Introduction
Glioblastoma multiforme (GBM) is the most common
malignant brain tumour in adults (1). The current standard of first-line
therapy is maximal safe resection followed by radiation therapy
concurrent with temozolomide and subsequent adjuvant temozolomide
chemotherapy (2). However, almost
all patients experience relapse after adjuvant therapy and overall
survival (OS) is dismal, despite the best available treatment
modalities (3). New adjuvant
treatment strategies, better patient selection and personalized
therapy are crucial for improving clinical outcomes.
Clinical factors such as age at presentation, tumour
location, Karnofsky performance status, extent of surgery and
isocitrate dehydrogenase (IDH) status are well-known prognostic
factors for GBM (4). Identification
of more accessible and cost-effective prognostic factors may better
guide adjuvant treatment decisions. The neutrophil-to-lymphocyte
ratio (NLR) is a marker of host inflammatory response, and its
elevation has recently been shown to be a poor prognostic factor in
a number of malignancies, including colon, prostate, lung and
bladder cancer (5–9). The platelet-to-lymphocyte ratio (PLR)
is another inflammatory marker, although it has been less
extensively investigated as a prognostic factor in cancer patients
compared with NLR.
A limited number of studies have evaluated the role
of NLR and PLR in GBM prognosis and survival, but the results are
controversial. Some studies found that higher NLR values were
associated with worse OS at the time of first diagnosis or prior to
second surgery, whereas one study demonstrated that lower NLR
values were associated with better prognosis only in IDH wild-type
patients. In addition, other studies did not observe any
association between NLR values and clinical outcome.
A total of 3 studies evaluated the prognostic effect
of PLR in GBM patients: One of those studies reported a negative
prognostic effect of increased PLR values, whereas the remaining 2
studies did not identify any association between PLR and OS.
The aim of the present study was to evaluate the
prognostic value of NLR and PLR for OS in a cohort of patients with
GBM.
Patients and methods
Patient information
In this retrospective, single-centre study, a total
of 80 patients who were diagnosed between January 2012 and June
2017 at the Departments of Radiation Oncology and Medical Oncology
of Samsun Training Hospital (Samsun, Turkey) were evaluated. The
Stupp protocol (primary radiotherapy with a total of 60 Gy with
concomitant temozolamide and subsequent temozolamide) was used for
all patients as the postoperative radiochemotherapy regimen. The
protocol of the present study was approved by the local Ethics
Committee.
Demographic data, clinicopathological data and
treatment parameters (i.e., extent of surgical resection,
radiotherapy and use of chemotherapy) were obtained from medical
records. Data on patient death were obtained from the National
Electronic Death Registration System, Turkey.
Patients with complete blood count results before
receiving corticosteroid therapy or surgery were included in the
study. NLR was calculated by dividing the neutrophil count by the
lymphocyte count, and the PLR was defined as the absolute platelet
count divided by the absolute lymphocyte count.
Statistical analysis
Progression-free survival (PFS) was calculated as
the time interval between the date of the initial surgery and the
detection of tumour progression documented on magnetic resonance
imaging or the date of death. The time interval between the date of
diagnosis and the date of death, or of the last follow-up for
surviving patients, was defined as the OS. Kaplan-Meier curves were
used to calculate OS and PFS. Patients who were alive at the last
visit were censored in the analysis. Univariate and multivariate
analyses were performed using the Cox proportional hazards model to
evaluate the effect of variables on PFS and OS. Both the NLR and
PLR were evaluated as continuous or dichotomous variables. A
cut-off value of 4 for NLR was selected according to previous
trials, as it had been shown to correlate with clinical outcomes in
GBM patients. P<0.05 was considered to indicate statistically
significant differences. All analyses were performed using SPSS
version 23 software (IBM SPSS, Armonk, NY, USA).
Results
Study population
Between 2012 and 2017, 104 patients with GBM were
assessed for consideration of adjuvant therapy at our institution,
among whom 80 patients with evaluable pre-corticosteroid full blood
count results were identified and included in the present study. Of
the 80 patients, 39 (48.7%) were male and 41 (51.3%) were female,
with a mean age of 56.8±13.1 years. The median tumour diameter was
42.3±14.8 mm. The majority of the patients (85%) had received
concurrent chemoradiotherapy after surgery. In addition, the
majority of the patients (72.5%) subsequently received adjuvant
temozolomide. Gross total excision was achieved in over half of the
patients (52.5%). The most common tumour localization was the
temporal lobe (27.5%). The median follow-up time was 12 months
(range, 3–55 months).
The mean pre-treatment neutrophil, platelet and
lymphocyte counts were 7.9±3.7×109/l (range,
2.4–21.6×109/l), 259.1±65.7×109/l (range,
133–462×109/l), and 1.7±0.7×109/l (range,
0.5–3.6×109/l), respectively.
The mean pre-treatment NLR was 6.3±5.5 (median,
4.39; range, 1.03–30.29), and the mean pre-treatment PLR was
182.9±95.4 (median, 163.1; range, 56.8–607.9). Baseline demographic
data are presented in Table I.
 | Table I.Demographic and clinical
characteristics. |
Table I.
Demographic and clinical
characteristics.
| Characteristics | n | % |
|---|
| Sex |
|
|
|
Female | 41 | 51.3 |
| Male | 39 | 48.7 |
| Age (years), mean ±
SD | 80 | 56.8±13.1 |
| Tumour location |
|
|
|
Temporal | 22 | 27.5 |
|
Parietal | 13 | 16.3 |
|
Frontal | 13 | 16.3 |
|
Frontoparietal | 9 | 11.3 |
|
Parietooccipital | 10 | 12.5 |
|
Frontotemporal | 5 | 6.3 |
|
Occipital | 3 | 3.8 |
|
Other | 5 | 6.4 |
|
Primary/secondary |
|
|
|
Primary | 77 | 96.3 |
|
Secondary | 3 | 3.8 |
| Hemisphere |
|
|
|
Right | 32 | 40 |
| Left | 45 | 56.3 |
|
Midline | 3 | 3.8 |
| Type of
operation |
|
|
|
Total | 42 | 52.5 |
|
Subtotal | 30 | 37.5 |
|
Biopsy | 7 | 8.8 |
|
Unknown | 1 | 1.3 |
| Adjuvant
treatment |
|
|
|
Chemoradiotherapy | 68 | 85 |
|
Radiotherapy | 5 | 6.3 |
| No
treatment | 3 | 3.7 |
|
Unknown | 2 | 2.5 |
|
Chemoradiotherapy +
Cyberknife | 2 | 2.5 |
| Temozolamide |
|
|
| Yes | 58 | 72.5 |
| No | 11 | 13.8 |
|
Unknown | 11 | 13.8 |
| Preoperative NLR,
mean ± SD | 80 | 6.3±5.5 |
| Preoperative PLR,
mean ± SD | 80 | 182.9±95.4 |
PFS
The median PFS was 9.1 months. On univariate
analysis, PFS did not differ significantly according to sex,
laterality, or pre-treatment neutrophil, lymphocyte and platelet
counts. However, age <65 years, Eastern Cooperative Oncology
Group (ECOG) performance status 1 and 2, administration of
concurrent radiotherapy compared with radiotherapy alone,
administration of second- and third-line systemic therapy, and
frontal compared with occipital tumour localization were considered
as prognostic factors. PFS did not differ significantly between
patients with higher (>4) and those with lower (<4) NLR
values.
On multivariate analysis, ECOG performance status,
localization, radiochemotherapy and second-line systemic therapy
remained as independent prognostic indicators (Table II).
 | Table II.Univariate and multivariate analysis
for progression-free survival. |
Table II.
Univariate and multivariate analysis
for progression-free survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 0.508 | 0.300–0.859 | 0.012a | 0.845 | 0.421–1.695 | 0.635 |
| Sex | 0.869 | 0.546–1.384 | 0.555 |
|
|
|
| ECOG PS |
|
|
|
|
|
|
| 1–2 |
|
|
|
|
|
|
| 3–4 | 0.220 | 0.130–0.371 | 0.000a | 6.629 | 3.096–14.194 | 0.000a |
| Localization |
|
|
|
|
|
|
|
Frontal |
|
|
|
|
|
|
|
Parietal/temporal | 1.293 | 0.764–2.189 | 0.339 | 1.926 | 1.057–3.510 | 0.032a |
|
Occipital | 5.936 | 1.632–21.596 | 0.007a | 3.965 | 0.829–18.950 | 0.084 |
|
Other | 3.579 | 1.306–9.807 | 0.013a | 2.320 | 0.782–6.884 | 0.129 |
| Laterality |
|
|
|
|
|
|
|
Right |
|
|
|
|
|
|
|
Left | 0.719 | 0.440–1.173 | 0.187 |
|
|
|
|
Midline | 1.590 | 0.480–5.261 | 0.448 |
|
|
|
| Type of
operation |
|
|
|
|
|
|
|
Biopsy |
|
|
|
|
|
|
|
Subtotal | 0.803 | 0.307–2.106 | 0.656 |
|
|
|
| Gross
total | 0.408 | 0.157–1.064 | 0.067 |
|
|
|
| Radiotherapy |
|
|
|
|
|
|
|
Concurrent |
|
|
|
|
|
|
|
Alone | 3.007 | 1.187–7.620 | 0.020a | 4.201 | 1.292–13.657 | 0.017a |
| Temozolamide |
|
|
|
|
|
|
|
Yes |
|
|
|
|
|
|
| No | 1.366 | 0.706–2.645 | 0.355 |
|
|
|
| Second-/third-line
systemic therapy |
|
|
|
|
|
|
|
Yes |
|
|
|
|
|
|
| No | 0.331 | 0.196–0.558 | 0.000a | 0.292 | 0.161–0.527 | 0.000a |
| NLR |
|
|
|
|
|
|
|
>4 |
|
|
|
|
|
|
|
<4 | 1.374 | 0.858–2.202 | 0.186 |
|
|
|
| PLR |
|
|
|
|
|
|
|
>135 |
|
|
|
|
|
|
|
<135 | 0.724 | 0.444–1.182 | 0.197 |
|
|
|
| Pre-treatment
neutrophils | 1.000 | 1.000–1.000 | 0.07 |
|
|
|
| Pre-treatment
lymphocytes | 1.000 | 1.000–1.001 | 0.236 |
|
|
|
| Pre-treatment
platelets | 1.000 | 1.000–1.000 | 0.556 |
|
|
|
| Pre-treatment
NLR | 1.015 | 0.968–1.065 | 0.532 |
|
|
|
| Tumour size | 0.993 | 0.972–1.014 | 0.525 |
|
|
|
OS
A total of 53 patients had succumbed to the disease
by the time of the analysis. The OS was 13.2 months in the entire
study population. Univariate and multivariate analyses demonstrated
that patients with ECOG 1–2, and those receiving concurrent
chemoradiotherapy or additional systemic therapy, had longer OS. On
multivariate analysis, frontal localization was also a significant
predictor of survival (Table
III).
 | Table III.Univariate and multivariate analysis
for overall survival. |
Table III.
Univariate and multivariate analysis
for overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.416 | 0.807–2.485 | 0.225 |
|
|
|
| Sex | 0.847 | 0.486–1.478 | 0.559 |
|
|
|
| ECOG PS |
|
|
|
|
|
|
|
1–2 |
|
|
|
|
|
|
|
3–4 | 2.647 | 1.505–4.658 | 0.001a | 2.526 | 1.256–5.080 | 0.009a |
| Localization |
|
|
|
|
|
|
|
Frontal |
|
|
|
|
|
|
|
Parietal/temporal | 1.197 | 0.652–2.197 | 0.562 | 2.006 | 0.963–4.179 | 0.063a |
|
Occipital | 4.042 | 0.872–18.733 | 0.074 | 5.432 | 1.034–28.533 | 0.046a |
|
Other | 8.331 | 2.078–33.403 | 0.003a | 2.394 | 0.513–11.178 | 0.267 |
| Laterality |
|
|
|
|
|
|
|
Right |
|
|
|
|
|
|
|
Left | 0.747 | 0.416–1.338 | 0.326 |
|
|
|
|
Midline | 6.154 | 0.742–51.067 | 0.092 |
|
|
|
| Operation type |
|
|
|
|
|
|
|
Biopsy |
|
|
|
|
|
|
|
Subtotal | 0.672 | 0.198–2.278 | 0.524 |
|
|
|
| Gross
total | 0.395 | 0.116–1.345 | 0.137 |
|
|
|
| Radiotherapy |
|
|
|
|
|
|
|
Concurrent |
|
|
|
|
|
|
|
Alone | 6.752 | 2.164–21.072 | 0.001a | 7.127 | 1.768–28.740 | 0.06a |
| Temozolamide |
|
|
|
|
|
|
|
Yes |
|
|
|
|
|
|
| No | 1.864 | 0.901–3.855 | 0.093 |
|
|
|
| Second-/third-line
systemic therapy |
|
|
|
|
|
|
|
Yes |
|
|
|
|
|
|
| No | 0.176 | 0.091–0.341 | 0.000a | 0.085 | 0.036–0.205 | 0.000a |
| NLR |
|
|
|
|
|
|
|
>4 |
|
|
|
|
|
|
|
<4 | 1.258 | 0.727–2.179 | 0.412 |
|
|
|
| PLR |
|
|
|
|
|
|
|
>135 |
|
|
|
|
|
|
|
<135 | 0.649 | 0.365–1.125 | 0.121 |
|
|
|
| Pre-treatment
neutrophils |
| 1.000 | 1.000–1.000 | 0.074 |
|
|
| Pre-treatment
lymphocytes |
| 1.000 | 1.000–1.001 | 0.236 |
|
|
| Pre-treatment
platelets |
| 1.000 | 1.000–1.000 | 0.556 |
|
|
| Pre-treatment
NLR |
| 1.015 | 0.968–1.065 | 0.532 |
|
|
| Tumour size |
| 0.993 | 0.972–1.014 | 0.525 |
|
|
NLR and PLR
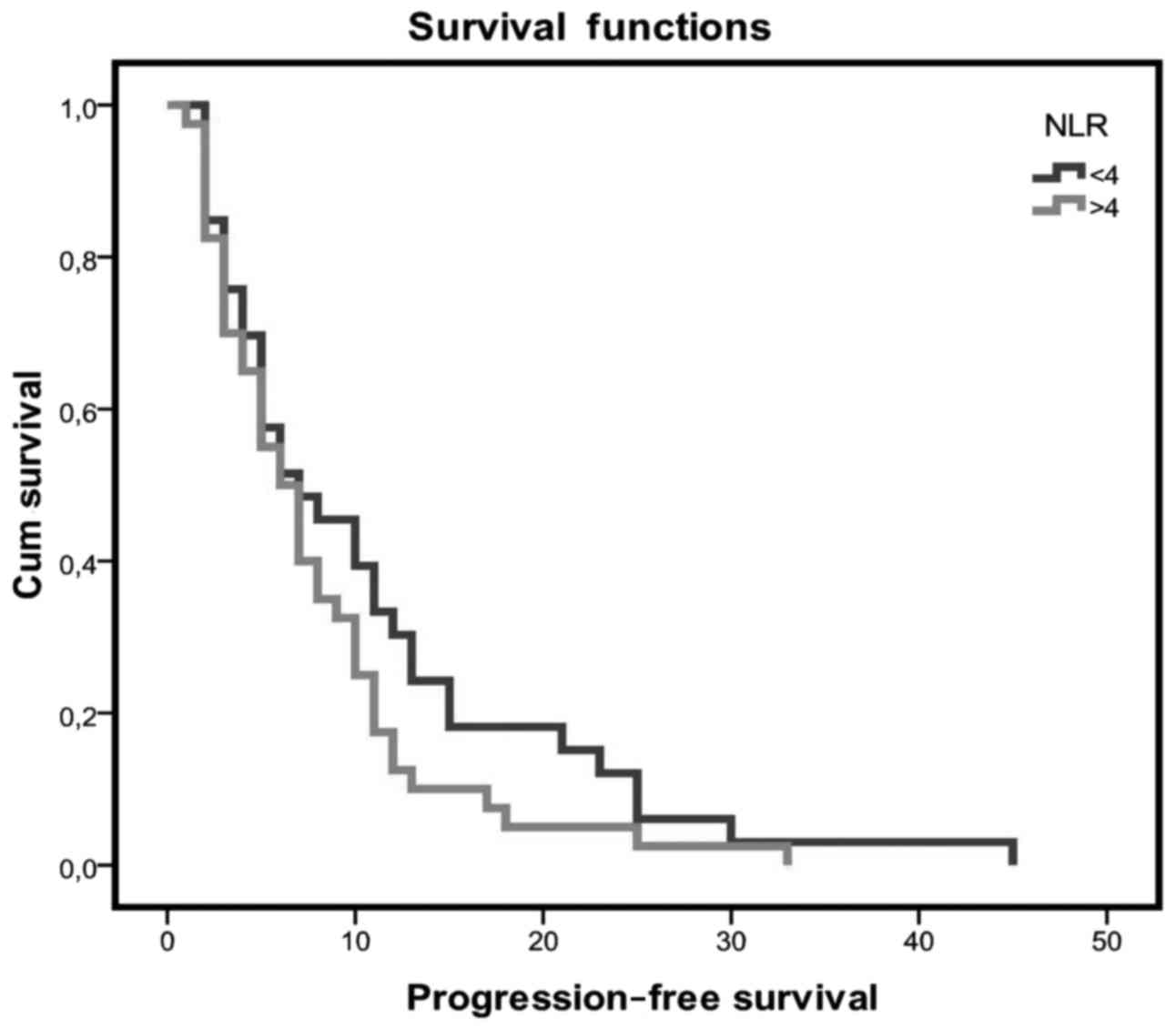
Patients with an NLR value of <4 had a longer PFS
when compared with patients with higher NLR values (10.7 vs. 7.8
months, respectively), but the difference was not statistically
significant (Fig. 1). The PFS for
patients with low PLR values was 7.4 months as compared with 10.02
months for those with high PLR values, but the difference was not
statistically significant (P=0.166).
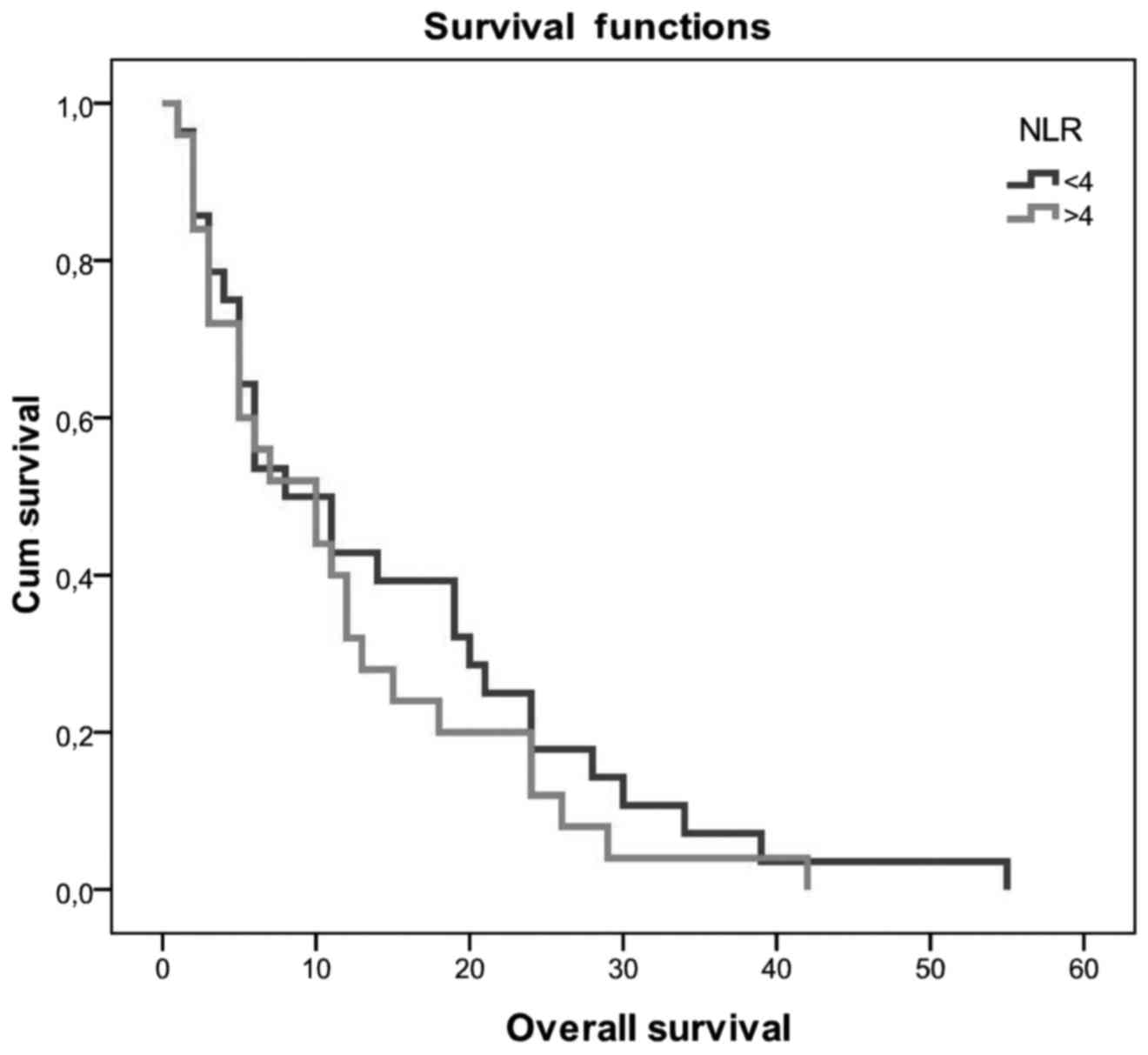
Patients with NLR <4 had a better OS of 14.5 vs.
11.6 months in patients with NLR >4. The difference was not
statistically significant (P>0.05; Fig. 2). OS did not differ significantly
between patients with low and those with high PLR values (10.2 vs.
15.2 months, respectively; P=0.105).
Discussion
The results of the present study demonstrated that
patients with lower NLR values exhibited a longer OS compared with
patients with higher NLR values, but the difference was not
statistically significant. PLR was not prognostic for clinical
outcome. Although NLR is an easily available and cost-effective
test, its clinical use in patients with GBM is associated with some
challenges. NLR should be calculated prior to steroid therapy and
surgery, as both these interventions may increase the neutrophil
count and lead to misinterpretation of the value (10). NLR may also be affected by various
factors, such as hypertension, autoimmune diseases, cardiovascular
diseases and insulin resistance. In addition, the optimal cut-off
value has not been established.
The identification of prognostic factors is crucial
for GBM patients and may guide clinical treatment. Several studies
have demonstrated an association between inflammatory status and
cancer development. Pre-treatment neutrophil, lymphocyte and
platelet counts are indicators of cancer-associated inflammation.
High neutrophil count has been demonstrated to be an independent
negative prognostic marker for recurrence and survival in gastric
cancer, metastatic renal cell carcinoma, metastatic melanoma and
advanced non-small-cell lung cancer (11). NLR is considered to reflect the
balance between activation of the inflammatory pathway and the
anti-tumour immune function (12).
As a marker of systemic inflammation, the prognostic
significance of pre-treatment NLR in GBM patients remains unclear.
NLR values <4 were shown to be significantly correlated with a
better OS for GBM patients in 3 retrospective studies (10,13,14). By
contrast, 2 retrospective studies did not identify any association
between NLR values and OS, in accordance with our results. Mason
et al evaluated NLR values in postoperative GBM patients who
mostly received corticosteroids, which affect the NLR values, and
used a cut-off value of 7.5 (15).
Lopes et al also did not observe any correlation of NLR with
OS, but they reported a shorter OS in patients with an NLR value
>7 who completed the Stupp protocol (16).
McNamara et al assessed the prognostic value
of NLR prior to second surgery in GBM patients and demonstrated
that low NLR values were associated with longer OS after the second
surgery compared with high NLR values (17).
PLR is also described as a prognostic factor in
different cancer types; however, it is less extensively
investigated compared with NLR in GBM patients. A total of 3
retrospective studies evaluated the prognostic value of PLR in GBM
patients: Wang et al reported that PLR had independent
prognostic value in GBM patients (10). Conversely, the other 2 trials could
not find any association between PLR and clinical outcome in GBM
patients (14,16).
Of the known prognostic factors in our study
population, ECOG performance status, localization, combined therapy
and second-line systemic treatment were identified as prognostic
factors for clinical outcome.
There were certain limitations to the present study.
First, this was a retrospective study and the sample size was not
sufficient to reach statistical significance for survival due to
worse disease outcomes; the results should be confirmed in a
prospective study. Second, the O6-methylguanine-DNA
methyltransferase methylation status of the patients was not known.
Moreover, the post-progression salvage treatments were
heterogeneous. Finally, cardiovascular diseases, infection, or drug
treatments may also have affected the neutrophil and lymphocyte
counts; however, we could not detect all confounders for patients
with such medical history.
In conclusion, pre-treatment NLR and PLR values were
not associated with prognosis in GBM patients and do not appear to
be useful markers for predicting prognosis in GBM patients. There
remains an unmet need to identify better prognostic factors for GBM
patients.
Acknowledgements
The authors would like to thank Ferhat Yıldız for
the statistical analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
OY made substantial contributions to conception and
design, and acquisition of data, and interpretation of data; EO
made substantial contributions to conception and acquisition of
data; OO made substantial contributions to acquisition of data; YK
involved in drafting the manuscript and revising it critically for
important intellectual content.
Ethics approval and consent to
participate
Samsun Research and Training Hospital approval no.
2017/14.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no potential
competing interests to disclose.
References
|
1
|
Behin A, Hoang-Xuan K, Carpentier AF and
Delattre JY: Primary brain tumours in adults. Lancet. 361:323–331.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawano H, Hirano H, Yonezawa H, Yunoue S,
Yatsushiro K, Ogita M, Hiraki Y, Uchida H, Habu M, Fujio S, et al:
Improvement in treatment results of glioblastoma over the last
three decades and beneficial factors. Br J Neurosurg. 29:206–212.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakagawa Y, Sasaki H, Ohara K, Ezaki T,
Toda M, Ohira T, Kawase T and Yoshida K: Clinical and molecular
prognostic factors for long-term survival of the patients with
glioblastomas in a single-institutional consecutive cohort. World
Neurosurg. 106:165–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ozdemir Y, Akin ML, Sucullu I, Balta AZ
and Yucel E: Pretreatment neutrophil/lymphocyte ratio as a
prognostic aid in colorectal cancer. Asian Pac J Cancer Prev.
15:2647–2650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buttigliero C, Pisano C, Tucci M, Vignani
F, Bertaglia V, Iaconis D, Guglielmini P, Numico G, Scagliotti GV
and Di Maio M: Prognostic impact of pretreatment
neutrophil-to-lymphocyte ratio in castration-resistant prostate
cancer patients treated with first-line docetaxel. Acta Oncol.
56:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bar-Ad V, Palmer J, Li L, Lai Y, Lu B,
Myers RE, Ye Z, Axelrod R, Johnson JM, Werner-Wasik M, et al:
Neutrophil to lymphocyte ratio associated with prognosis of lung
cancer. Clin Transl Oncol. 19:711–717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang X, Du P and Yang Y: The clinical use
of neutrophil-to-lymphocyte ratio in bladder cancer patients: A
systematic review and meta-analysis. Int J Clin Oncol. 22:817–825.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang PF, Song HW, Cai HQ, Kong LW, Yao K,
Jiang T, Li SW and Yan CX: Preoperative inflammation markers and
IDH mutation status predict glioblastoma patient survival.
Oncotarget. 8:50117–50123. 2017.PubMed/NCBI
|
|
11
|
Dolan RD, McSorley ST, Horgan PG, Laird B
and McMillan DC: The role of the systemic inflammatory response in
predicting outcomes in patients with advanced inoperable cancer:
Systematic review and meta-analysis. Crit Rev Oncol Hematol.
116:134–146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Faria SS, Fernandes PC Jr, Silva MJ, Lima
VC, Fontes W, Freitas-Junior R, Eterovic AK and Forget P: The
neutrophil-to-lymphocyte ratio: A narrative review.
Ecancermedicalscience. 10:7022016.PubMed/NCBI
|
|
13
|
Bambury RM, Teo MY, Power DG, Yusuf A,
Murray S, Battley JE, Drake C, O'Dea P, Bermingham N, Keohane C, et
al: The association of pre-treatment neutrophil to lymphocyte ratio
with overall survival in patients with glioblastoma multiforme. J
Neurooncol. 114:149–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han S, Liu Y, Li Q, Li Z, Hou H and Wu A:
Pre-treatment neutrophil-to-lymphocyte ratio is associated with
neutrophil and T-cell infiltration and predicts clinical outcome in
patients with glioblastoma. BMC Cancer. 15:6172015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mason M, Maurice C, McNamara MG, Tieu MT,
Lwin Z, Millar BA, Menard C, Laperriere N, Milosevic M, Atenafu EG,
et al: Neutrophil-lymphocyte ratio dynamics during concurrent
chemo-radiotherapy for glioblastoma is an independent predictor for
overall survival. J Neurooncol. 132:463–471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopes M, Carvalho B, Vaz R and Linhares P:
Influence of neutrophil-lymphocyte ratio in prognosis of
glioblastoma multiforme. J Neurooncol. 136:173–180. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McNamara MG, Lwin Z, Jiang H, Templeton
AJ, Zadeh G, Bernstein M, Chung C, Millar BA, Laperriere N and
Mason WP: Factors impacting survival following second surgery in
patients with glioblastoma in the temozolomide treatment era,
incorporating neutrophil/lymphocyte ratio and time to first
progression. J Neurooncol. 117:147–152. 2014. View Article : Google Scholar : PubMed/NCBI
|