Introduction
Large-cell neuroendocrine carcinoma (LCNEC) was
first reported in the lung (1).
LCNECs have since been reported in several locations, including the
mediastinum, head and neck, pancreas, gallbladder, intestine and
gynecological organs (2,3). Among gynecological organs, LCNECs more
frequently occur in the uterine cervix rather than the uterine
corpus. To the best of our knowledge, after the first such case was
reported in 2004 (4), only 18 cases
of LCNEC arising in the uterine corpus have been published to date
(5–8). Due to the small number of reported
cases, LCNECs may be difficult to diagnose. We herein present a
case of LCNEC arising in the endometrium, with aim to describe the
histological characteristics of this tumor and emphasize its rapid
progression and poor prognosis.
Case report
A 52-year-old woman (gravida 6, para 4), with no
history of gynecological disorders, was referred to Toyooka
Hospital (Toyooka, Japan) due to genital bleeding in February 2016.
The patient had been taking a low-dose contraceptive pill for 18
months prior to the consultation to control functional bleeding.
Regular gynecological physical examinations 3 months prior to the
consultation failed to identify any abnormalities. The patient
consulted her primary care doctor due to continuous genital
bleeding for 50 days. The doctor diagnosed the patient with anemia
[hemoglobin (Hb) level, 8.8 g/dl] and detected a uterine cervical
polyp; the patient was subsequently referred to Toyooka Hospital.
Upon pelvic examination at the Department of Gynecology, a
cauliflower-shaped tumor, ~80 mm in diameter, was identified in the
vaginal cavity. Continuous bleeding from the tumor surface was
observed. Anemia was also observed on blood examination. The tumor
appeared to develop from the uterine os, suggesting that it
originated in the uterine cavity and extended into the vaginal
cavity. Examination using ultrasound revealed a tumor occupying the
entire uterine cavity and was attached to the fundus through a
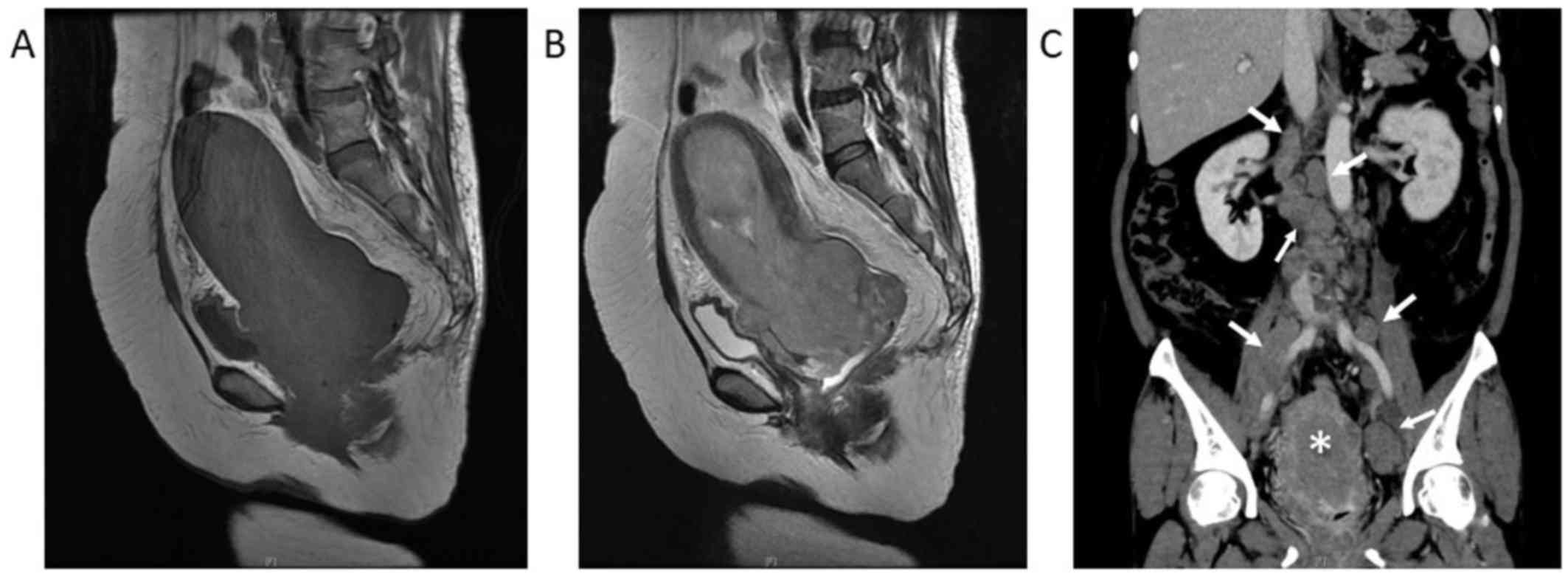
stalk. Magnetic resonance imaging examination revealed a
homogeneous tumor, sized 16.9×8.4×7.8 mm, in the endometrial
cavity, protruding into the vaginal cavity. A coronal
contrast-enhanced computed tomography scan revealed a
heterogeneously enhanced uterine tumor and enlargement of the
paraaortic lymph nodes, external iliac lymph nodes, and partially
necrotic internal iliac lymph nodes, suggesting lymph node
metastasis. There was no detectable invasion of the tumor into the
bladder or rectum on diagnostic imaging (Fig. 1). Laboratory data on admission
included Hb 8.7 g/dl, C-reactive protein 8.73 mg/dl, creatinine
0.77 mg/dl, lactate dehydrogenase 814 U and carbohydrate
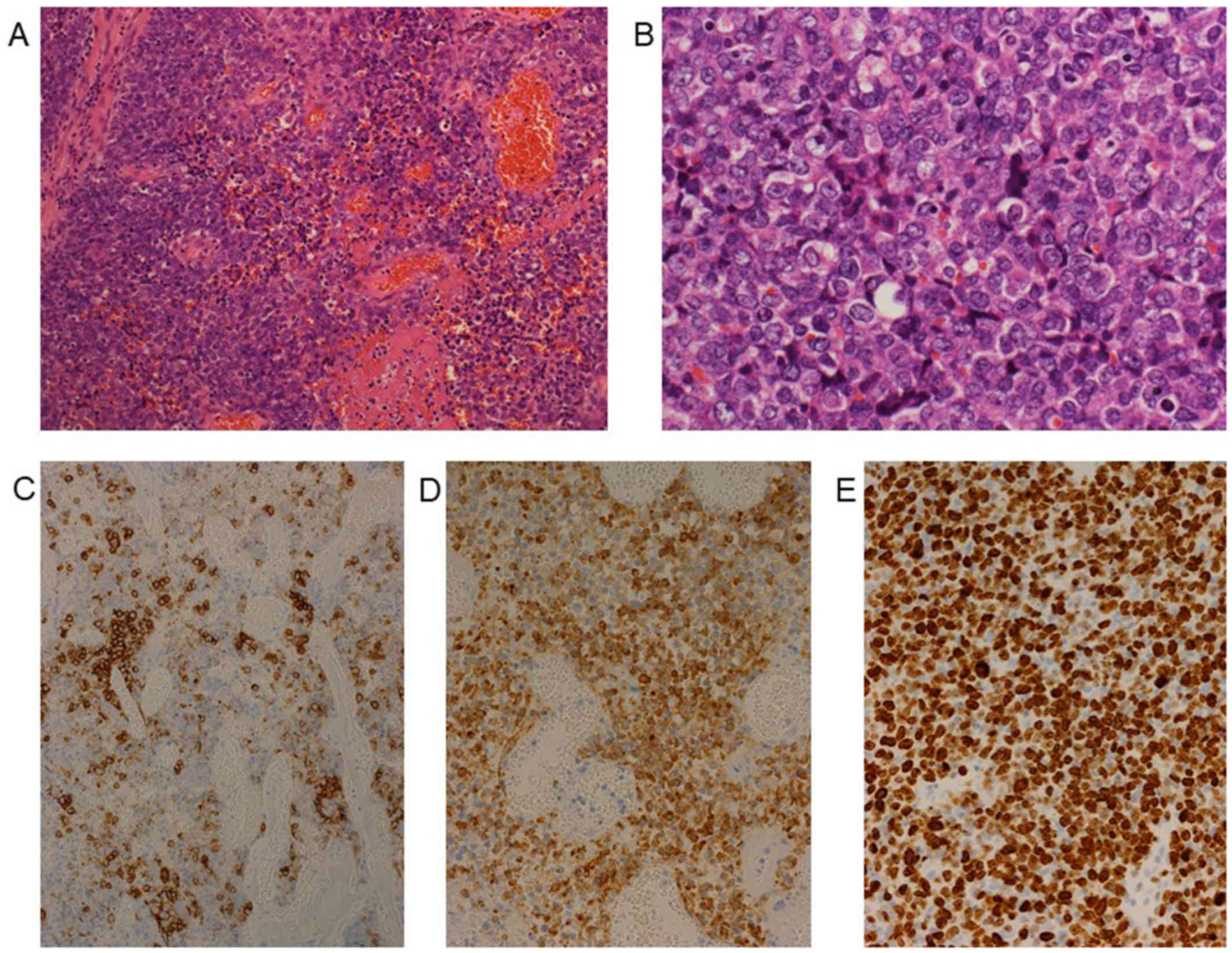
antigen-125 36.5 U/ml. Histological examination of a biopsy sample
from the tumor revealed atypical cells and necrotic tissue. The
tumor cells were medium-large in size, with abundant cytoplasm and
prominent nucleoli (Figs. 2A and B).
Immunohistochemically, the tumor cells were positive for CD56 and
synaptophysin (Figs. 2C and D), and
negative for cytokeratin AE1/AE3, chromogranin A, P40, α-smooth
muscle actin (SMA), S100, CD10 and CD34. The Ki-67 index was ~85%
(Fig. 2E). Based on the histological
characteristics and results of the immunochemical staining, the
tumor was diagnosed as LCNEC. The blood levels of neuron-specific
enolase (NSE) and proGRP was measured, and revealed that the level
of proGRP was normal, but the level of NSE was extremely high
(240.4 ng/ml; normal range, ≤10 ng/ml). Due to the poor condition
of the patient and extent of tumor spread, palliative care using
morphine hydrochloride (600 µg/kg/day) was administered in an
attempt to alleviate the symptoms. However, the tumor rapidly
progressed and the patient succumbed to the disease 36 days after
admission.
Discussion
Erhan et al first reported LCNEC arising in
the endometrium in 2004 (4). To the
best of our knowledge, only 18 cases (excluding the present case)
have been reported in the literature to date. In 2013, Nguyen et
al summarized 13 cases of endometrial LCNEC (5), and Kobayashi et al (6) reviewed 16 cases in 2017. While
reviewing the relevant literature, 2 additional reports were
identified (7,8). Thus, including the case presented
herein, there are a total of 19 reported cases of endometrial LCNEC
in patients aged 40–88 years [mean, 62 years; standard deviation
(SD), 14.8 years].
Cases of LCNEC arising in the cervix have also been
reported. Gilks et al reported 12 cases of LCNEC arising in
the uterine cervix (cervical LCNEC) in patients aged 21–62 years
(mean, 34 years) (9). Wang et
al reported a mean age of 42 years and a SD of 11.3 years
across 4 cases (10). Therefore, the
mean age of patients with endometrial LCNEC appears to be higher
compared with that of patients with cervical LCNEC.
The mean age at diagnosis of endometrioid carcinoma
of the endometrium (the most common type of endometrial carcinoma)
is ~63 years, while the majority of patients with microinvasive
squamous cell carcinoma (SqCC) arising in the uterine cervix are
aged 35–46 years (11).
Microinvasive SqCC of the uterine cervix represents the early stage
of SqCC, which is the most common type of carcinoma in the uterine
cervix. These results suggest that the susceptible age range for
LCNEC is similar to that of endometrioid carcinoma of the
endometrium and SqCC of the uterine cervix. Of the 19 cases of
endometrial LCNEC, 2 cases, including our patient, were diagnosed
by biopsy only. Therefore, it remains unclear whether the tumors
were composed purely of LCNEC cells, or incorporated other
histological type(s). Of the 17 resected tumors, 6 contained an
adenocarcinoma component, 5 contained endometrioid carcinoma and 1
contained serous carcinoma (8,12,13).
These results suggest that adenocarcinoma of the endometrium may
transform into LCNEC in a proportion of the cases. Even if the
histological diagnosis is pure LCNEC, this may be the result of
LCNEC cells overwhelming any other type of carcinoma and dominating
the entire tumor. As LCNEC has a high Ki-67 index and exhibits
rapid growth, the prognosis of endometrial LCNEC is poor. Of the 19
patients, 8 (42%) succumbed to the disease during the follow-up
period. The mean period of follow-up of the 8 patients was 7 months
(SD, 7.7 months). The survival rate was 46% at 1 year and 11% at 5
years after the diagnosis of LCNEC. In the present case, the
patient succumbed to rapid tumor growth ~1 month after the hospital
consultation. Similar rapidly progressing cases of endometrial
LCNEC have been reported by Nguyen et al (5) and Makihara et al (14).
For diagnosis of LCNEC, histological examination is
necessary. However, it has been reported that neuroendocrine
carcinomas, including small-cell carcinoma and LCNEC, do not always
express cytokeratin AE1/AE3, which is commonly used as an
epithelial marker (15). In the
present case, the tumor was negative for cytokeratin AE1/AE3.
Therefore, sarcoma was first suspected and staining with
anti-a-SMA, anti-S100 and anti-CD10 antibodies was performed; the
tumor was negative for all three. Thereafter, staining for
neuroendocrine markers was performed and the blood levels of NSE
and proGRP were measured, which confirmed the diagnosis of LCNEC.
If endometrial LCNEC had been considered earlier in the
differential diagnosis, the patient may have been diagnosed sooner.
Therefore, when a rapidly growing tumor is detected, even if that
tumor is negative for cytokeratin, the possibility of LCNEC should
be taken into consideration.
In conclusion, we herein present a rare case of
LCNEC with rapid growth and poor prognosis. When the tumor is
cytokeratin-negative, it must be carefully examined before a
definitive diagnosis is reached. Other cytokeratin-negative
carcinomas in addition to neuroendocrine tumors, such as sarcoma
and hematopoietic tumors, should also be considered.
Acknowledgements
The authors would like to thank Ms. H. Ogaki, Mr. K
Nagaoka, Mr. T. Kuge and Mr. H. Takenaka of the Toyooka Hospital
for their expert technical assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JO, YA, KYas, AO, HN, KK, KYam, KS, TK and SI
designed the study. JO, KYas, HN, KK, KYam, KS, TK and YA analyzed
and interpreted the patient data. JO and YA were major contributors
in writing the manuscript. AO, SI and YA performed the histological
examination of the tumor and performed histological diagnosis.
Ethics approval and consent to
participate
The Ethics Committee of the Toyooka Hospital
(Toyooka, Japan) approved the present study and the patient
provided written informed consent.
Consent for publication
The patient approved the publication of their data
within this case report.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Travis WD, Linnoila RI, Tsokos MG, et al:
Neuroendocrine tumors of the lung with proposed criteria for
large-cell neuroendocrine carcinoma. An ultrastructural,
immunohistochemical, and flow cytometric study of 35 cases. Am J
Surg Pathol. 15:529–553. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lukina O, Gorbunkov S, Dvorakovskaja I,
Varlamov V and Akopov A: Fast-growing large cell neuroendocrine
carcinoma of mediastinum. Ann Thorac Surg. 91:1618–1620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faggiano A, Sabourin JC, Ducreux M, et al:
Pulmonary and extrapulmonary poorly differentiated large cell
neuroendocrine carcinomas: diagnostic and prognostic features.
Cancer. 110:265–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Erhan Y, Dikmen Y, Yucebilgin MS, Zekioglu
O, Mgoyi L and Terek MC: Large cell neuroendocrine carcinoma of the
uterine corpus metastatic to brain and lung: case report and review
of the literature. Eur J Gynaecol Oncol. 25:109–112.
2004.PubMed/NCBI
|
|
5
|
Nguyen ML, Han L, Minors AM, et al: Rare
large cell neuroendocrine tumor of the endometrium: A case report
and review of the literature. Int J Surg Case Rep. 4:651–655. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobayashi A, Yahata T, Nanjo S, et al:
Rapidly progressing large-cell neuroendocrine carcinoma arising
from the uterine corpus: A case report and review of the
literature. Mol Clin Oncol. 6:881–885. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Froio E, D' Adda T, Fellegara G, et al:
Uterine carcinosarcoma metastatic to the lung as large-cell
neuroendocrine carcinoma with synchronous sarcoid granulomatosis.
Lung Cancer. 64:371–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ono K, Yokota NR, Yoshioka E, et al:
Metastatic large cell neuroendocrine carcinoma of the lung arising
from the uterus: A pitfall in lung cancer diagnosis. Pathol Res
Pract. 212:654–657. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gilks CB, Young RH, Gersell DJ and Clement
PB: Large cell neuroendocrine [corrected] carcinoma of the uterine
cervix: a clinicopathologic study of 12 cases. Am J Surg Pathol.
21:905–914. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang KL, Yang YC, Wang TY, et al:
Neuroendocrine carcinoma of the uterine cervix: A clinicopathologic
retrospective study of 31 cases with prognostic implications. J
Chemother. 18:209–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kurman RJ, Ronnett B.M..Sherman
M.E..Wilkinson E.J.: Tumor of the cervix. ARP press; Silver Spring,
Maryland: 2010
|
|
12
|
Mulvany NJ and Allen DG: Combined large
cell neuroendocrine and endometrioid carcinoma of the endometrium.
Int J Gynecol Pathol. 27:49–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Posligua L, Malpica A, Liu J, Brown J and
Deavers MT: Combined large cell neuroendocrine carcinoma and
papillary serous carcinoma of the endometrium with pagetoid spread.
Arch Pathol Lab Med. 132:1821–1824. 2008.PubMed/NCBI
|
|
14
|
Makihara N, Maeda T, Nishimura M, et al:
Large cell neuroendocrine carcinoma originating from the uterine
endometrium: a report on magnetic resonance features of 2 cases
with very rare and aggressive tumor. Rare Tumors. 4:e372012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCluggage WG, Oliva E, Connolly LE,
McBride HA and Young RH: An immunohistochemical analysis of ovarian
small cell carcinoma of hypercalcemic type. Int J Gynecol Pathol.
23:330–336. 2004. View Article : Google Scholar : PubMed/NCBI
|