Introduction
Sunitinib malate (Sutent, Pfizer Inc., New York, NY,
USA), a small-molecule multi-targeted inhibitor of tyrosine
kinases, is widely recognized as a standard treatment option for
patients with advanced renal cell carcinoma (RCC). In a randomized,
multicenter, phase III trial, 750 patients with previously
untreated metastatic RCC were enrolled to receive either sunitinib
or interferon α (IFN-α). Sunitinib was found to be superior to
IFN-α with respect to objective response rate (47 vs. 12%),
progression-free survival (median, 11.0 vs. 5.0 months), and
overall survival (median, 26.4 vs. 21.8 months) (1,2).
Elderly patients constitute a large proportion of
the advanced RCC population, and the incidence of RCC peaks between
ages 60 and 70 years (3). Overall
survival in RCC patients is negatively correlated with the severity
and number of comorbidities (4).
Comorbid conditions are generally more prevalent in elderly
patients compared with younger patients (5,6). In
addition, elderly patients with cancer are more likely to have a
compromised performance status (PS) (7). The presence of comorbidities and
decreased PS in elderly patients may result in their
under-representation in clinical trials (8,9).
Clinical trials have not directly compared the efficacy and safety
of targeted agents in the elderly population, and outcomes in
elderly subsets may be informative about how age affects the
efficacy and tolerability of individual targeted agents. In the
present study, we evaluate the actual tolerability of sunitinib in
elderly Japanese patients with advanced RCC.
Patients and methods
The present study was performed with the approval of
the Kitasato University Medical Ethics Organization (approval no.
KMEO B16-156); the requirement for informed consent was waived due
to the retrospective nature of the analyses. The data of 56
consecutive patients with advanced RCC who underwent sunitinib
treatment between December 2008 and December 2012 was collected and
analyzed in the present study. Eligible patients had measurable
tumors (metastatic or primary). All patients had undergone surgical
treatment or biopsy of the primary lesion and had histologically
proven RCC. The sample comprised 40 men and 16 women with a median
age of 64 years (range, 36–80 years) at the time of sunitinib
initiation. In general, 50 mg sunitinib was administered orally
once daily in a 6-week cycle consisting of 4 weeks of treatment
followed by 2 weeks without treatment. Dose reductions were
permitted on the basis of individual tolerability.
Response and progression were assessed by the
treating physician according to the Response Evaluation Criteria in
Solid Tumors (RECIST), version 1.1, with computed tomography or
magnetic resonance imaging performed every 4 to 8 weeks. Adverse
events were evaluated by means of physical examination and
laboratory assessments such as hematologic and serum chemistry
every 2 to 4 weeks during treatment with sunitinib and were graded
according to the National Cancer Institute Common Terminology
Criteria for Adverse Events (NCI CTCAE), version 4.0.
Patient charts were retrospectively reviewed.
Patients were divided into two groups according to the age at the
time of sunitinib initiation: (i) elderly cohort (≥70 years), and
(ii) younger cohort (<70 years). Comorbidity in each patient was
evaluated by the Charlson comorbidity index (10). Disease control rates, which were
defined as complete response, partial response, and stable disease,
were evaluated. Progression-free survival, overall survival, and
relative dose intensity (RDI) were analyzed for each cohort. The
RDI was determined as the ratio of the cumulative dose that was
received during the cycle to 1,400 mg.
The groups were analyzed using the t-test for
differences of means between groups. The chi-square test was used
to evaluate differences for categorical variables. Non-parametric
estimates of survival were performed using Kaplan-Meier curves.
Survival curves were generated on the basis of progression-free
survival or overall survival from the initiation of sunitinib to
the date of disease progression or death. Log-rank tests were used
for statistical comparisons. All analyses were performed with
StatView, version 5.0 (SAS Institute, Cary, NC, USA), and P<0.05
was considered to indicate a statistically significant
difference.
Results
The clinical characteristics of each cohort treated
with sunitinib are summarized in Table
I. The elderly cohort (≥70 years; range, 71–80 years) comprised
10 men and 4 women (total 14 patients; 25.0%). The younger cohort
(<70 years; range, 36–69 years) comprised 30 men and 12 women
(total 42 patients; 75.0%). The elderly cohort had a significantly
higher Charlson comorbidity index than the younger cohort (mean,
9.7 vs. 7.9; P<0.0001). Other pretreatment variables, including
Eastern Cooperative Oncology Group PS, Memorial Sloan-Kettering
Cancer Center risk classification, C-reactive protein, prior
nephrectomy, prior immunotherapy, prior targeted therapy, and
number of metastatic sites, did not reach statistically significant
differences (Table I).
 | Table I.Patient characteristics grouped by age
at the time of sunitinib initiation. |
Table I.
Patient characteristics grouped by age
at the time of sunitinib initiation.
| Characteristics | ≥70 years | <70 years | P-value |
|---|
| Total patients, n
(%) | 14 (25.0) | 42 (75.0) | – |
| Age, years | 71–80 | 36–69 | – |
| Sex, n (%) |
|
| – |
| Male | 10 (71.4) | 30 (71.4) |
|
|
Female | 4 (28.6) | 12 (28.6) |
|
| Charlson comorbidity
index |
|
| <0.0001 |
|
Median | 9 | 8 |
|
|
Range | 9–11 | 6–10 |
|
| Mean | 9.7 | 7.9 |
|
| ECOG PS |
|
| 0.6249 |
| 0 | 10 (71.4) | 27 (64.3) |
|
| ≥1 | 4 (28.6) | 15 (35.7) |
|
| MSKCC risk
classification, n (%) |
|
| 0.6952 |
|
Favorable | 3 (21.4) | 7 (16.7) |
|
|
Intermediate | 8 (57.1) | 21 (50.0) |
|
| Poor | 3 (21.4) | 14 (33.3) |
|
| Pretreatment CRP, n
(%) |
|
| 0.2402 |
| ≤0.30
mg/dl | 6 (42.9) | 11 (26.2) |
|
| >0.30
mg/dl | 8 (57.1) | 31 (73.8) |
|
| Prior nephrectomy, n
(%) |
|
| 0.1325 |
| Yes | 13 (92.9) | 31 (73.8) |
|
| No | 1 (7.1) | 11 (26.2) |
|
| Histological
classification, n (%) |
|
| 0.3040 |
|
Clear-cell | 14 (100) | 39 (92.9) |
|
|
Papillary | 0 (0) | 3 (7.1) |
|
| T stage, n (%) |
|
| 0.2773 |
| T1 or
T2 | 8 (57.1) | 17 (40.5) |
|
| ≥T3 | 6 (42.9) | 25 (59.5) |
|
| Grade, n (%) |
|
| 0.1742 |
| 1 or
2 | 10 (71.4) | 23 (54.8) |
|
| 3 | 2 (14.3) | 14 (33.3) |
|
| Prior immunotherapy,
n |
|
| 0.4401 |
|
IFN-α | 6 | 11 |
|
| IL-2 and
IFN-α | 2 | 8 |
|
| Prior targeted
therapy, n |
|
| 0.8667 |
|
Sorafenib | 4 | 13 |
|
| Metastatic sites,
n |
|
| – |
| Lung | 8 | 31 |
|
| Bone | 4 | 15 |
|
| Lymph
nodes | 2 | 11 |
|
|
Brain | 0 | 4 |
|
|
Pancreas | 2 | 4 |
|
|
Adrenal | 2 | 4 |
|
|
Skin | 0 | 3 |
|
|
Liver | 1 | 3 |
|
|
Kidney | 1 | 2 |
|
|
Local | 1 | 2 |
|
|
Prostate | 0 | 1 |
|
| No. of metastatic
sites, n (%) |
|
| 0.9410 |
| 1 | 5 (35.7) | 17 (40.5) |
|
| ≥2 | 7 (50.0) | 25 (59.5) |
|
| Treatment, n
(%) |
|
| 0.3887 |
|
First-line | 5 (35.7) | 21 (50.0) |
|
|
Second-line | 6 (42.9) | 10 (23.8) |
|
|
Third-line | 3 (21.4) | 11 (26.2) |
|
Disease control rates according to the RECIST were
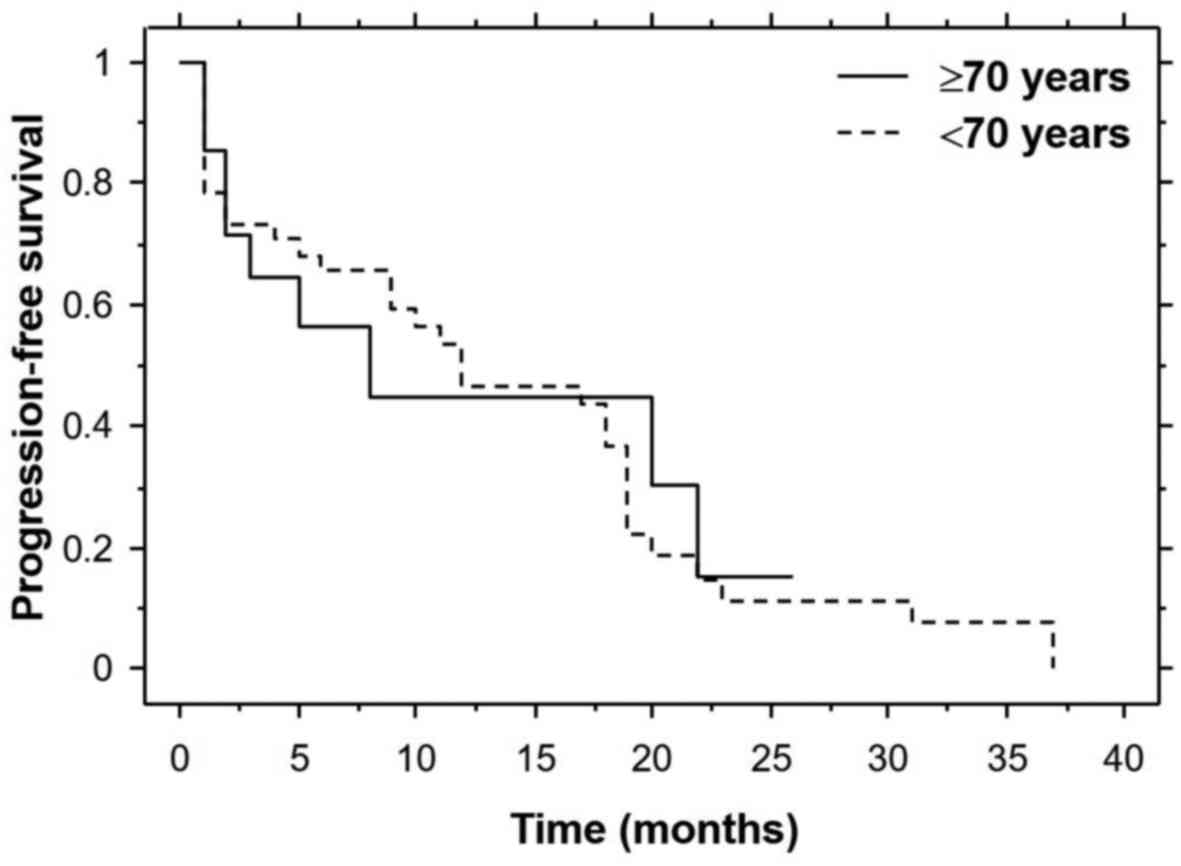
57.1% in both cohorts. The median progression-free survival time
was 8.0 months in the elderly cohort and 12.0 months in the younger
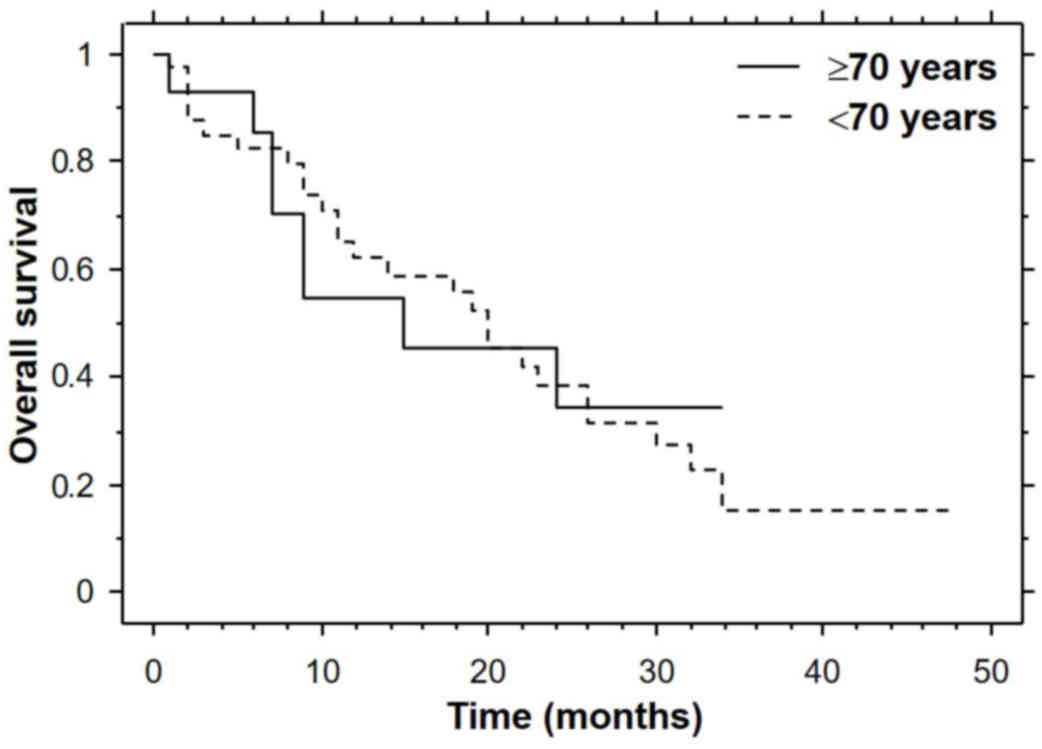
cohort (Fig. 1). The median overall
survival time was 15.0 months in the elderly cohort and 20.0 months
in the younger cohort (Fig. 2). The
differences did not reach statistical significance.
Starting doses of sunitinib were not significantly
different between the elderly and younger cohorts (P=0.4838). In
the elderly cohort, the starting dose was 50 mg in 2 patients
(14.3%), 37.5 mg in 8 patients (57.1%), and 25 mg in 4 patients
(28.6%). In the younger cohort, the starting dose was 50 mg in 10
patients (23.8%), 37.5 mg in 17 patients (40.5%), 25 mg in 11
patients (26.2%), and 12.5 mg in 4 patients (9.5%). Dose reductions
were needed for all patients in the elderly cohort and 90.5% of the
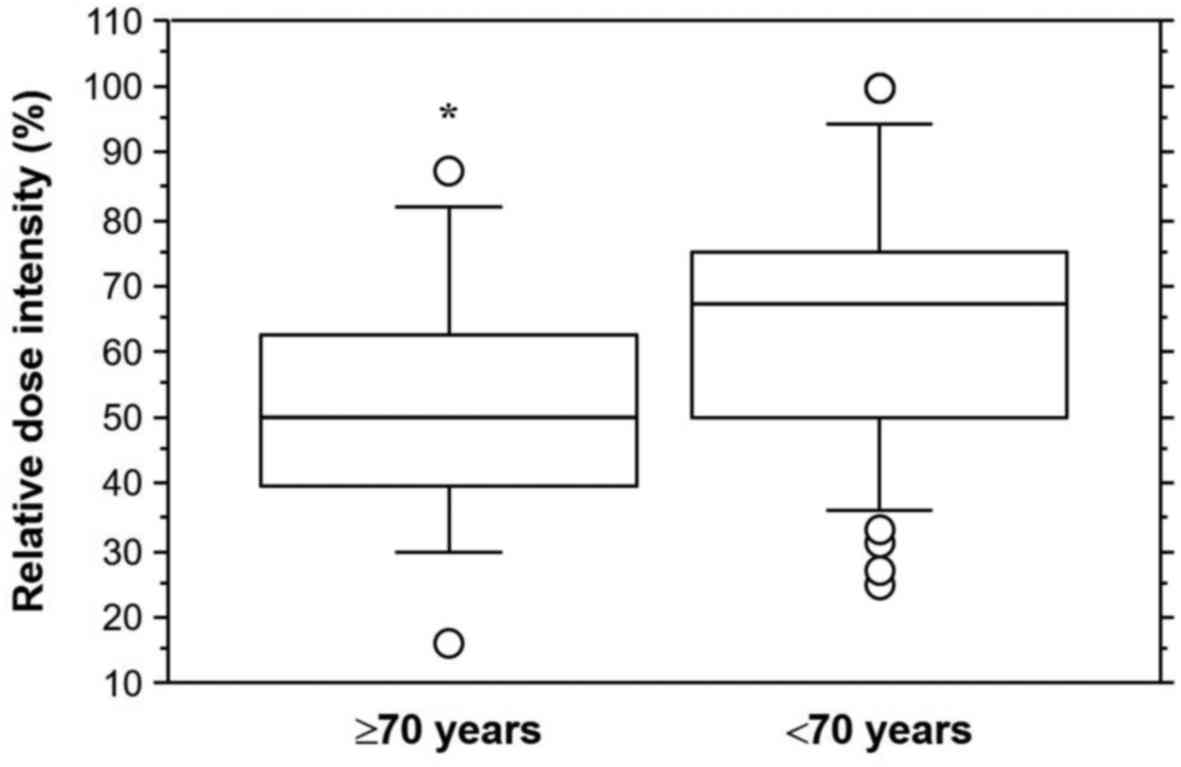
patients in the younger cohort. The elderly cohort had a
significantly lower RDI than the younger cohort (mean ± standard
deviation, 51.7 ± 19.0% vs. 65.0 ± 20.2%; P=0.0340, Fig. 3).
Selected treatment-related adverse events that
occurred during the study are summarized in Table II. The elderly cohort demonstrated
significantly lower frequency of altered taste than the younger
cohort (P=0.0383). No other adverse events showed statistically
significant differences. In addition, there were no NCI CTCAE grade
4 or 5 adverse events in either cohort. No patients discontinued
sunitinib treatment because of adverse events.
 | Table II.Selected treatment-associated adverse
events (all grades). |
Table II.
Selected treatment-associated adverse
events (all grades).
| Event | ≥70 years | <70 years | P-value |
|---|
| Adverse event,
% |
|
Hypertension | 64.3 | 61.9 | 0.8734 |
|
Hand-foot syndrome | 35.7 | 47.6 | 0.4378 |
|
Stomatitis | 57.1 | 40.5 | 0.2773 |
|
Fatigue | 35.7 | 40.5 | 0.7520 |
|
Diarrhea | 42.9 | 38.1 | 0.7520 |
| Altered
taste | 14.3 | 45.2 | 0.0383 |
|
Edema | 35.7 | 23.8 | 0.3837 |
|
Nausea | 14.3 | 9.5 | 0.6179 |
|
Fever | 14.3 | 9.5 | 0.6179 |
| Cardiac
dysfunction | 7.1 | 4.8 | 0.7319 |
|
Cholecystitis | 0 | 7.1 | 0.3040 |
|
Enteritis | 0 | 2.4 | 0.5602 |
| Nasal
bleeding | 0 | 2.4 | 0.5602 |
| Laboratory
abnormalities, % |
|
Leukopenia | 85.7 | 83.3 | 0.8336 |
|
Anemia | 85.7 | 76.2 | 0.4520 |
|
Thrombocytopenia | 92.9 | 92.9 | – |
|
Increased creatinine | 50.0 | 45.2 | 0.7570 |
|
Increased alanine
transaminase | 14.3 | 9.5 | 0.6179 |
|
Increased alkaline
phosphatase | 0 | 4.8 | 0.4057 |
|
Increased lipase | 0 | 9.5 | 0.2308 |
|
Hypothyroidism | 50.0 | 64.3 | 0.3432 |
|
Proteinuria | 64.3 | 78.6 | 0.2850 |
Discussion
This retrospective study demonstrated that sunitinib
efficacy and safety are comparable between elderly and younger
Japanese patients with advanced RCC. Disease control rates,
progression-free survival times, and overall survival times were
not significantly different between the elderly and younger
cohorts. Previous studies revealed similar efficacy and
tolerability of sunitinib in patients aged ≥70 years (11,12).
Brunello et al (11) reported
that median progression-free survival was 13.6 months and median
overall survival was 18.3 months in 68 elderly Italian patients
with metastatic RCC treated with sunitinib. They concluded that
treatment with sunitinib is feasible and effective in elderly
patients (11). Hutson et al
(12) reported a large pooled data
set from six clinical trials. Of 1,059 patients with metastatic RCC
treated with sunitinib, 202 (19%) were ≥70 years. In the first-line
treatment setting, median progression-free survival was 11.0 months
and median overall survival was 25.6 months. In the
cytokine-refractory treatment setting, median progression-free
survival was 8.4 months and overall survival was 15.8 months. The
adverse events profile was broadly similar in elderly and younger
patients. Advanced age alone should not be a deterrent to using
sunitinib in this population (12).
Despite comparable results in survival analysis in
the current study, sunitinib doses were different. RDI (mean ±
standard deviation) was 51.7±19.0% for the elderly cohort and
65.0±20.2% for the younger cohort. The elderly cohort had a
significantly lower RDI than the younger cohort (P=0.0340). The
significantly lower RDI of sunitinib is a novel finding from the
present study. Elderly Japanese patients obtained optimal
therapeutic efficacy from a relatively low dose of sunitinib. In
contrast to this result, Hutson et al (12) reported that exposure to sunitinib was
comparable in elderly and younger age groups. In their study, the
median RDI of sunitinib was 89.7% for patients aged ≥70 years and
97.1% for patients aged <70 years (12). In addition, an expanded-access trial
reported that mean RDI was 95.2% (13). However, difficulty continuing an
initial dose of sunitinib therapy without drug withdrawal has been
reported in Japanese patients (14).
Thus, genetic differences in sunitinib metabolism should be
considered.
The adverse events profiles are comparable between
elderly and younger patients in this study, higher incidence of
adverse events than phase III clinical trial (1) was observed. Hutson et al
(12) already published large pooled
data sets of sunitinib-treated patients with RCC and have shown
that the adverse events profiles are similarly in elderly and
younger patients. Kato et al (15) reported a high incidence of
sunitinib-induced hematotoxicity in Japanese patients with RCC,
resulting in difficulties maintaining the original 4-weeks on
2-weeks off dosing regimen because of a high incidence of adverse
events. There are no established criteria to decide the starting
and maintenance doses in molecular-targeted agents (15). Modified dosing regimen should be
designed especially for elderly patients with advanced RCC.
Elderly patients with RCC may be more responsive to
sunitinib treatment. A recent RCC tumor biopsy study revealed
age-related differences in tumor vasculature, with clear cell RCC
tumors from patients aged ≥65 years having significantly higher
microvascular density than those from patients aged <65 years;
in addition, markers of angiogenic activity also differed (16). Potential explanations for the elderly
being more responsive to sunitinib are that the higher
microvascular density results in greater sensitivity to
anti-angiogenic treatment or that the higher vessel density is
inversely associated with tumor aggressiveness (17).
Several studies have suggested that increasing age
is an adverse prognostic factor in RCC, with older age being
associated with higher tumor stage and grade (18–21).
However, efficacy and safety of sunitinib were comparable between
elderly and younger patients with advanced RCC. The lack of
clinical trial data and the associated dearth of evidence-based
guidelines for elderly patients mean that physicians have little to
guide them, with the result that patients may not receive the
optimal therapy (22).
Characterizing the efficacy and safety of sunitinib treatment in
elderly patients is important.
The potential limitations of this study are that it
was a retrospective single institutional study and the sample size
was small. The major drawback of this study is the small patient
numbers, which makes it unlikely to expect differences that reach
statistical relevance. From the only 14 ‘elderly’ patients, 8
patients are in tumor stage T1 or T2 and 6 patients are in ≥T3. One
patient did not undergo surgery prior treatment, meaning, the
elderly cohort is inhomogeneously. In a post-marketing Japanese
study of sunitinib including 1,689 patients with advanced RCC, 43%
of patients were ≥65 years and the maximum age was 88 years old.
This real-world analysis revealed that median progression-free
survival was 22.7 weeks and median overall survival was not reached
(23). These limitations are
unlikely to have affected the validity of our results.
Although the elderly cohort (≥70 years) had a
significantly lower RDI, relatively shorter progression-free and
overall survival times than the younger cohort (<70 years),
clinical benefit might be obtained from sunitinib treatment.
In conclusion, treatment with sunitinib is feasible
and effective in elderly Japanese patients. However, the RDI of
elderly patients is significantly lower than that of younger
patients. The current sunitinib dosing schedule is likely not
optimal for elderly patients and most of them may be over-treated.
Starting sunitinib treatment at a reduced dose should be considered
for elderly patients with advanced RCC.
Acknowledgements
The authors would like to thank Dr Neil M. Singer
for providing expert editorial assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
TF designed the present study. TH, DI, KY and MI
collected and interpreted the patients' data. TF and KM analyzed
the patients' data. TF was a major contributor in writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Kitasato
University Medical Ethics Organization (approval no: KMEO B16-156).
Patient written informed consent was not required for the present
study as it was conducted retrospectively.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili
R, Bjarnason GA, et al: Overall survival and updated results for
sunitinib compared with interferon alfa in patients with metastatic
renal cell carcinoma. J Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berger DA, Megwalu II, Vlahiotis A, Radwan
MH, Serrano MF, Humphrey PA, Piccirillo JF and Kibel AS: Impact of
comorbidity on overall survival in patients surgically treated for
renal cell carcinoma. Urology. 72:359–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coebergh JW, Janssen-Heijnen ML, Post PN
and Razenberg PP: Serious co-morbidity among unselected cancer
patients newly diagnosed in the southeastern part of The
Netherlands in 1993–1996. J Clin Epidemiol. 52:1131–1136. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coebergh JW, Janssen-Heijnen ML and
Razenberg PP: Prevalence of co-morbidity in newly diagnosed
patients with cancer: A population-based study. Crit Rev Oncol
Hematol. 27:97–100. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Repetto L, Venturino A, Vercelli M, Gianni
W, Biancardi V, Casella C, Granetto C, Parodi S, Rosso R and
Marigliano V: Performance status and comorbidity in elderly cancer
patients compared with young patients with neoplasia and elderly
patients without neoplastic conditions. Cancer. 82:760–765. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lewis JH, Kilgore ML, Goldman DP, Trimble
EL, Kaplan R, Montello MJ, Housman MG and Escarce JJ: Participation
of patients 65 years of age or older in cancer clinical trials. J
Clin Oncol. 21:1383–1389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Surbone A: Ethical considerations in
conducting clinical trials for elderly cancer patients. Aging
Health. 4:253–260. 2008. View Article : Google Scholar
|
|
10
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brunello A, Basso U, Sacco C, Sava T, De
Vivo R, Camerini A, Barile C, Roma A, Maruzzo M, Falci C, et al:
Safety and activity of sunitinib in elderly patients (≥ 70 years)
with metastatic renal cell carcinoma: A multicenter study. Ann
Oncol. 24:336–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hutson TE, Bukowski RM, Rini BI, Gore ME,
Larkin JM, Figlin RA, Barrios CH, Escudier B, Lin X, Fly K, et al:
Efficacy and safety of sunitinib in elderly patients with
metastatic renal cell carcinoma. Br J Cancer. 110:1125–1132. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gore ME, Szczylik C, Porta C, Bracarda S,
Bjarnason GA, Oudard S, Hariharan S, Lee SH, Haanen J, Castellano
D, et al: Safety and efficacy of sunitinib for metastatic
renal-cell carcinoma: An expanded-access trial. Lancet Oncol.
10:757–763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawashima A, Tsujimura A, Takayama H, Arai
Y, Nin M, Tanigawa G, Yasunaga Y, Mukai M, Uemura M, Nakai Y, et
al: Osaka Renal Cell Carcinoma Clinical Study Collaboration:
Importance of continuing therapy and maintaining one-month relative
dose intensity in sunitinib therapy for metastatic renal cell
carcinoma. Med Oncol. 29:3298–3305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kato R, Kato Y, Matsuura T, Kanehira M,
Takata R and Obara W: Characteristics of early-onset hematotoxicity
of sunitinib in Japanese patients with renal cell carcinoma. BMC
Cancer. 17:2142017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meehan B, Appu S, St Croix B,
Rak-Poznanska K, Klotz L and Rak J: Age-related properties of the
tumour vasculature in renal cell carcinoma. BJU Int. 107:416–424.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yildiz E, Ayan S, Goze F, Gokce G and
Gultekin EY: Relation of microvessel density with microvascular
invasion, metastasis and prognosis in renal cell carcinoma. BJU
Int. 101:758–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Denzinger S, Otto W, Burger M,
Hammerschmied C, Junker K, Hartmann A, Wieland WF and Walter B:
Sporadic renal cell carcinoma in young and elderly patients: Are
there different clinicopathological features and disease specific
survival rates? World J Surg Oncol. 5:162007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verhoest G, Veillard D, Guillé F, De La
Taille A, Salomon L, Abbou CC, Valéri A, Lechevallier E, Descotes
JL, Lang H, et al: Relationship between age at diagnosis and
clinicopathologic features of renal cell carcinoma. Eur Urol.
51:1298–1304; discussion 1304–1305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karakiewicz PI, Jeldres C, Suardi N,
Hutterer GC, Perrotte P, Capitanio U, Ficarra V, Cindolo L, de La
Taille A, Tostain J, et al: Age at diagnosis is a determinant
factor of renal cell carcinoma-specific survival in patients
treated with nephrectomy. Can Urol Assoc J. 2:610–617. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung EJ, Lee HJ, Kwak C, Ku JH and Moon
KC: Young age is independent prognostic factor for cancer-specific
survival of low-stage clear cell renal cell carcinoma. Urology.
73:137–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aapro MS, Köhne CH, Cohen HJ and Extermann
M: Never too old? Age should not be a barrier to enrollment in
cancer clinical trials. Oncologist. 10:198–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akaza H, Naito S, Ueno N, Aoki K, Houzawa
H, Lowenthal Pitman S and Lee SY: Real-world use of sunitinib in
Japanese patients with advanced renal cell carcinoma: Efficacy,
safety and biomarker analyses in 1689 consecutive patients. Jpn J
Clin Oncol. 45:576–583. 2015. View Article : Google Scholar : PubMed/NCBI
|