Introduction
Castleman's disease (CD), first described by
Castleman in 1954 (1), is commonly
encountered in the chest and neck, but is less common in the
abdomen and rare in the liver. Primary hepatic CD is very rare,
with <20 cases reported in the literature to date (2). Furthermore, description of computed
tomography (CT) and magnetic resonance imaging (MRI)
characteristics of hepatic CD is rarely found in the radiological
literature, particularly from diffusion-restricted on
diffusion-weighted imaging (DWI), and CD may be misdiagnosed as a
malignant lesion (3). Therefore, the
radiological diagnosis of hepatic CD remains difficult. We herein
describe a case of hepatic CD detected by CT and MRI, along with a
review of the previous cases reported in the literature, with the
aim to help improve our understanding and radiological diagnosis of
hepatic CD.
Case report
An asymptomatic 70-year-old woman underwent a
routine physical examination in a local hospital, during which a
focal lesion was identified in the right lobe of the liver via
ultrasound. The patient received no immediate treatment and was
referred for further evaluation to the First Affiliated Hospital of
Zhejiang Chinese Medical University (Hangzhou, China). The findings
on physical examination and the results of the laboratory tests,
including complete blood count and basic metabolic panels, were
normal. Subsequently, the patient underwent CT (Somatom, Sensation
64; Siemens, Waltershausen, Germany) and MRI (3.0T Discovery 750;
GE Healthcare, Chicago, IL, USA) scans of the abdomen for further
evaluation and, finally, the focal lesion was surgically
resected.
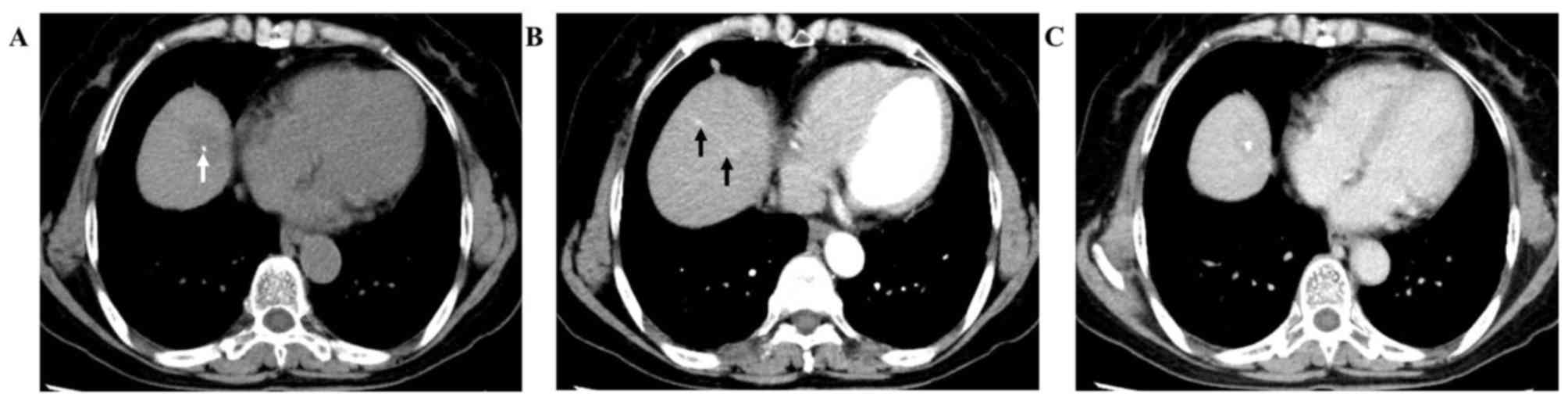
A non-enhanced abdominal CT was first conducted and
revealed well-defined nodular hypoattenuation with punctate
hyperattenuation (Fig. 1A, arrow)
within a lesion in the eighth segment of the liver. On enhanced CT
imaging, the lesion exhibited nodular enhancement (2.3×1.9 cm), and
a strip-like enhanced area was found to extend from the nodule in
the arterial phase 30 sec after injection of iodine contrast agent
(Fig. 1B, arrows), with persistent
enhancement in the portal venous phase 65 sec after injection of
iodine contrast agent (Fig. 1C).
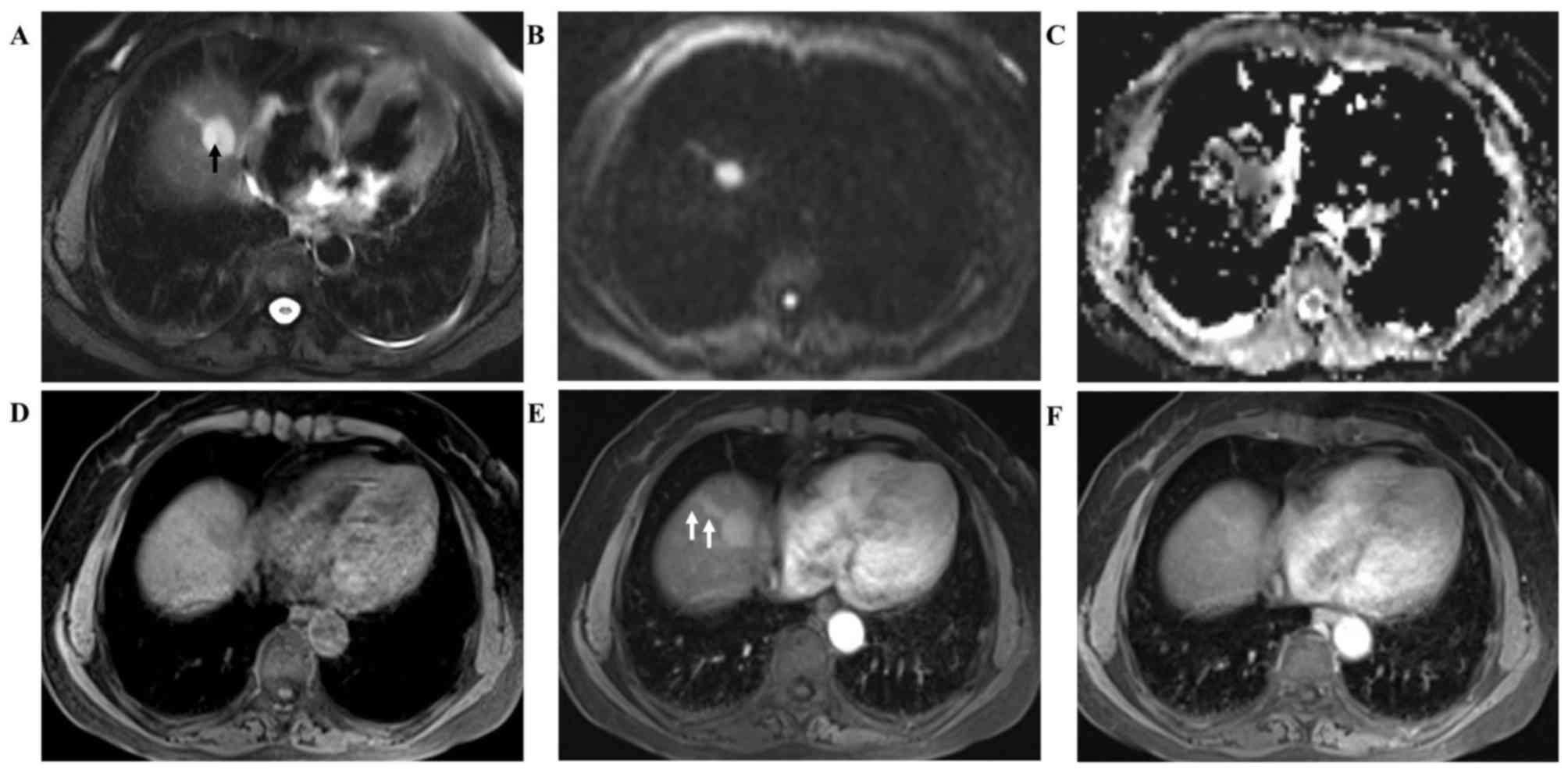
A follow-up MRI was performed 10 days later and
revealed a well-defined nodule (2.6×2.0 cm) with an extended strip
in the eighth segment of the liver on T2-weighted imaging. Punctate
hypointensity was also observed in the center of the nodule
(Fig. 2A, arrow). Both the nodule
and the strip displayed hyperintensity on DWI (b=800
sec/mm2, Fig. 2B) with a
low apparent diffusion coefficient (ADC) value
(0.622×10−3 and 0.693×10−3
mm2/sec, respectively; Fig.
2C). Compared with non-enhanced T1-weighted MR images (Fig. 2D), post-contrast MRI revealed
significant enhancement of the nodule and the strip in the arterial
phase 30 sec after gadolinium injection (Fig. 2E, arrows), and persistent enhancement
in the parenchymal phase 120 sec after gadolinium injection
(Fig. 2F).
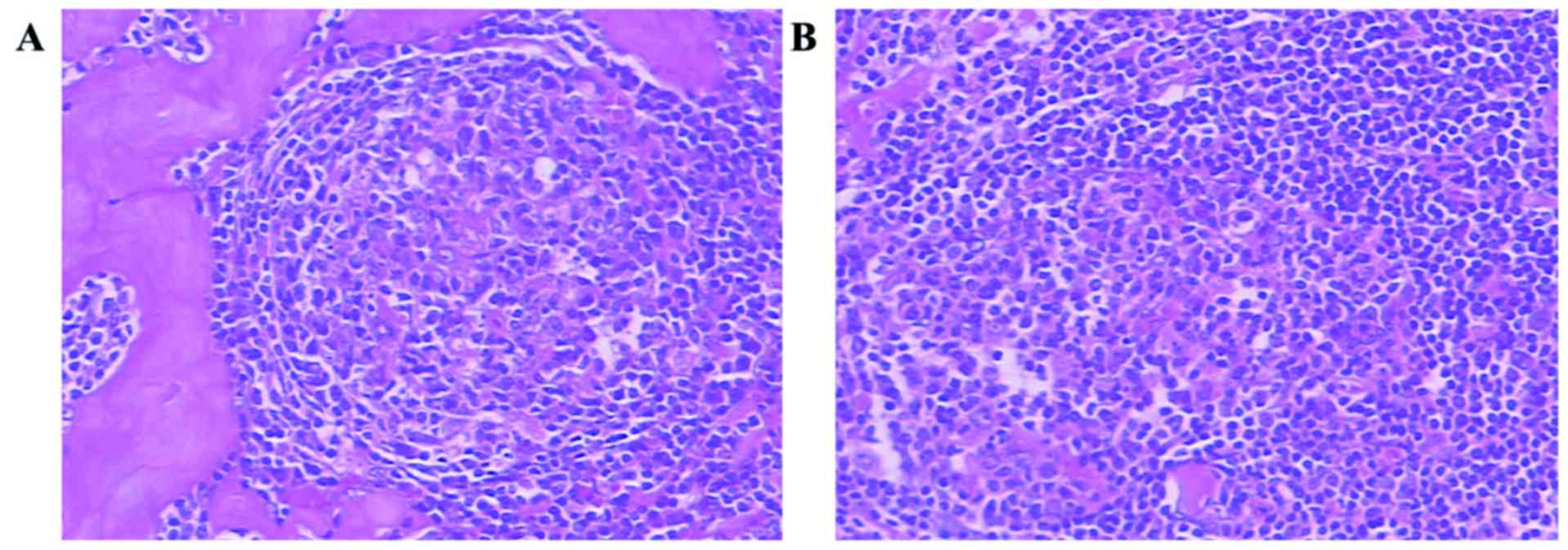
The lesion in the right liver was resected by
surgical laparoscopy under general anesthesia. Postoperative
pathological examination demonstrated that the pathological
components of the nodular mass were lymphocytes and hyalinized
vessels (Fig. 3A), with the
strip-like area being composed mainly of lymphocytes (Fig. 3B).
Postoperative treatment of the patient was
administration with Saimeijie (an atypical β-lactams antibiotic,
2.0 g bid) and Ornidazole (0.5 g bid) to prevent infection, and
support treatment with hemostasis and rehydration within 2 weeks.
Postoperative recovery was good and there has been no recurrence on
the last follow-up before the present study was submitted.
Discussion
Castleman's disease (CD) (4) may be histologically divided into
hyaline vascular (HV), plasma cell (PC) and mixed variants. The HV
variant (5), which is composed of
follicles and follicular lymph tissue, is commonly found in women
aged 30–40 years and accounts for 90% of diagnosed cases.
Phenotypically, this variant is defined by the presence of abundant
hyperplastic hyaline capillaries between follicles and lymph
sinuses. Furthermore, small lymphocytes in the mantle zone are
arranged in concentric rings around the germinal center. The
prognosis of the HV variant is mostly favorable following surgical
resection. The PC variant (6) is a
systemic disease that is more common among patients aged 50–60
years, and accounts for ~10% of diagnosed cases. This variant is
defined by enlargement of follicular cells and an abundance of
mature plasma cells, but a lower degree of angiogenesis. Treatment
is administered systemically using hormone or immunosuppressive
agents, but the prognosis of the PC variant is poor. Two clinical
subtypes are classified as unicentric CD (UCD) and multicentric CD
(MCD) (7–9). UCD occurs most frequently as a focal
lesion of the HV variant that is not associated with obvious
clinical symptoms and is often incidentally found during routine
physical examination. MCD is a systemic disease, with the majority
of the cases being of the PC variant, accompanied by systemic
symptoms including fatigue, fever, night sweats, weight loss, joint
pain and hepatosplenomegaly.
The imaging findings of UCD (10) often present as a single, well-defined
soft tissue lesion, with rare cystic degeneration and focal
necrosis. This finding may be associated with substantial
angiogenesis and development of an extensive collateral
circulation, so the lymph follicle tissue is not prone to necrosis
(11). Non-enhanced CT images
revealed homogeneous mild hypoattenuation within the lesion.
Non-enhanced MR T1WI revealed a slightly hyperintensity compared
with muscle. Furthermore, T2WI and DWI displayed high signals,
suggesting diffusion restriction and supporting abundant cell
proliferation (12).
Contrast-enhanced CT/MRI of CD lesions often displays mild to
moderate enhancement during the arterial phase, and persistent
enhancement during the venous phase (13). This finding may be associated with
the small arterial lumen and the subsequent reduction in blood
velocity. Polymorphic calcification is commonly found in the
lesions (14), which may be
attributed to capillary proliferation with wall thickening and
accompanied by glassy degeneration, fibrotic degeneration and the
formation of calcareous deposits along the vessel wall. Imaging
findings in MCD (15) include
multiple enlarged lymph nodes with homogeneous density and mild to
moderate enhancement on a contrast-enhanced scan, which
pathologically reflects enlarged follicles and follicular plasma
cell infiltration. In MCD is of the HV variant, the degree of
enhancement is similar to that of UCD.
The reported imaging findings of hepatic CD
(16–23) (Table
I) are commonly consistent with UCD. These findings and the
calcification of the lesion may be helpful for radiological
diagnosis of CD in the liver. Moreover, we observed that the
signal, density, and pathological components of the strip-like area
were consistent with those of the nodule. To the best of our
knowledge, this finding has not been previously reported in the
literature on hepatic CD to date. We hypothesized that the
strip-like area may be attributed to lymphoid tissue, and
lymphoproliferative disease may develop anywhere where lymphoid
tissue is present (20). This
strip-like area extending from the nodule may help with the
differential diagnosis of hepatic CD from other diseases. However,
pathological examination is required for a definitive
diagnosis.
 | Table I.Imaging characteristics of hepatic
Castleman's disease. |
Table I.
Imaging characteristics of hepatic
Castleman's disease.
|
|
|
|
| Imaging
characteristics |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Authors | Sex/age (years) | Symptoms | Size (cm) | Margins | Ultrasound | CT/MRI | Pathology | (Refs.) |
|---|
| Rahmouni et
al | Female/48 | Abdominal pain | 5 | Well-defined | Hypoechoic | Hypervascular,
calcifications | HV | (16) |
|
| Male/28 | Asymptomatic | 3 | Lobulated | Hypoechoic | Hypervascular | HV |
|
| Cirillo et
al | Female/43 | Abdominal pain | 13 | Well-defined | NA | Nonvascular,
calcifications | HV | (17) |
| Uzunlar et
al | Female/56 | Vague abdominal
pain | 3.5 | Well-defined | Hypoechoic | Hypervascular | HV | (18) |
| Karami et
al | Female/5 | Abdominal pain | 3.7 | Lobulated | Solid mass | Hypervascular | HV | (19) |
| Jang et
al | Female/40 | Abdominal pain, fever
and chills | 2.2 | Well-defined | NA | Hypervascular | HV | (20) |
| Miyoshi et
al | Female/70 | Asymptomatic | 1.5 | Well-demarcated | Hypoechoic | Hypovascular | HV | (21) |
| Dong et
al | Female/57 | Asymptomatic | 3.3 | Well-defined | NA | Hypervascular,
diffusion restricted | HV | (22) |
| Maundura et
al | Female/64 | Asymptomatic | 1.4 | Well-defined | Hypoechoic | Hypervascular,
diffusion restricted | HV | (23) |
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Competing interests
The authors declare that they have no competing
interests.
Ethics approval and consent to
participate
This article does not contain any studies with human
participants or animals performed by any of the authors.
Authors' contributions
All authors contributed to this paper and approved
the final version. LK wrote the case report. ZCL offered the
assistance for the pathologic diagnosis of this case. XMS and LK
revised and edited the final version.
Consent for publication
The patient and/or her family consented to the
publication of the case details and associated images.
References
|
1
|
Castleman B and Towne VW: Case records of
the massachusetts general hospital; weekly clinicopathological
exercises; founded by Richard C. Cabot. N Engl J Med. 251:396–400.
1954.PubMed/NCBI
|
|
2
|
Talat N, Belgaumkar AP and Schulte KM:
Surgery in Castleman's disease: A systematic review of 404
published cases. Ann Surg. 255:677–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malayeri AA, El Khouli RH, Zaheer A,
Jacobs MA, Corona-Villalobos CP, Kamel IR and Macura KJ: Principles
and applications of diffusion-weighted imaging in cancer detection,
staging, and treatment follow-up. Radiographics. 31:1773–1791.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soumerai JD, Sohani AR and Abramson JS:
Diagnosis and management of Castleman disease. Cancer Control.
21:266–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keller AR, Hochholzer L and Castleman B:
Hyaline-vascular and plasma-cell types of giant lymph node
hyperplasia of the mediastinum and other locations. Cancer.
29:670–683. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferry JA and Harris NL: Atlas of lymphoid
hyperplasia and lymphoma. WB. Saunders Company; pp. 1–1042.
1997
|
|
7
|
Gaba AR, Stein RS, Sweet DL and Variakojis
D: Multicentric giant lymph node hyperplasia. Am J Clin Pathol.
69:86–90. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Casper C: The aetiology and management of
Castleman disease at 50 years: Translating pathophysiology to
patient care. Br J Haematol. 129:3–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Rhee F, Stone K, Szmania S, Barlogie B
and Singh Z: Castleman disease in the 21st century: An update on
diagnosis, assessment, and therapy. Clin Adv Hematol Oncol.
8:486–498. 2010.PubMed/NCBI
|
|
10
|
Zhou LP, Zhang B, Peng WJ, Yang WT, Guan
YB and Zhou KR: Imaging findings of Castleman disease of the
abdomen and pelvis. Abdom Imaging. 33:482–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McAdams HP, Rosado-de-Christenson M,
Fishback NF and Templeton PA: Castleman disease of the thorax:
Radiologic features with clinical and histopathologic correlation.
Radiology. 209:221–228. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
White NS, McDonald C, Farid N, Kuperman J,
Karow D, Schenker-Ahmed NM, Bartsch H, Rakow-Penner R, Holland D,
Shabaik A, et al: Diffusion-weighted imaging in cancer: Physical
foundation and application of restriction spectrum imaging. Cancer
Res. 74:4638–4652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang XH, Song HM, Liu QY, Cao Y, Li GH
and Zhang WD: Castleman disease of the neck: CT and MR imaging
findings. Eur J Radiol. 83:2041–2050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonekamp D, Horton KM, Hruban RH and
Fishman EK: Castleman disease: The great mimic. Radiographics.
31:1793–1807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johkoh T, Müller NL, Ichikado K, Nishimoto
N, Yoshizaki K, Honda O, Tomiyama N, Naitoh H, Nakamura H and
Yamamoto S: Intrathoracic multicentric Castleman disease: CT
findings in 12 patients. Radiology. 209:477–481. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rahmouni A, Golli M, Mathieu D, Anglade
MC, Charlotte F and Vasile N: Castleman disease mimicking liver
tumor: CT and MR features. J Comput Assist Tomogr. 16:699–703.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cirillo RL Jr, Vitellas KM, Deyoung BR and
Bennett WF: Castleman disease mimicking a hepatic neoplasm. Clin
Imaging. 22:124–129. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uzunlar AK, Ozateş M, Yaldiz M,
Büyükbayram H and Ozaydin M: Castleman's disease in the porta
hepatis. Eur Radiol. 10:1913–1915. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karami H, Sahebpour AA, Ghasemi M, Karami
H, Dabirian M, Vahidshahi K, Masiha F and Shahmohammadi S: Hyaline
vascular-type Castleman's disease in the hilum of liver: A case
report. Cases J. 3:742010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jang SY, Kim BH, Kim JH, Ha SH, Hwang JA,
Yeon JW, Kim KH and Paik SY: A case of Castleman's disease
mimicking a hepatocellular carcinoma: A case report and review of
literature. Korean J Gastroenterol. 59:53–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyoshi H, Mimura S, Nomura T, Tani J,
Morishita A, Kobara H, Mori H, Yoneyama H, Deguchi A, Himoto T, et
al: A rare case of hyaline-type Castleman disease in the liver.
World J Hepatol. 5:404–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong A, Dong H and Zuo C: Castleman
disease of the porta hepatis mimicking exophytic hepatocellular
carcinoma on CT, MRI, and FDG PET/CT. Clin Nucl Med. 39:e69–e72.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maundura M, Hayward G, McKee C and Koea
JB: Primary Castleman's disease of the liver. J Gastrointest Surg.
21:417–419. 2017. View Article : Google Scholar : PubMed/NCBI
|