
analyses identified NLR ≥3.92 (AUC: 0.809, 81.0% sensitive, 56.1%
specific), PLR ≥300.00 (AUC: 0.775, 76.2% sensitive, 70.7%
specific), and PNI ≤40.64 (AUC: 0.845, 84.5% sensitive, 66.7%
specific), as the most accurate cut-off values for predicting
12-month survival; NLR ≥2.90 (AUC: 0.736, 72.7% sensitive, 57.1%
specific), PLR ≥265.00 (AUC: 0.669, 65.9% sensitive, 60.0%
specific), and PNI ≤46.70 (AUC: 0.791, 80.0% sensitive, 75.0%
specific), as the most accurate cutoff values for predicting
24-month survival; and NLR ≥2.80 (AUC: 0.693, 70.4% sensitive,
52.0% specific), PLR ≥260.00 (AUC: 0.616, 61.1% sensitive, 56.0%
specific), and PNI ≤46.90 (AUC: 0.730, 72.0% sensitive, 68.5%
specific), as the most accurate cutoff values for predicting
overall survival (Fig. 1B).
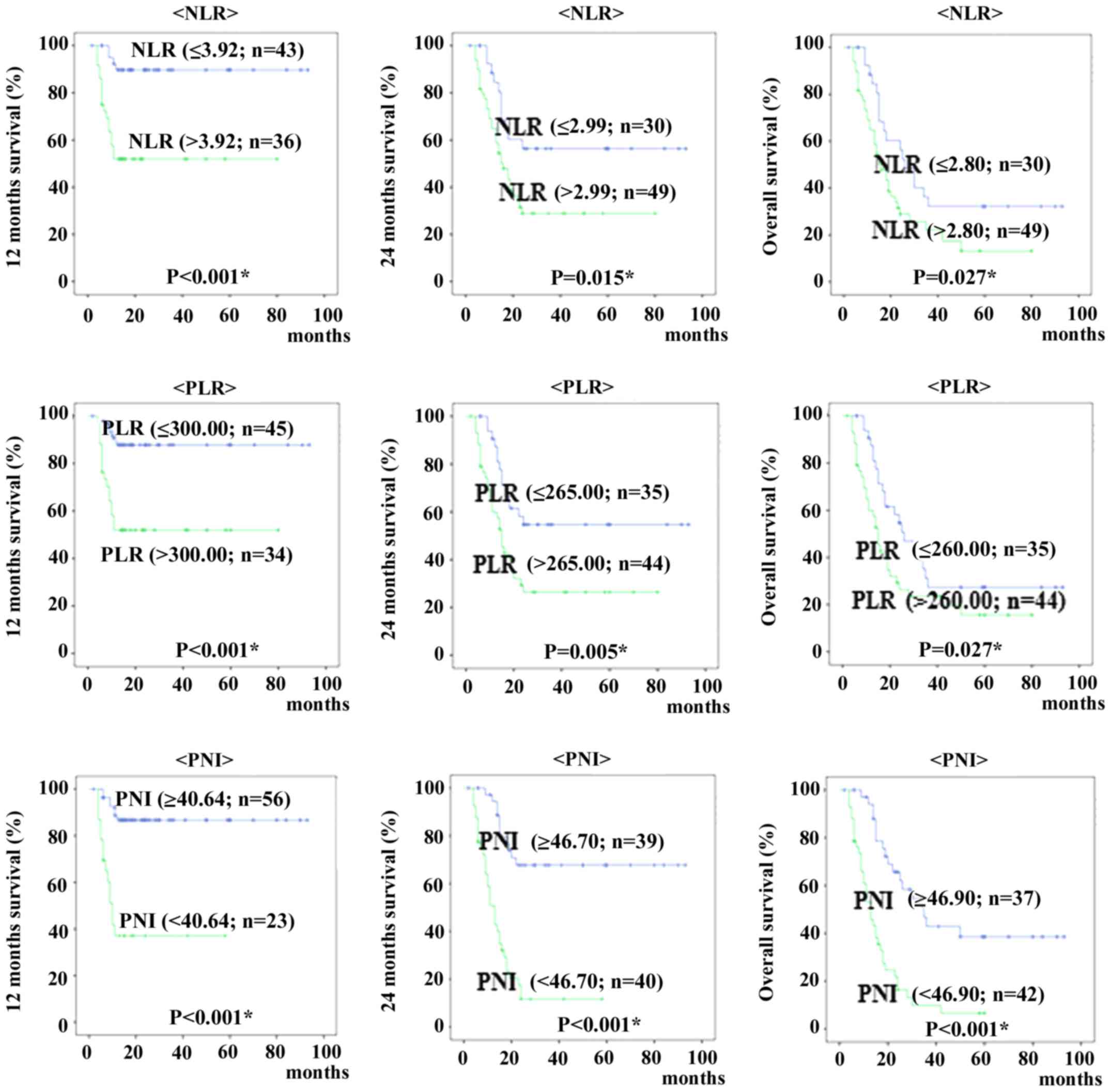
When patients were classified into those above and
below each cut-off value for 12 months, 24 months and overall
survival, Kaplan-Meyer curves of survival show patients with high
NLR and PLR were significantly shorter than rates of patients with
low NLR and PLR (12 months survival; P<0.001 and P<0.001, 24
months survival; P=0.015 and P=0.005, overall survival; P=0.027 and
P=0.027, respectively). The Kaplan-Meier curves showed that
patients with low PNI were shorter than for patients with high PNI
(12 months survival; P<0.001, 24 months survival; P<0.001,
overall survival; P<0.001, respectively) (Fig. 2).
Correlations between clinical factors and 12-month,
24-month and overall survival were assessed in univariate and
multivariate analyses (Table III).
Treatment-free interval (P=0.036), treatment type (P=0.015), NLR
(P=0.001), PLR (P=0.001) and PNI (P<0.001) were significantly
associated with 12-month survival in univariate analyses;
treatment-free interval (P=0.012) and PNI (P=0.001) were
independent predictors of 12-month survival in multivariate
analyses.
 | Table III.Prognostic factors for 12, 24 months
survival and overall survival with recurrent cervical cancer
selected by Cox's univariate and multivariate analysis. |
Table III.
Prognostic factors for 12, 24 months
survival and overall survival with recurrent cervical cancer
selected by Cox's univariate and multivariate analysis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| 12 months
survival |
|
|
|
|
|
|
|
Histology | 0.808 | 0.326–2.002 | 0.645 |
|
|
|
|
Treatment free interval | 2.568 | 1.064–6.201 | 0.036a | 3.322 | 1.300–8.489 | 0.012a |
|
Multiple metastasis | 1.852 | 0.780–4.397 | 0.162 |
|
|
|
|
Hematogenous metastasis | 1.295 | 0.545–3.074 | 0.558 |
|
|
|
|
Lymphogenous metastasis | 1.355 | 0.575–3.191 | 0.487 |
|
|
|
|
Treatment (Cx or PC) | 12.115 | 1.625–90.298 | 0.015a | 5.588 | 0.680–45.943 | 0.109 |
| NLR
(>3.92) | 6.422 | 2.156–19.125 | 0.001a | 2.332 | 0.457–11.894 | 0.308 |
| PLR
(>300.00) | 5.19 | 1.897–14.199 | 0.001a | 1.475 | 0.348–6.252 | 0.598 |
| PNI
(<40.64) | 7.205 | 2.887–17.987 |
<0.001a | 4.983 | 1.889–13.146 | 0.001a |
| 24 months
survival |
|
|
|
|
|
|
|
Histology | 0.595 | 0.310–1.140 | 0.118 |
|
|
|
|
Treatment free interval | 2.748 | 1.500–5.035 | 0.001a | 2.44 | 1.316–4.526 | 0.005a |
|
Multiple metastasis | 2.3 | 1.258–4.206 | 0.007a | 1.099 | 0.565–2.139 | 0.781 |
|
Hematogenous metastasis | 1.302 | 0.717–2.366 | 0.385 |
|
|
|
|
Lymphogenous metastasis | 1.301 | 0.718–2.357 | 0.386 |
|
|
|
|
Treatment (Cx or PC) | 6.772 | 2.649–17.314 |
<0.001a | 4.17 | 1.463–11.885 | 0.008a |
| NLR
(>2.90) | 2.252 | 1.136–4.464 | 0.020a | 1.002 | 0.342–2.931 | 0.997 |
| PLR
(>265.00) | 2.378 | 1.258–4.495 | 0.008a | 1.093 | 0.408–2.927 | 0.859 |
| PNI
(<46.70) | 5.495 | 2.749–10.984 |
<0.001a | 3.767 | 1.750–8.109 | 0.001a |
| Overall
survival |
|
|
|
|
|
|
|
Histology | 0.713 | 0.406–1.252 | 0.239 |
|
|
|
|
Treatment free interval | 2.446 | 1.421–4.209 | 0.001a | 2.244 | 1.285–3.918 | 0.004a |
|
Multiple metastasis | 2.564 | 1.478–4.447 | 0.001a | 1.07 | 0.578–1.980 | 0.829 |
|
Hematogenous metastasis | 1.245 | 0.726–2.136 | 0.426 |
|
|
|
|
Lymphogenous metastasis | 1.302 | 0.760–2.231 | 0.336 |
|
|
|
|
Treatment (Cx or PC) | 7.196 | 3.293–15.727 |
<0.001a | 5.709 | 2.297–14.190 |
<0.001a |
| NLR
(>2.80) | 1.89 | 1.056–3.380 | 0.032a | 1.051 | 0.435–2.539 | 0.912 |
| PLR
(>260.00) | 1.821 | 1.051–3.155 | 0.032a | 0.817 | 0.353–1.891 | 0.637 |
| PNI
(<46.90) | 3.621 | 2.026–6.473 |
<0.001a | 3.229 | 1.689–6.173 |
<0.001a |
Treatment-free interval (P=0.001), multiple
metastases (P=0.007), treatment type (P<0.001), NLR (P=0.020),
PLR (P=0.008) and PNI (P<0.001) were significantly associated
with 24-month survival in univariate analyses; treatment-free
interval (P=0.005), treatment type (P=0.008) and PNI (P=0.001) were
independent predictors of 24-month survival in multivariate
analyses.
Treatment-free interval (P=0.001), multiple
metastases (P=0.001), treatment type (P<0.001), NLR (P=0.032),
PLR (P=0.032) and PNI (P<0.001) were significantly associated
with overall survival in univariate analyses; treatment-free
interval (P=0.004), treatment type (P<0.001) and PNI
(P<0.001) were independent predictors of overall survival in
multivariate analyses.
Discussion
Among patients with recurrent cancer, prognosis is
based on different criteria for those with advanced disease than
for those with earlier-stage disease, in whom prognosis depends
mainly on the primary site and histology. However, the prognostic
values of SIRs are still unknown for recurrence of cervical cancer.
This is the first study to evaluate whether NLR, PLR and PNI are
predictors of survival for patients whose cervical cancer has
recurred after undergoing treatment.
SIRs have been examined as possible predictors of
prognosis in various types of cancer. Neutrophils release
inflammatory cytokines, leukocyte chemotactic factors and other
phagocytic mediators that can damage cellular DNA, inhibit
apoptosis and promote angiogenesis (14–17).
Platelets can release potent mitogens or adhesive glycoprotein,
such as platelet-derived growth factor, transforming growth
factor-β, and vascular endothelial growth factor (18–20).
Albumin levels decrease with increased levels of pro-inflammatory
cytokines such as IL-1, IL-6, and tumor necrosis factor, which
modulate albumin production (21).
Lymphocytes can affect growth and metastasis such as
CD3+ T cells and NK cells (22). Recent evidence has shown that
relative differences in neutrophil, platelet, albumin and
lymphocyte counts, NLR, PLR, and PNI are systemic indicators of
prognosis. The PNI is based on albumin and absolute lymphocyte
count, which are measured routinely in clinical practice. It was
originally derived to assess immunologic and nutritional condition
of patients undergoing surgical treatment for disease of the
digestive tract. Mizunuma et al reported that NLR was a
significant prognostic factor for PFS and OS in patients with
cervical cancer who had been treated with CCRT or RT (23). Reportedly, PLR is an important
predictor of prognosis in patients whose cervical cancers recur
after undergoing CCRT (8). The
current investigation of correlations among clinicopathological
parameters and NLR, PLR and PNI found NLR and PLR were significant
associated with treatment type; and PNI was significantly
associated with multiple metastases, hematogenous metastasis and
treatment type.
This study mainly evaluated correlations between the
SIR parameters, such as NLR, PLR and PNI, and survival (12 months,
24 months and overall survival) of patients whose cervical cancer
recurred after undergoing CCRT or radical hysterectomy (with or
without CCRT). We found that 12-month, 24-month and overall
survival for patients with higher NLR and PLR were significantly
shorter than for patients with lower NLR and PLR. We also found
that 12-month, 24-month and overall survival for patients with
lower PNI were significantly shorter than for patients with higher
PNI. We not only found PNI to be an independent prognostic factor
for 12-month, 24-month and overall survival in multivariate
analysis, but PNI was also superior to NLR or PLR as a predictor of
survival in all patient cohorts with recurrent cervical cancer.
Each patient underwent varied treatment
combinations, some of which are subjected to alter the bone marrow
hematopoietic function, further to affect the results of blood
tests, like dosage of radiotherapy, coverage of irradiated field,
numbers of chemotherapies, regimens of chemotherapies and different
combination. In this study, pretreatment of SIR was also superior
to during treatment as a predictor of survival with recurrent
cervical cancer. Therefore, the PNI may be useful in reflecting the
frailty and nutritional decline in patients with recurrent cervical
cancer. Our results suggest that the PNI will provide additional
value to the routine assessment of survival of patients with
recurrent cervical cancer.
We acknowledge that our study has some limitations.
The number of patients was relatively few, and the duration of
follow-up was relatively short. Further prospective studies with
more patients and longer follow-up periods would provide more
definitive data to clarify the significance of our findings.
In conclusion, our results show that PNI can serve
as a useful indicator of survival in patients with recurrent
cervical cancer.
References
|
1
|
Quinn MA, Benedet JL, Odicino F,
Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY and
Pecorelli S: Carcinoma of the cervix uteri. FIGO 26th annual report
on the results of treatment in gynecological cancer. Int J Gynaecol
Obstet. 95 Suppl 1:S43–S103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Acmaz G, Aksoy H, Unal D, Ozyurt S,
Cingillioglu B, Aksoy U and Muderris I: Are neutrophil/lymphocyte
and platelet/lymphocyte ratios associated with endometrial
precancerous and cancerous lesions in patients with abnormal
uterine bleeding? Asian Pac J Cancer Prev. 15:1689–1692. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babu SN, Chetal G and Kumar S: Macrophage
migration inhibitory factor: A potential marker for cancer
diagnosis and therapy. Asian Pac J Cancer Prev. 13:1737–1744. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Absenger G, Szkandera J, Stotz M,
Postlmayr U, Pichler M, Ress AL, Schaberl-Moser R, Loibner H,
Samonigg H and Gerger A: Preoperative neutrophil-to-lymphocyte
ratio predicts clinical outcome in patients with stage II and III
colon cancer. Anticancer Res. 33:4591–4594. 2013.PubMed/NCBI
|
|
5
|
Migita K, Takayama T, Saeki K, Matsumoto
S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N and
Nakajima Y: The prognostic nutritional index predicts long-term
outcomes of gastric cancer patients independent of tumor stage. Ann
Surg Oncol. 20:2647–2654. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanda M, Fujii T, Kodera Y, Nagai S,
Takeda S and Nakao A: Nutritional predictors of postoperative
outcome in pancreatic cancer. Br J Surg. 98:268–274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang
J, Wang T, Zhu W and Liu P: Prognostic value of PLR in various
cancers: A meta-analysis. PLoS One. 9:e1011192014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakamura K, Nishida T, Haruma T, Haraga J,
Omichi C, Ogawa C, Kusumoto T, Seki N, Masuyama H and Hiramatsu Y:
Pretreatment platelet-lymphocyte ratio is an independent predictor
of cervical cancer recurrence following concurrent chemoradiation
therapy. Mol Clin Oncol. 3:1001–1006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nozoe T, Ninomiya M, Maeda T, Matsukuma A,
Nakashima H and Ezaki T: Prognostic nutritional index: A tool to
predict the biological aggressiveness of gastric carcinoma. Surg
Today. 40:440–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mann HB and Whitney DR: On a test of
whether one of two random variables is stochastically larger than
the other. Ann Math Stat. 18:50–60. 1947. View Article : Google Scholar
|
|
11
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assn.
53:457–481. 1958. View Article : Google Scholar
|
|
12
|
Breslow NE: Analysis of survival data
under the proportional hazards model. Int Stat Rev. 43:45–57. 1975.
View Article : Google Scholar
|
|
13
|
Cox DR: Regression Models and Life-Tables.
J R Stat Soc B. 34:187–220. 1972.
|
|
14
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jackson JR, Seed MP, Kircher CH,
Willoughby DA and Winkler JD: The codependence of angiogenesis and
chronic inflammation. FASEB J. 11:457–465. 1997.PubMed/NCBI
|
|
17
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Assoian RK and Sporn MB: Type beta
transforming growth factor in human platelets: Release during
platelet degranulation and action on vascular smooth muscle cells.
J Cell Biol. 102:1217–1223. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dubernard V, Arbeille BB, Lemesle MB and
Legrand C: Evidence for an alpha-granular pool of the cytoskeletal
protein alpha-actinin in human platelets that redistributes with
the adhesive glycoprotein thrombospondin-1 during the exocytotic
process. Arterioscler Thromb Vasc Biol. 17:2293–2305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaplan KL, Broekman MJ, Chernoff A,
Lesznik GR and Drillings M: Platelet alpha-granule proteins:
Studies on release and subcellular localization. Blood. 53:604–618.
1979.PubMed/NCBI
|
|
21
|
Gupta D and Lis CG: Pretreatment serum
albumin as a predictor of cancer survival: A systematic review of
the epidemiological literature. Nutr J. 9:692010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin X, Li W, Lai J, Okazaki M, Sugimoto S,
Yamamoto S, Wang X, Gelman AE, Kreisel D and Krupnick AS: Five-year
update on the mouse model of orthotopic lung transplantation:
Scientific uses, tricks of the trade, and tips for success. J
Thorac Dis. 4:247–258. 2012.PubMed/NCBI
|
|
23
|
Mizunuma M, Yokoyama Y, Futagami M, Aoki
M, Takai Y and Mizunuma H: The pretreatment
neutrophil-to-lymphocyte ratio predicts therapeutic response to
radiation therapy and concurrent chemoradiation therapy in uterine
cervical cancer. Int J Clin Oncol. 20:989–996. 2015. View Article : Google Scholar : PubMed/NCBI
|