Introduction
Neuroendocrine gastrointestinal tumors constitute a
heterogeneous group of tumors and share a common phenotype, with
positive immunostaining for the neuroendocrine markers chromogranin
A and synaptophysin, among others. Neuron-specific enolase and CD56
are often, but not always, positive (1). Pure small cell carcinomas of the
gastrointestinal tract are in fact poorly differentiated,
high-grade gastro-enteropancreatic neuroendocrine carcinomas
(2). Primary small cell gastric
carcinomas (SCGC) account for 15–20% of all gastric neuroendocrine
tumors (3), 0.1% of extra-pulmonary
small cell carcinomas (ESCC) and <0.1% of all gastric cancer
cases (4,5), and occur primarily in males (5.4:1
ratio) (6).
Small cell carcinoma of the gastrointestinal tract
was first described in 1952 (7), and
primary SCGC was initially reported in 1976 (6). Since then, only a few hundred cases of
SCGC have been reported, mainly in Asian populations (6,8–10). SCGC is characterized by early,
widespread metastases and a poor overall prognosis (11). Due to the rarity of the disease, and
therefore the inability to conduct prospective and randomized
clinical trials, clear and thorough guidelines for SCGC treatment
have not yet been established. Nevertheless, the similarities
between SCGC and small cell lung carcinoma (SCLC) with respect to
their histopathology, molecular biology and clinical course have
resulted in the use of the same therapeutic strategies for SCGC and
SCLC (6,11).
Herein, two cases of primary SCGC in young Caucasian
males are presented, along with a review of the literature.
Informed consent was obtained from both patients for whom
identifying information is included in this article.
Case reports
Case 1
In December 2014, a 44-year old male patient was
admitted to the 251 General Air Force Hospital in Athens (Greece),
after a 2-month history of intermittent and persistent epigastric
pain. The patient was afebrile with stable vital signs, no reported
weight loss and the clinical examination revealed nothing else of
note. The medical history was insignificant and the results of the
laboratory tests were within the normal range, including the tumor
biomarkers carcinoembryonic antigen (CEA), cancer antigen (CA) 19-9
and α-fetoprotein (α-FP).
Esophagogastroduodenoscopy revealed a large
fungating mass in the gastro-esophageal junction extending from the
cardia of the stomach. The biopsy revealed a high-grade small cell
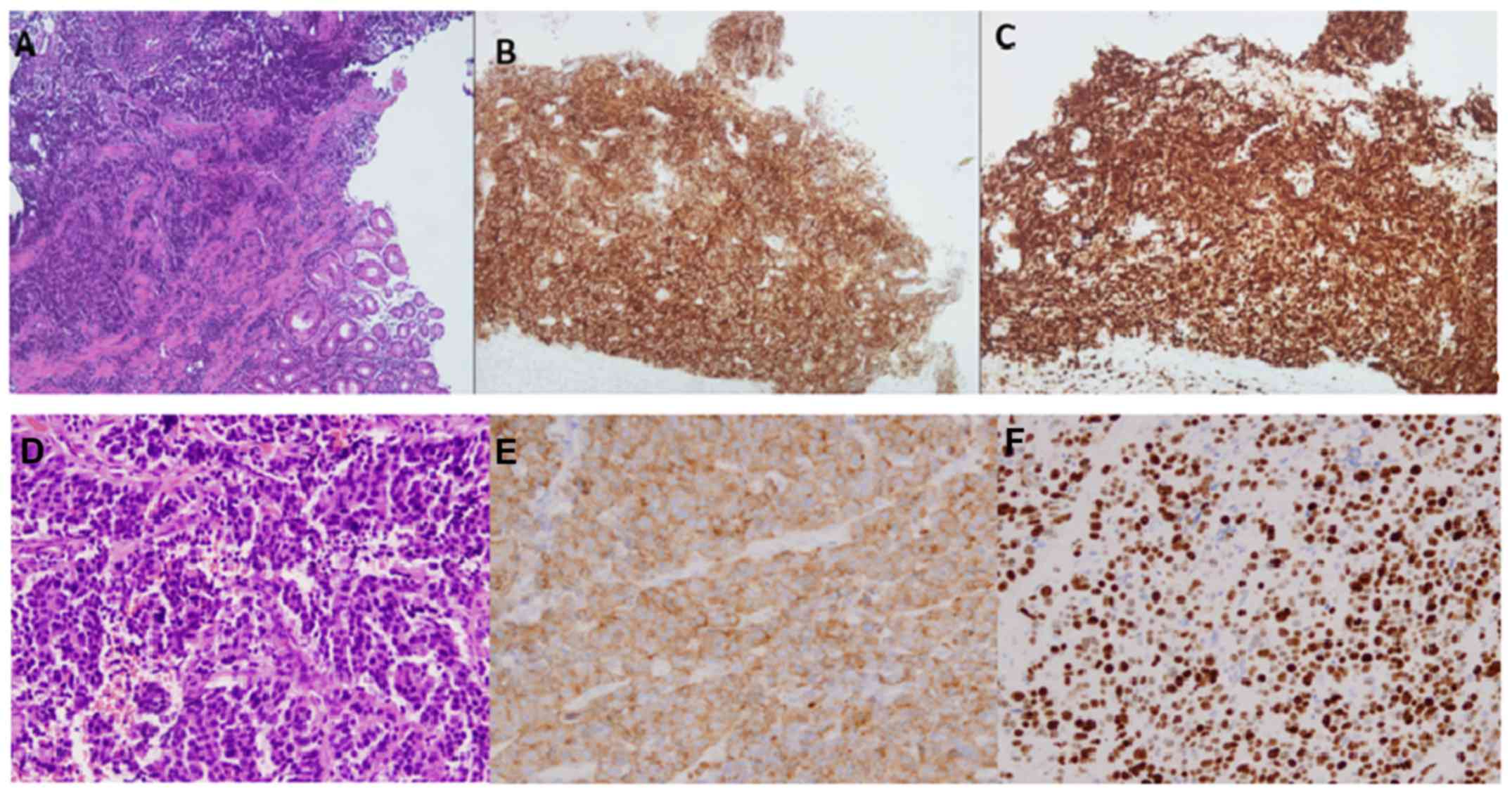
carcinoma, without evidence of Helicobacter pylori gastritis
(Fig. 1A). Immunohistochemistry
(IHC) revealed that the tumor cells were positive for
synaptophysin, chromogranin, cytokeratin 8 and 18, AE3 and CD117,
and negative for CD56, cytokeratin 7 and 20 and CEA, whereas almost
all cells (95–100%) were Ki-67 positive (Fig. 1B and C).
Computed tomography (CT) of the thorax was negative,
but a CT scan of the abdomen and pelvis revealed a round 18 mm
lesion at the gastro-esophageal junction with regional thickening
of the gastric wall around the cardia of the stomach. Two enlarged
lymph nodes were also identified at the right groin and at the
upper third of the right external iliac chain, measuring 25 and 15
mm, respectively.
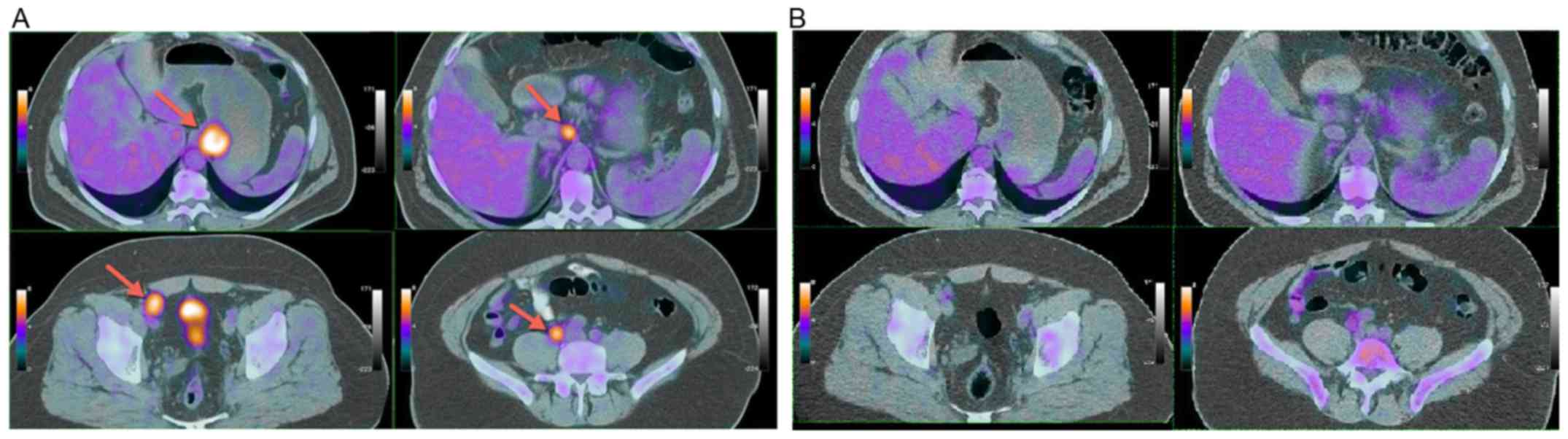
Positron emission tomography (PET)/CT revealed
increased fludeoxyglucose (FDG) uptake along the gastro-esophageal
junction and cardia of the stomach [maximum standardized uptake
value (SUVmax), 10.4], at one paraaortic lymph node (14
mm with SUVmax, 10.2) and at multiple pelvic (right
ileac and inguinal) lymph nodes (Fig.
2A). Furthermore, a highly hypermetabolic (SUVmax,
24.2) nodule of 29 mm was detected at the right thyroid lobe, and
fine needle aspiration revealed a papillary thyroid cancer.
The Multidisciplinary Team (MDT) of the Institution
(251 General Air Force Hospital) proposed systemic chemotherapy
with standard etoposide/cisplatin combination. The patient received
six cycles of chemotherapy with cisplatin 70 mg/m2 on
day 1 and etoposide 100 mg/m2 on days 1, 2 and 3, every
3 weeks, with excellent tolerance for 4 months (from December 2014
until April 2015). After the second chemotherapy cycle, a control
PET/CT scan revealed a significant metabolic partial response
(Fig. 2B) with only the two residual
right external ileac lymph nodes (one of 12 mm in diameter, with
SUVmax, 5.5; one of 9 mm in diameter, with
SUVmax, 2.2). One month following treatment completion,
imaging studies with whole body CT scans indicated a complete
clinical response. Further treatment with external radiotherapy was
selected due to better local disease control. The patient received
5,040 cGy in 28 fractions of 180 cGy to the gastro-esophageal
junction region, to the celiac, paraaortic, common ileac, internal
ileac, external ileac and inguinal lymph node regions. Radiation
was administered using intensity-modulated radiation therapy with
concurrent cisplatin (40 mg/m2) every 7 days for 3
weeks.
Approximately two months following the completion of
radiotherapy, the patient was underwent a total thyroidectomy for
papillary thyroid cancer and received post-operative radioiodine
therapy. A total of 20 months following treatment completion, the
patient remains in complete remission and asymptomatic; an
esophagogastroduodenoscopy with blind biopsies, as well as imaging
studies that included a whole body PET/CT scan, did not reveal any
residual disease.
Case 2
In October 2013, a 45-year old male was admitted to
the Iaso General Hospital in Athens, with a 3-week history of
dysphagia and pain in the upper abdomen. The patient was afebrile
and asymptomatic between meals. Clinical examination was normal and
the results of laboratory tests, including CEA, CA 19-9 and α-FP,
were within the normal range. Upper gastrointestinal endoscopy
revealed an ulcerative tumor in the gastro-esophageal junction. The
histological report indicated a high-grade small cell carcinoma
positive for synaptophysin and CD56, but negative for chromogranin
(Fig. 1D). Furthermore, the cells
were CK7(+), CK18(+), CK20(−) and Ki-67(+) (80–90% of the cells)
(Fig. 1E and F).
Thoracic and abdominal CT scans were negative for
metastatic disease, and a radical gastrectomy with D2 lymph node
dissection was performed. Pathological examination of the surgical
specimen confirmed the diagnosis of a small cell carcinoma with
metastatic involvement in six lymph nodes of the lesser omentum, in
one of the left gastric artery and in one of the hepatic artery.
The tumor invaded the muscularis propria but did not penetrate the
serosa. Thus, the TNM classification was T2N1M0 according to the
European Neuroendocrine Tumor Society (12), and T2N3M0 stage IIIA according to the
American Joint Committee on Cancer (AJCC) (12).
At 2 months following surgery, the patient was
referred to the 1st Department of Medical Oncology and received 6
cycles of chemotherapy with etoposide (100 mg/m2 on days
1, 2 and 3) and cisplatin (60 mg/m2 on day 1) every 3
weeks, for 4 months and without any treatment modifications. During
the last cycle of chemotherapy, the patient experienced an acute
thoracic pain; a subsequent CT scan and MRI of the mediastinum
revealed a mass measuring 3.4×2.5 cm at the lower left posterior
mediastinum. An endoscopic ultrasound (EUS) confirmed this finding
and a whole body PET/CT scan indicated increased FDG uptake along
the mediastinal lesion (SUVmax, 8) without other
hypermetabolic localization.
Based on these findings, a metastasectomy was
attempted, but the patient only underwent subtotal surgical
resection of the mediastinal lesion as the mass had infiltrated the
adjacent thoracic aorta. External image guided radiation therapy
(5,580 cGy in 28 fractions) with concurrent carboplatin (AUC=2)
every week for 5 weeks was subsequently administered, resulting in
disease stabilization. A total of 3 months later, thoracic and
abdominal CT scans revealed massive disease progression with
multiple lung and liver metastases and subcutaneous nodules. A
further 2 cycles of weekly paclitaxel (100 mg/m2 on days
1, 8 and 15 every 28 days), in combination with bevacizumab (10
mg/kg every 15 days), were administered without success. The
patient succumbed to disease almost 3 months later due to acute
respiratory failure causes by a lung infection and disease
progression, 20.2 months after the initial diagnosis.
Discussion
Epidemiology. Extra-pulmonary small cell carcinomas
are rare types of tumors, 18.2% of which are localized in the upper
gastrointestinal tract (13). SCGC
accounts for 0.1–1% of all GI malignancies (11), whereas primary SCGC represents ~0.1%
of all gastric cancer cases (4,5). Wu
et al (6) retrospectively
evaluated 205 patients with SCGC from January 1999 until December
2012, all from China, and reported a predominance of SCGC in males
(male to female ratio, 5.4:1) (6,11).
Molecular biology and pathology. To the best of our
knowledge, there are no published data regarding the molecular
basis of SCGC pathogenesis and its molecular biology. However, the
available data, though limited, from cases of esophageal small cell
cancer, suggest a similar molecular profile for gastrointestinal
and pulmonary small cell cancer, with universal high rates of
proliferative activity and telomerase inactivation, p53
overexpression (65–83 and 90% respectively) and pRb inactivation
(67–95 and 90% respectively). K-Ras mutations (0.17 and 0%
respectively) are rare and the loss of p16 expression (33 and 10%
respectively) is infrequent (11).
The Ki67 (MIB-1) index in SCGC is, by definition, >20%, ranging
from 30–95.5% (14). The
pathological characteristics and the immunohistochemical features
of SCGC are essentially identical to those of SCLC (4,6,11,15–21).
However, almost half of SCGC cases are ‘mixed’ or ‘combined’
tumors, comprised of SCC and nonSCC components (6,11,22,23).
Staging and prognosis. In 2007, the European
Neuroendocrine Tumour Society (ENETS) proposed a staging system of
gastrointestinal neuroendocrine neoplasms, adapted for the several
primary sites: i) Stomach; ii) duodenum, ampulla of Vater, proximal
jejunum; iii) lower jejunum; ileum; iv) pancreas; and v) colon and
rectum. It takes into account the following: i) Depth of invasion;
ii) tumor size; and iii) the presence of regional lymph nodes or
distant metastases (14,24). This staging system was later endorsed
and modified by the AJCC (12).
Patient survival depends on the treatment approach
selected. Patients who received curative surgery experienced an
~6-fold increase in survival compared with those who did not (46.45
and 7.65 months respectively) (25,26); in
addition, patients who received post-operative chemotherapy
survived ≥2× longer (48.50 vs. 19.00 months) (27). The median overall survival time for
patients with SCGC is ~18.50 months (6), with 1-, 2- and 5-year survival rates of
66.75, 37.13 and 20.10%, respectively (25–29).
Treatment. Wu et al (6) reported that 97.6% (200) of the 205
studied Chinese patients with SCGC underwent surgical resection,
leading to a median overall survival time of 46.45 months (range,
10.00–63.00 months). Although patients who received adjuvant
chemotherapy obtained a relatively prolonged survival time; thus,
curative surgery can be regarded as a standard treatment for
locoregional non-metastatic SCGC (6,27).
Post-operative chemotherapy is considered a standard
treatment and several regimens are in clinical use (Table I) (11); however, platinum based chemotherapy
forms the backbone of treatment for early and metastatic disease
(30). Huang et al (27) demonstrated a survival advantage of
almost 30 months for surgery plus chemotherapy (48.5 months) vs.
surgery alone (19 months) (27).
Pre-operative chemotherapy with the same regimen is also a rational
option (11,31). Cisplatin plus irinotecan is an
alternative first line treatment (32), but there is no standard regimen for
second line therapy. Brain metastases are infrequent and
prophylactic cranial irradiation is not recommended (33).
 | Table I.Single case reports and retrospective
series of patients with small cell gastric cancer. |
Table I.
Single case reports and retrospective
series of patients with small cell gastric cancer.
|
|
|
| Initial
treatment |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Authors/(Refs.),
year | Type of
article | Patients (n) | Surgery | Chemotherapy | Radiotherapy | CMT regimen | OS |
|---|
| Wu et al
(6), 2015 | Review article | 205 | Yes (n=200) | Yes (n=139) | Yes (n=2) | N/A | N/A |
| Namikawa et
al (15), 2005 | Case report | 1 | Yes | Yes | – | CDDP+VP-16 | >36 months |
| Frances et
al (23), 2013 | Case report | 1 | – | Yes | Yes | CDDP+VP-16 | 7.5 months |
| Dong et al
(25), 2010 | Series of
patients | 23 | Yes | N/A | – | N/A | 17.7 months |
| Huang et al
(26), 2013 | Series of
patients | 41 | Yes (n=25) | Yes (n=25) | – |
CDDP/Carboplatin+VP-16 | 19 months |
| Liu et al
(28), 2013 | Series of
patients | 17 | Yes (n=17) | Yes (n=11) | – |
CDDP/Carboplatin+VP-16 | 13 months |
| Kou et al
(29), 2013 | Series of
patients | 42 | Yes | Yes | – | 5-FU+LOHP | 25 months |
| Terada et al
(34), 2013 | Case report | 1 | – | Yes | – | CDDP based | N/A |
| Kuo et al
(35), 2009 | Case report | 1 | Yes | Yes | – |
CDDP/Carboplatin+VP-16 | 17 months |
| Kai Xin et
al (36), 2014 | Case report | 1 | Yes | Yes | – | CPT-11+LOHP | N/A |
| Iwamuro et
al (37), 2009 | Case report | 2 | – | Yes | – |
Carboplatin+VP-16- | 14.5 months |
| Koide et al
(38), 2007 | Case report | 1 | Yes | Yes | – | CDDP+S-1 | N/A |
| Hussein et
al (39), 1990 | Case report | 1 | Yes | Yes | – | N/A | 10 |
| Hamano et al
(40), 2007 | Case report | 1 | Yes | – | – | – | N/A |
| Funahashi et
al (41), 2013 | Case report | 1 | Yes | Yes | – | CDDP+CPT-11 | 12 months |
| Tanemura et
al (42), 2002 | Case report | 1 | Yes | – | – | – | >43 months |
| Moise et al
(43), 2010 | Case report | 1 | – | – | – | – | 0.5 months |
| Cioppa et al
(44), 2007 | Case report | 1 | Yes | Yes | – | CDDP based CMT | 15 months |
| Okita et al
(45), 2011 | Series of
patients | 22 | Yes | Yes (at
relapse) | – | CDDP+CPT-11 | 22.6 months |
| Peng et al
(46), 2013 | Series of
patients | 27 | Yes (n=27) | Yes (n=22) | – | CDDP+VP-16 (n=12)
Cyclophosphamide+Doxorubicin+CDDP (n=10) | 10 months |
| Nakamura et
al (47), 2005 | Case report | 1 | Yes | Yes | – |
Carboplatin+Epirubin+VP-16+5-FU | >36 months |
| Onoyama et
al (48), 2011 | Case report | 1 | – | Yes | – | CDDP+CPT-11 | >28 months |
Although concurrent or sequential chemoradiotherapy
is associated with satisfactory results in patients with
gastrointestinal small cell carcinoma, particularly esophageal-SCC,
data regarding the effect of radiotherapy on SCGC are currently
limited. In the larger published series of patients with SCGC by Wu
et al (6) only two patients
received radiotherapy.
The two patient cases presented herein have been
treated according to current practice but had opposing outcomes.
The patient with disseminated disease at diagnosis (case 1) was
evaluated in the MDT and it was decided to administer first-line
chemotherapy with the standard etoposide/cisplatin regimen.
Chemotherapy resulted in a major objective response that was
followed by radiotherapy. It is considered, as in the case of SCLC,
that involved-field radiation may further disease control, thus
explaining the favorable clinical outcome of the patient.
Conversely, the second patient did not have the opportunity to be
discussed in the MDT and underwent a ‘curative’ gastrectomy for
locoregional disease with post-operative chemotherapy applied after
a 2-month delay. However, it should be noted that the pre-surgical
staging of the disease was inadequate as there was no more detailed
evaluation of the regional lymph nodes status by either a PET/CT
scan or a laparoscopy and lymph node sampling. Such an evaluation
could change the therapeutic plan administering first systemic
chemotherapy followed by, depending on the results, either curative
surgery or involved-field radiotherapy. On the other hand, it
cannot be excluded that the 2-month delay in the administration of
chemotherapy was an important adverse contributor to the patient's
clinical outcome and the early disease dissemination based on the
assumption of the possible presence of occult metastatic disease at
the time of initial diagnosis.
To conclude, primary SCGC is a rare type of disease
in Caucasians, and the two cases discussed indicate the treatment
challenges presented by this disease. SCGC is aggressive with poor
prognosis and currently small retrospective case series are the
main source of data for this malignancy, which shares the same
histopathological, molecular, clinical and treatment
characteristics with SCLC. Careful evaluation of each patient's
case by the MDT and a multimodal therapeutic approach are strongly
recommended for this disease, with platinum-based chemotherapy
regimens to represent the standard of care. The MDT must determine
for each individual case the optimal therapeutic strategy, and
critically evaluate the role and the sequence of local treatments
(surgery or radiotherapy) administered alone or in combination with
chemotherapy. This is particularly important considering the
absence of prospective randomized studies as the only data
currently available are from treated patients.
Acknowledgements
The authors would like to thank Mrs. Vasso
Athanasaki, Scientific Secretary of the Hellenic Oncology Research
Group, for her attentive editing of this manuscript.
References
|
1
|
Öberg K, Knigge U, Kwekkeboom D and Perren
A: ESMO Guidelines Working Group: Neuroendocrine
gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up. Ann Oncol 23 Suppl.
7:vii124–130. 2012.
|
|
2
|
Basturk O, Tang L, Hruban RH, Adsay V,
Yang Z, Krasinskas AM, Vakiani E, La Rosa S, Jang KT, Frankel WL,
et al: Poorly differentiated neuroendocrine carcinomas of the
pancreas: A clinicopathologic analysis of 44 cases. Am J Surg
Pathol. 38:437–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hassan MM, Phan A, Li D, Dagohoy CG, Leary
C and Yao JC: Risk factors associated with neuroendocrine tumors: A
U.S.-based case-control study. Int J Cancer. 123:867–873. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kusayanagi S, Konishi K, Miyasaka N,
Sasaki K, Kurahashi T, Kaneko K, Akita Y, Yoshikawa N, Kusano M,
Yamochi T, et al: Primary small cell carcinoma of the stomach. J
Gastroenterol Hepatol. 18:743–747. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim KO, Lee HY, Chun SH, Shin SJ, Kim MK,
Lee KH, Hyun MS, Bae SH and Ryoo HM: Clinical overview of
extrapulmonary small cell carcinoma. J Korean Med Sci. 21:833–837.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu QQ, Qiang WG, Wang F, Dai KJ, Xu EC,
Luo JD, Li Q, Tang H, Zhou XF and Lu XJ: Management of primary
gastric small cell carcinoma in China. Int J Clin Exp Med.
8:1589–1597. 2015.PubMed/NCBI
|
|
7
|
McKeown F: Oat-cell carcinoma of the
oesophagus. J Pathol Bacteriol. 64:889–891. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takaku H, Oka K, Naoi Y, Santoh N, Setsu Y
and Mori N: Primary advanced gastric small cell carcinoma: A case
report and review of the literature. Am J Gastroenterol.
94:1402–1404. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujisawa K, Iwashita A and Ueno H:
Multiple cancers of the stomach including oatcell carcinoma, an
autopsy case. Ito Cho. 16:1349–1354. 1981.(In Japanese).
|
|
10
|
Fukuda T, Ohnishi Y, Nishimaki T, Ohtani H
and Tachikawa S: Early gastric cancer of the small cell type. Am J
Gastroenterol. 83:1176–1179. 1988.PubMed/NCBI
|
|
11
|
Brenner B, Tang LH, Klimstra DS and Kelsen
DP: Small-cell carcinomas of the gastrointestinal tract: A review.
J Clin Oncol. 22:2730–2739. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gennatas S, Noble J, Stanway S, Gunapala
R, Chowdhury R, Wotherspoon A, Benepal T and Popat S: Patterns of
relapse in extrapulmonary small cell carcinoma: Retrospective
analysis of outcomes from two cancer centres. BMJ Open.
5:e0064402015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
World Health Organization, . WHO
Classification of Tumours of the Digestive SystemBosman FT,
Carneiro F, Hruban RH and Theise ND: 4th edition. IARC WHO
Classification of Tumours; Lyon: pp. 4172010
|
|
15
|
Namikawa T, Kobayashi M, Okabayashi T,
Ozaki S, Nakamura S, Yamashita K, Ueta H, Miyazaki J, Tamura S,
Ohtsuki Y and Araki K: Primary gastric small cell carcinoma: Report
of a case and review of the literature. Med Mol Morphol.
38:256–261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wick MR, Weatherby RP and Weiland LH:
Small cell neuroendocrine carcinoma of the colon and rectum:
Clinical, histologic, and ultrastructural study and
immunohistochemical comparison with cloacogenic carcinoma. Hum
Pathol. 18:9–21. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burke AB, Shekitka KM and Sobin LH: Small
cell carcinomas of the large intestine. Am J Clin Pathol.
95:315–321. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ho KJ, Herrera GA, Jones JM and Alexander
CB: Small cell carcinoma of the esophagus: Evidence for a unified
histogenesis. Hum Pathol. 15:460–468. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takubo K, Nakamura K, Sawabe M, Arai T,
Esaki Y, Miyashita M, Mafune K, Tanaka Y and Sasajima K: Primary
undifferentiated small cell carcinoma of the esophagus. Hum Pathol.
30:216–221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maitra A, Tascilar M, Hruban RH, Offerhaus
GJ and Albores-Saavedra J: Small cell carcinoma of the gallbladder:
A clinicopathologic, immunohistochemical, and molecular pathology
study of 12 cases. Am J Surg Pathol. 25:595–601. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sarsfield P and Anthony PP: Small cell
undifferentiated (‘neuroendocrine’) carcinoma of the colon.
Histopathology. 16:357–363. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Joyce EA, Kavanagh J, Sheehy N, Beddy P
and O'Keeffe SA: Imaging features of extrapulmonary small cell
carcinoma. Clin Radiol. 68:953–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frances N, Zeichner SB, Francavilla M and
Cusnir M: Gastric small-cell carcinoma found on
esophagogastroduodenoscopy: A case report and literature review.
Case Rep Oncol Med. 2013:4759612013.PubMed/NCBI
|
|
24
|
Pape UF, Jann H, Müller-Nordhorn J,
Bockelbrink A, Berndt U, Willich SN, Koch M, Röcken C, Rindi G and
Wiedenmann B: Prognostic relevance of a novel TNM classification
system for upper gastroenteropancreatic neuroendocrine tumors.
Cancer. 113:256–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong RZ, Shi YQ, Ye YW, Fu H and Zhao GF:
Clinicopathological and prognostic analysis of 23 poorly
differentiated neuroendocrine carcinomas of the stomach. Zhonghua
Wei Chang Wai Ke Za Zhi. 13:583–586. 2010.(In Chinese). PubMed/NCBI
|
|
26
|
Huang S, Zheng ZX, Xu Q and Yuan XH: The
diagnosis, treatment and prognosis evaluation of gastric small cell
carcinoma: Analysis of 41 cases. Zhonghua Wai Ke Za Zhi.
51:225–229. 2013.(In Chinese). PubMed/NCBI
|
|
27
|
Huang J, Zhou Y, Zhao X, Zhang H, Yuan X
and Wang J: Primary small cell carcinoma of the stomach: An
experience of two decades (1990–2011) in a Chinese cancer
institute. J Surg Oncol. 106:994–998. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Xie YB, Xu Q, Zhang JW, Tian YT,
Zhao DB, Wang CF, Shan Y, Zhou ZX and Yuan XH: Clinical analysis of
17 cases of gastric small cell carcinoma. Zhonghua Zhong Liu Za
Zhi. 35:292–294. 2013.(In Chinese). PubMed/NCBI
|
|
29
|
Kou Y, Gao YB, Ma J, Yang K, Fu Q and Xie
JG: Prognostic analysis of 42 patients with gastric neuroendocrine
carcinoma. Zhonghua Wei Chang Wai Ke Za Zhi. 16:570–573. 2013.(In
Chinese). PubMed/NCBI
|
|
30
|
Sorbye H, Strosberg J, Baudin E, Klimstra
DS and Yao JC: Gastroenteropancreatic high-grade neuroendocrine
carcinoma. Cancer. 120:2814–2823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sorbye H: Neoadjuvant chemotherapy in
extra-pulmonary neuroendocrine carcinoma. Neoadjuvant Chemotherapy.
Current Applications in Clinical Practice. Oliver F Bathe:
http://www.intechopencom/articles/show/title/neoadjuvant-chemotherapy-in-poorly-differentiated-neuroendocrine-carcinoma2012.
|
|
32
|
Yamaguchi T, Machida N, Morizane C, Kasuga
A, Takahashi H, Sudo K, Nishina T, Tobimatsu K, Ishido K, Furuse J,
et al: Multicenter retrospective analysis of systemic chemotherapy
for advanced neuroendocrine carcinoma of the digestive system.
Cancer Sci. 105:1176–1181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cicin I, Karagol H, Uzunoglu S, Uygun K,
Usta U, Kocak Z, Caloglu M, Saynak M, Tokatli F and Uzal C:
Extrapulmonary small-cell carcinoma compared with small-cell lung
carcinoma: A retrospective single-center study. Cancer.
110:1068–1076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Terada T: Primary small cell carcinoma of
the stomach: a case report with an immunohistochemical and
molecular genetic analysis. Int J Clin Exp Pathol. 6:524–530.
2013.PubMed/NCBI
|
|
35
|
Kuo SC, Chao Y, Luo JC, Lee KC, Wu CW, Li
AFY, Lee RC and Li CP: Primary small cell carcinoma of the stomach
successfully treated with cisplatin and etoposide. J Chin Med
Assoc. 72:598–602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xin K, Wei J, Wang H, Guan W and Liu B:
Neoadjuvant chemotherapy followed by D2 gastrectomy and
esophagojejunal Roux en Y anastomosis in gastric small cell
carcinoma: A case report. Oncol Lett. 8:2549–2552. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iwamuro M, Tanakab S, Besshob A, Takahashi
H, Ohtab T, Takadac R and Murakami I: Two cases of primary small
cell carcinoma of the stomach. Acta Med Okayama. 63:293–298.
2009.PubMed/NCBI
|
|
38
|
Koide N, Suzuki A, Saito H, Sato T,
Murakami M, Ota H and Miyagawa S: Gastric small cell carcinoma
successfully treated by surgery and postoperative chemotherapy
consisting of cisplatin and S-1: report of a case. Surg Today.
37:989–994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hussein AM, Otrakji CL and Hussein BT:
Small cell carcinoma of the stomach. Case report and review of the
literature. Dig Dis Sci. 35:513–518. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hamano R, Hirao T, Tokuoka M, Masuzawa T,
Shibata K and Kobayashi T: A case report of gastric small cell
carcinoma with long survival time by adjuvant chemotherapy-reports
of chemotherapy regimens for gastric small cell carcinoma. Gan To
Kagaku Ryoho. 34:609–613. 2007.PubMed/NCBI
|
|
41
|
Funahashi H, Miyai H, Wakasugi T, Ishiguro
H, Matsuo Y, Kimura M and Takeyama H: Successful combination
chemotherapy with irinotecan hydrochloride and cisplatin for
primary gastric small cell carcinoma: report of a case. World J
Surg Oncol. 11:2632013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ohshita H, Kanno A, Kusakabe M, Tomita E,
Nishigaki Y, Sugiyama A and Yamada T: A patient with small-cell
carcinoma of the stomach with long survival after percutaneous
microwave coagulating therapy (PMCT) for liver metastasis. Int J
Clin Oncol. 7:128–132. 2002.PubMed/NCBI
|
|
43
|
Moise D, Singh J, Dahl K, Rashid S, Prasad
A, Siddiqui G, Subramani K, Mustacchia P and Rizvon K:
Extrapulmonary small cell carcinoma of the stomach: a lethal
entity. Case Rep Gastroenterol. 4:298–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cioppa T, Marrelli D, Neri A, Caruso S,
Pedrazzani C, Malagnino V, Pinto E and Roviello F: A case of
small-cell gastric carcinoma with an adenocarcinoma component and
hepatic metastases: treatment with systemic and intra-hepatic
chemotherapy. Eur J Cancer Care (Engl). 16:453–457. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Okita NT, Kato K, Takahari D, Hirashima Y,
Nakajima TE, Matsubara J, Hamaguchi T, Yamada Y, Shimada Y,
Taniguchi H and Shirao K: Neuroendocrine tumors of the stomach:
chemotherapy with cisplatin plus irinotecan is effective for
gastric poorly-differentiated neuroendocrine carcinoma. Gastric
Cancer. 14:161–165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peng C, Shen S, Zhang X and Zou X: Limited
stage small cell carcinoma of the gastrointestinal tract: a
clinicopathologic and prognostic analysis of 27 cases. Rare Tumors.
5:e62013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nakamura Y, Otani S, Otaka M, Shimada T,
Takahashi S, Saito M, Takahashi T, Komatsu M, Suzuki T, Okubo S,
Hayashi M and Sasano H: Gastric small cell carcinoma with marked
response to neoadjuvant chemotherapy. Int J Clin Oncol. 10:348–352.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Onoyama H, Iwasaki Y, Ohashi M, Iwanaga T,
Ohinata R, Maeda Y, Omuro Y, Sasaki E, Shimoyama T and Tateishi Y:
A case of gastric neuroendocrine cell carcinoma successfully
treated by neoadjuvant chemotherapy. Gan To Kagaku Ryoho.
38:2131–2133. 2011.PubMed/NCBI
|