Introduction
Salivary duct carcinoma (SDC) is a relatively rare
highly aggressive tumor, accounting for approximately 10% of all
salivary gland malignancies (1).
This type of tumor is characterized histopathologically by
proliferation of neoplastic cells containing large round to oval
nuclei with conspicuous nucleoli and rich eosinophilic cytoplasm,
occasionally accompanied by comedonecrosis, which resembles
high-grade breast carcinoma (1). The
immunohistochemical characteristics of SDC include overexpression
of human epidermal growth factor receptor (HER) 2 and positivity
for androgen receptor (AR) (1,2).
Although SDC shows a high frequency of lymph node
metastases and distant metastases including in the lung, liver,
brain, and bone (1), metastasis in
the pleural effusion is extremely rare (3–5).
In this report, we describe the first documented
cytological case of metastatic SDC in the cardiac and pleural
effusion with immunocytochemical analyses for AR and HER2.
Case report
A 50-year-old Japanese male underwent surgery for a
right submandibular gland tumor, which was diagnosed as SDC, and
chemo-radiation therapy was added. At the age of 52, he was found
to have nodules in the bilateral lungs, and subsequently cardiac
tamponade and respiratory discomfort developed due to cardiac and
pleural effusions. Aspiration of the cardiac and pleural effusions
was performed. He has been followed-up at our hospital until now,
and is alive with disease (brain metastasis) 4 months after
development of cardiac tamponade. No resection of the lung nodules
and brain metastasis was performed. The present patient has not
been received HER2-targeted or AR deprivation therapy yet. The
study was approved by the institutional review board of our
hospital (approval no. 2016646), and we obtained patient consent
according to our hospital policy.
Specimens of the cardiac and pleural effusions were
stained with Papanicolaou stain. Formalin-fixed and
paraffin-embedded specimens of the resected submandibular gland
tumor were processed for routine histological examination and
immunohistochemical analyses.
In this report, immunohistochemical and
immunocytochemical analyses were performed using an autostainer (XT
System Benchmark, Roche Diagnostics, Basel, Switzerland). The
primary antibodies used in this report were a rabbit monoclonal
antibody against AR (SP107, Roche Diagnostics) and a rabbit
monoclonal antibody against HER2 (4B5, Roche Diagnostics).
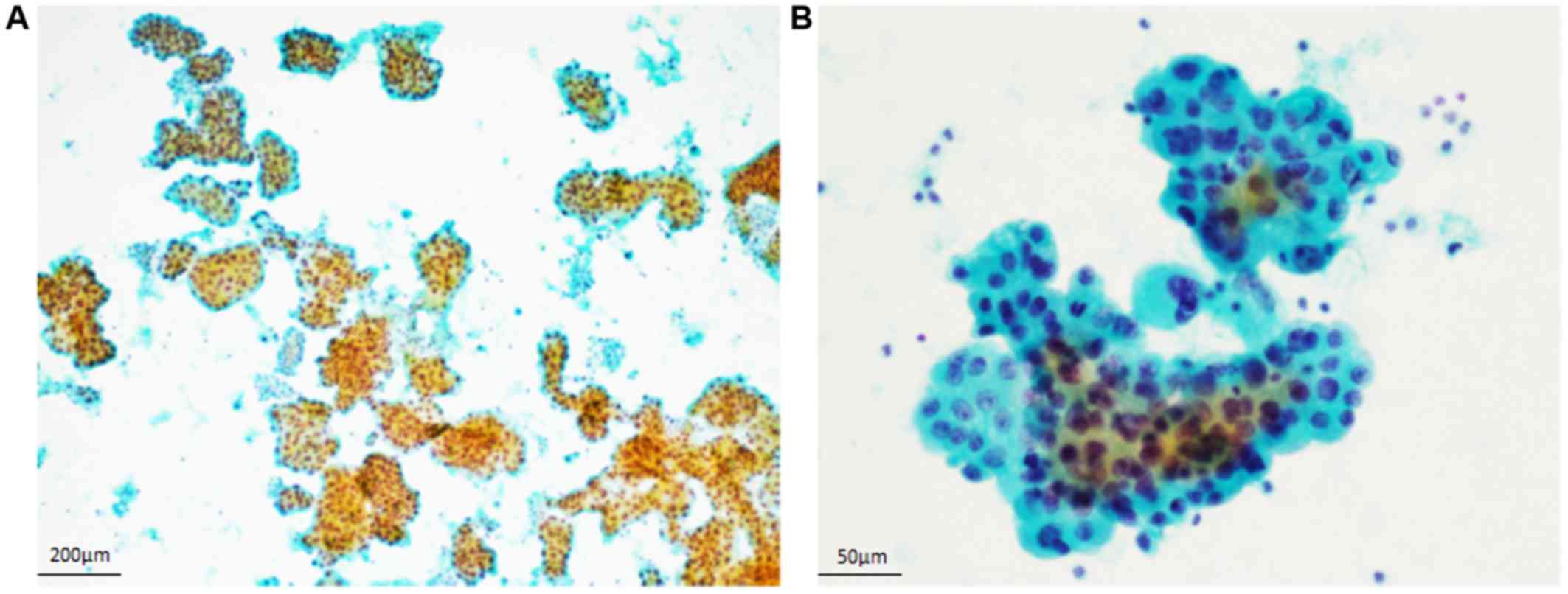
Cytological findings of the cardiac and pleural
effusions. The cytological specimens of the cardiac and pleural
effusions showed the same features. The Papanicolaou smears
demonstrated numerous small ball-like or papillary clusters in an
inflammatory background (Fig. 1A).
These neoplastic cells had large round to oval nuclei with
conspicuous nucleoli and coarse chromatin, and relatively rich
granular cytoplasm (Fig. 1B).
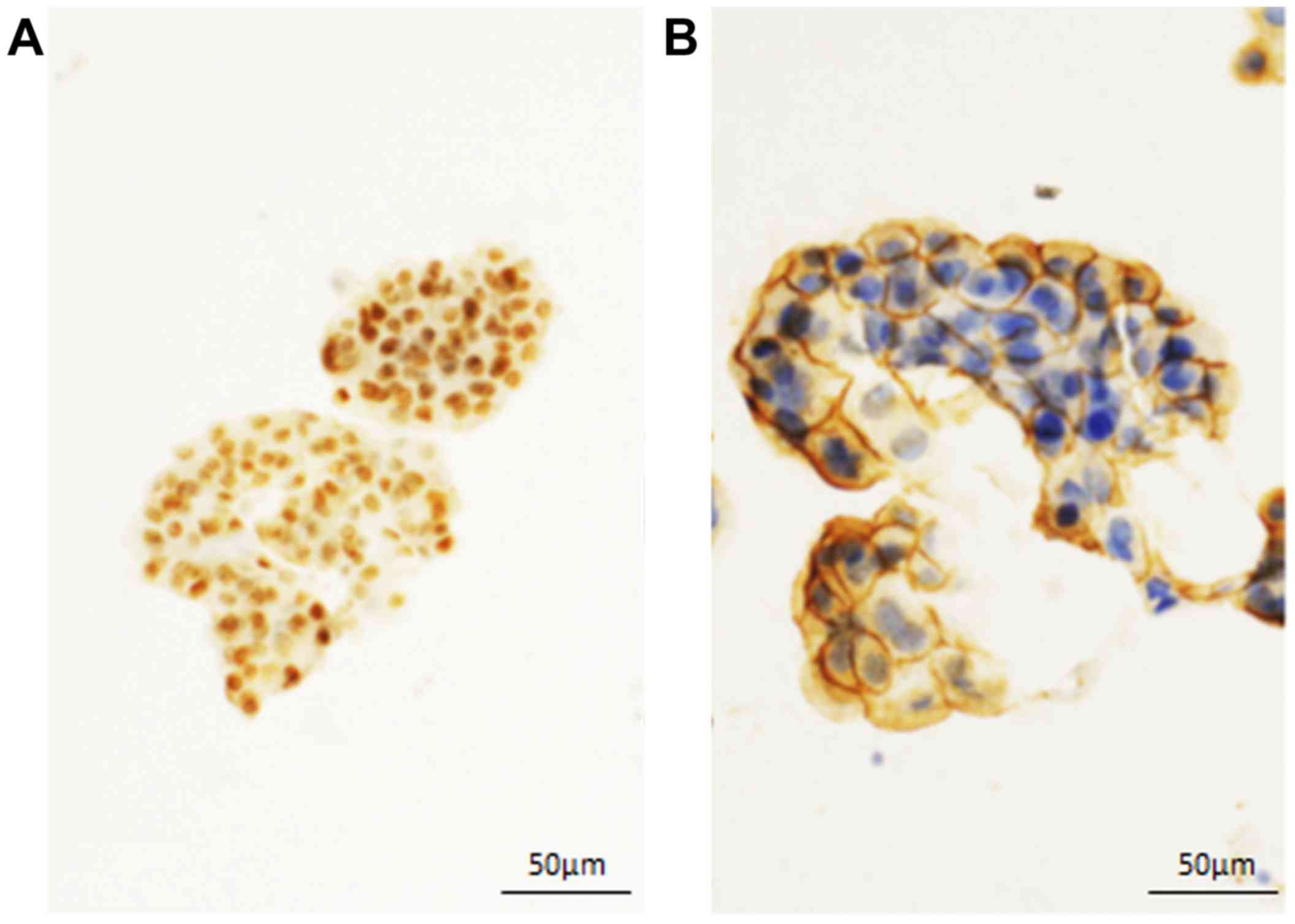
Immunocytochemical findings. AR was diffusely
expressed in the nuclei of the neoplastic cells (Fig. 2A). Strong membranous expression of
HER2 was noted in the tumor cells (Fig.
2B). Accordingly, a cytodiagnosis of metastatic SDC in the
cardiac and pleural effusions was made with consideration of the
clinical history.
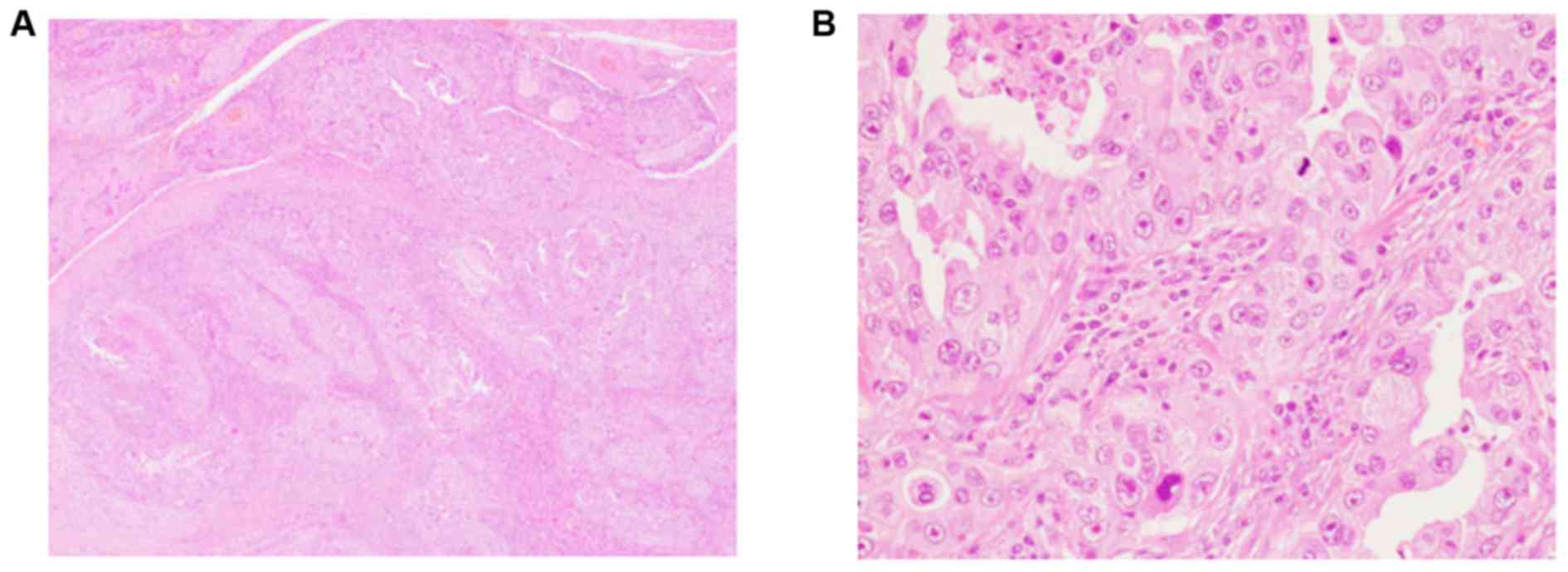
Histopathological findings of the submandibular
gland tumor. The resected specimen showed infiltrative neoplastic
growth composed of variable-sized nests or irregular glandular
formation with invasion into the fatty tissue and nerve bundles
surrounding the submandibular gland (Fig. 3A). The neoplastic cells had rich
eosinophilic cytoplasm and large round to oval nuclei with
conspicuous nucleoli (Fig. 3B).
Mitotic figures were easily found, and comedonecrosis was also
noted. Metastatic carcinoma was present in the right level III, IV,
and V lymph nodes (10/45).
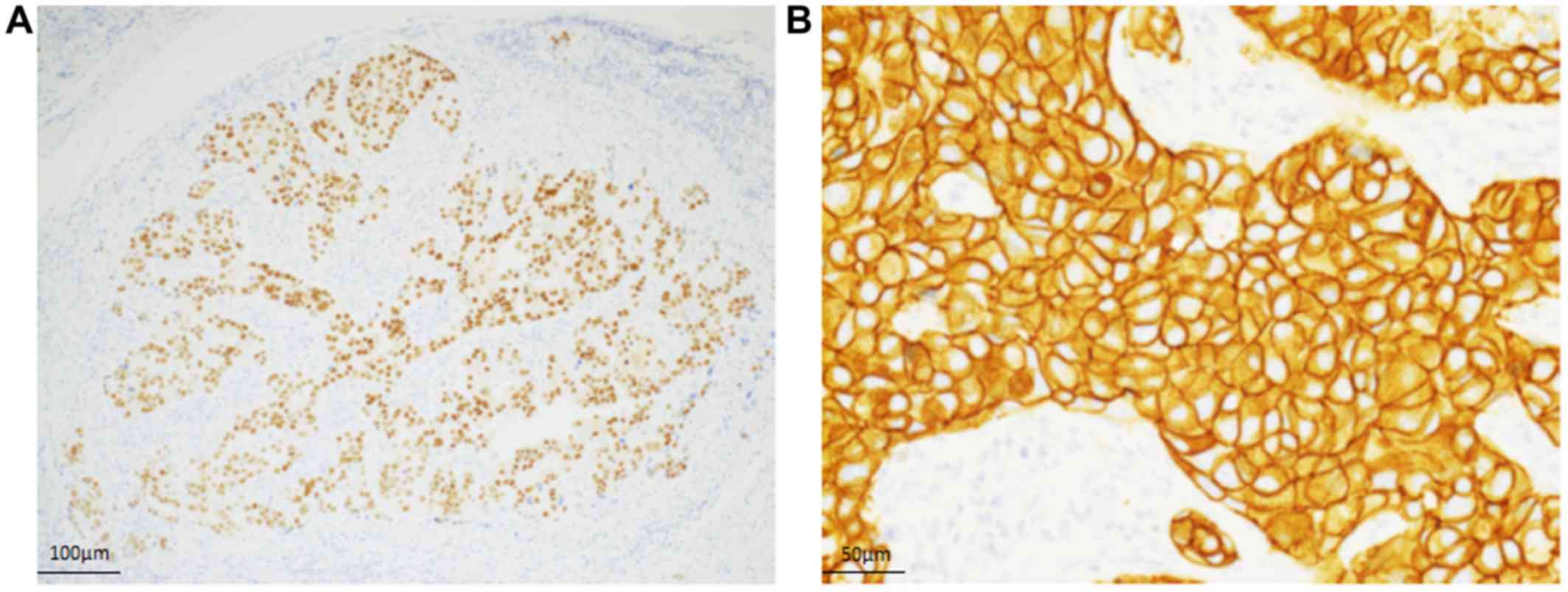
Immunohistochemical findings. The neoplastic cells
diffusely expressed for AR (Fig.
4A). Strong membranous expression of HER2 was noted in the
neoplastic cells (Fig. 4B).
Accordingly, a final diagnosis of SDC was made.
Discussion
In this article, we describe the first documented
case of metastatic SDC in cardiac and pleural effusions. Although
SDC shows an aggressive clinical course and lung metastasis
occasionally occurs, the presence of carcinoma cells of SDC in the
pleural effusion is extremely rare. It is very important to detect
the carcinoma cells in cardiac and pleural effusions because of
staging and therapeutic strategy for patients. Presence of cardiac
effusion is clinically important because cardiac tamponade can be
developed, as seen in the present patient. Only three cytological
cases of pleural effusion have been reported in the literature
(3–5), and no cytological report of SDC in
cardiac effusion has been published. Table I summarizes the clinicocytological
features of the previously reported cases as well as the present
one. The characteristic cytological features of fine-needle
aspiration of SDC of the salivary gland are as follows: i) Presence
of three-dimensional clusters, flat sheets, cribriform glands, or
dissociated neoplastic cells in a necrotic background; and ii)
large polygonal to spindle-shaped neoplastic cells with rich and
finely granular (apocrine-like) cytoplasm and large round to oval
nuclei containing coarse chromatin and conspicuous nucleoli
(6,7). Although the cytological features of the
pleural and cardiac effusions of the previously reported cases and
the present case were fundamentally the same as the above-mentioned
cytological features, the presence of ball-like neoplastic cell
clusters (and papillary clusters) may be a characteristic feature
of the neoplastic cells present in the pleural and cardiac
effusions (Table I). Murata et
al pointed out that other histological subtypes of salivary
gland carcinoma can present ball-like clusters in the pleural
effusion (5). Therefore, recognition
of this finding may be important for making differential diagnoses
of metastatic carcinoma present in the pleural and cardiac
effusions.
 | Table I.Clinicocytological features of
metastatic salivary duct carcinoma in the pleural effusion. |
Table I.
Clinicocytological features of
metastatic salivary duct carcinoma in the pleural effusion.
| Case no. | Age | Sex | Original site | Cytological
features | Immunocytochemical
features | (Refs.) |
|---|
| 1 | 76 | Male | Submandibular
gland | Small clusters with
subtle cribriform features. The tumor cells have finely granular
apocrine-type cytoplasm. | AR(+), HER2(+) (cell
block) | (3) |
| 2 | 57 | Male | Submandibular
gland | Numerous cell
clusters. The neoplastic cells have rich granular cytoplasm and
eccentric large round to oval nuclei containing conspicuous
nucleoli. | AR(+) | (4) |
| 3 | 46 | Male | Parotid gland | Small ball-like cell
clusters. The tumor cells have apocrine-like cytoplasm and pyknotic
chromatin. | AR(+), HER2(+),
HER3(+) | (5) |
| Present Case | 52 | Male | Submandibular
gland | Numerous papillary
and ball-like cell clusters. The tumor cells have large nuclei with
conspicuous nucleoli and rich cytoplasm. | AR(+), HER2(+) |
|
The most common type of malignant cells present in
the pleural effusion is adenocarcinoma, and the most common
original sites are breast and lung (8). Development of pleural metastasis of
salivary gland carcinoma is extremely rare, and only a few cases of
adenoid cystic carcinoma (9,10), mucoepidermoid carcinoma (11), and myoepithelial carcinoma (12) as well as SDC (3–5) have
been reported. Therefore, metastatic SDC in the pleural effusion
must be differentiated from metastatic breast carcinoma because SDC
resembles high-grade breast carcinoma. Expression of AR is a
characteristic finding of SDC (2),
which was useful for making the cytodiagnosis of metastatic SDC in
the present case. However, approximately 70% of breast cancer shows
positive immunoreactivity for AR (13). Therefore, cytomorphological and
immunocytochemical features as well as consideration of clinical
history are important for making a correct diagnosis.
It is well recognized that overexpression of HER2 is
occasionally observed in SDC (2).
Murata et al demonstrated overexpression of HER2 and HER3 in
metastatic SDC using a cytological specimen of the pleural effusion
(5). Our presented case also clearly
demonstrated AR and HER2 expression in SDC using the cytological
specimens of the cardiac and pleural effusions. Recently, some
classifications of molecular subtypes of SDC have been proposed
according to that of breast carcinoma. Di Palma et al
proposed molecular subgroups of SDC according to the immunoprofiles
of HER2, AR, epidermal growth factor receptor, and keratin 5/6:
Namely, luminal androgen receptor-positive, HER2, and basal-like
subtypes (14). Takase et al
proposed molecular subgroups of SDC: Namely, apocrine A
(AR+/HER2−/Ki-67 low), apocrine B
(AR+/HER2−/Ki-67 high), apocrine HER2
(AR+/HER2+), HER2 enriched
(AR−/HER2+), and double negative subtypes
(2). Moreover, HER2-targeted therapy
or AR deprivation therapy have been introduced for patients with
SDC (15,16). Although the present patient has not
been received HER2-targeted or AR deprivation therapy, the
determination of the above-mentioned molecular subtypes may be
crucial for developing a treatment strategy for patients with SDC.
According to the results in the present report, immunocytochemical
analyses for AR and HER2 in the effusion specimens may be useful
for determination of the treatment strategy in patients with
metastatic SDC, especially in the patients who are not performed
biopsy or resection of the metastatic tumor, as the present
patient.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HIt, MI, KS, KO contributed to cytological diagnosis
and manuscript preparation. YE performed immunocytochemical and
immunohistochemical stainings. MI, KT performed histopathological
diagnosis. TF, HIw contributed to patient data collection. The
final version of the manuscript has been read and approved by all
authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nagao T, Licitra L, Loening T, Vielh P and
Williams MD: Salivary duct carcinoma. In: WHO Classification of
Tumours of Head and Neck Tumours (4th edition). El-Naggar AK, Chan
JKC, Grandis JR, Takata T and Slootweg PJ: IARC; Lyon: pp. 173–174.
2017
|
|
2
|
Takase S, Kano S, Tada Y, Kawakita D,
Shimura T, Hirai H, Tsukahara K, Shimizu A, Imanishi Y, Ozawa H, et
al: Biomarker immunoprofile in salivary duct carcinomas:
Clinicopathological and prognostic implications with evaluation of
the revised classification. Oncotarget. 8:59023–59035. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huss J, Conrad R, Hirschowitz S and
Moatamed N: Pleural fluid metastases of salivary duct carcinoma: A
case report and review of the literature. Cytojournal. 11:42014.
View Article : Google Scholar
|
|
4
|
Iwamoto N, Ishida M, Kagotani A, Kasuga N,
Hayashi Y, Iwai M, Miyahira Y and Kushima R: A case of salivary
duct carcinoma in the pleural effusion successfully diagnosed by
immunocytochemical analysis. J Jpn Soc Clin Cytol. 55:412–415.
2016. View Article : Google Scholar
|
|
5
|
Murata K, Kawahara A, Ono T, Takase Y, Abe
H, Naito Y and Akiba J: HER2/HER3-positive metastatic salivary duct
carcinoma in the pleural effusion: A case report. Diagn Cytopathol.
46:429–433. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moriki T, Ueta S, Takahashi T, Mitani M
and Ichien M: Salivary duct carcinoma: Cytologic characteristics
and application of androgen receptor immunostaining for diagnosis.
Cancer. 93:344–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elsheikh TM, Bernacki EG Jr and Pisharodi
L: Fine-needle aspiration cytology of salivary duct carcinoma.
Diagn Cytopathol. 11:47–51. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sears D and Hajdu SI: The cytologic
diagnosis of malignant neoplasms in pleural and peritoneal
effusions. Acta Cytol. 31:85–97. 1987.PubMed/NCBI
|
|
9
|
Florentine BD, Fink T, Avidan S,
Braslavsky D, Raza A and Cobb CJ: Extra-salivary gland
presentations of adenoid cystic carcinoma: A report of three cases.
Diagn Cytopathol. 34:491–494. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Torre W, Comellas M and Cuesta M: Massive
pleural effusion as isolated manifestation of metastatic spread of
salivary adenoid cystic carcinoma. Respir Med. 91:169–170. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsushita I, Takeda T, Kobayashi TK,
Tanaka B and Sawaragi I: Mucoepidermoid carcinoma of the salivary
gland in pleural fluid. A case report. Acta Cytol. 27:525–528.
1983.PubMed/NCBI
|
|
12
|
Bhambra AC, Zhang Y, Huang EC, Bishop J,
Matin M and Afify A: Pleural fluid metastases of myoepithelial
carcinoma: A case report and review of the literature. Cytojournal.
13:132016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cimino-Mathews A, Hicks JL, Illei PB,
Halushka MK, Fetting JH, De Marzo AM, Park BH and Argani P:
Androgen receptor expression is usually maintained in initial
surgically resected breast cancer metastases but is often lost in
end-stage metastases found at autopsy. Hum Pathol. 43:1003–1011.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Di Palma S, Simpson RH, Marchiò C, Skálová
A, Ungari M, Sandison A, Whitaker S, Parry S and Reis-Filho JS:
Salivary duct carcinomas can be classified into luminal androgen
receptor-positive, HER2 and basal-like phenotypes. Histopathology.
61:629–643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Limaye SA, Posner MR, Krane JF, Fonfria M,
Lorch JH, Dillon DA, Shreenivas AV, Tishler RB and Haddad RI:
Trastuzumab for the treatment of salivary duct carcinoma.
Oncologist. 18:294–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Locati LD, Perrone F, Cortelazzi B, Lo
Vullo S, Bossi P, Dagrada G, Quattrone P, Bergamini C, Potepan P,
Civelli E, et al: Clinical activity of androgen deprivation therapy
in patients with metastatic/relapsed androgen receptor-positive
salivary gland cancers. Head Neck. 38:724–731. 2016. View Article : Google Scholar : PubMed/NCBI
|