Introduction
Breast cancer is the most commonly diagnosed
invasive cancer in women and the second most common cause of
cancer-related mortality (1). In
2016, 246,660 new cases of invasive breast cancer were diagnosed in
the USA, with an estimated 40,000 deaths (1). Significant advances in the
understanding and therapeutic approaches to breast cancer have been
made over the past several decades, which have led to improved
patient outcomes and decreased breast cancer-related mortality
(2). Historically, neoadjuvant
therapy has been reserved for locally advanced disease as a means
of reducing the tumor burden and increasing the efficacy of
surgical resection (3). The National
Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 trial was
one of the first to demonstrate that neoadjuvant therapy is as
effective as adjuvant therapy in terms of disease-free survival
(DFS) and overall survival (OS), and it provides the added benefit
of increasing the rate of breast-conserving surgery (4). In recent years, drug development in the
neoadjuvant setting has become an important research focus,
allowing for a potentially more rapid investigation of tumor
biology and predictive markers (3–9).
Bevacizumab (Bev) was the first vascular endothelial growth factor
(VEGF)-A inhibitor to be studied in breast cancer and other solid
cancers (10–14), and is currently approved by the USA
Food and Drug Administration (FDA) for use in several cancer types
(12–14). Following provisional approval for use
in metastatic breast cancer in the USA, the FDA subsequently
revoked approval in November 2011, due to the risks of Bev therapy
outweighing the benefits (15,16).
Currently, Bev is only approved for the treatment of breast cancer
outside the USA. In the neoadjuvant setting, while the effects of
Bev on increasing the rate of pathological complete response (pCR)
are well-documented, there is currently no clear evidence on the
effects of Bev on long-term outcome, or its side effects. Despite
the potential benefits of Bev as adjuvant therapy, it is not
approved for human epidermal growth factor receptor (HER)2-negative
breast cancer patients due to its side effects. However, no study
has yet compared all side effects between Bev vs. non-Bev groups. A
recent meta-analysis (17) was
undertaken to determine the effect of Bev on pCR in patients with
HER2-negative breast cancer. However, that study was based on mixed
randomized and non-randomized trials and included heterogeneous
populations; it also did not evaluate the long-term effects or
adverse effects of Bev compared with non-Bev. In view of the
controversy surrounding Bev, and given the efficacy of Bev in early
and advanced breast cancer but the lack of a clearly defined role
in this disease, we sought to conduct a meta-analysis of large,
high-quality randomized trials to compare the effect of neoadjuvant
chemotherapy with and without Bev on HER2-negative breast cancer.
The aim was to determine whether the achievement of higher rates of
pCR with combined neoadjuvant chemotherapy and Bev compared with
chemotherapy alone is associated with improved long-term
survival.
Materials and methods
Search strategy
We systematically searched for any phase II or III
randomized clinical trials evaluating the effect of Bev combined
with chemotherapy in the neoadjuvant setting for early or locally
advanced breast cancer. The PubMed and Ovid Medline databases were
searched between January 1990 and August 2016, using the search
terms ‘breast’ AND ‘neoadjuvant OR preoperative’ AND ‘bevacizumab
OR avastin’ AND ‘randomized OR randomised OR random OR RCT OR
trial’. After an initial search, the articles were reviewed and
only large (n≥200) prospective trials on HER2-negative breast
cancer were included in this meta-analysis. The PRISMA guidelines
were followed for reporting and conducting this meta-analysis
(18,19). G. Botrus and A. Dwivedi independently
analyzed the studies eligible for inclusion. Any disagreements
between the two reviewers were resolved by consulting a senior
investigator (Z. Nahleh).
Inclusion and exclusion criteria
Articles were included in the present analysis based
on the following critieria: i) Randomized controlled trials; ii)
evaluating neoadjuvant chemotherapy treatment with and without Bev;
iii) including stage I–III breast cancer; iv) HER2-negative and v)
providing data on pCR, DFS and OS. Articles were excluded if they
were animal studies, not written in English, based on endocrine
therapy rather than chemotherapy, not published as full
manuscripts, not including Bev, not randomized controlled trials,
and including stage IV breast cancer cases.
Outcome measures and data
extraction
The primary endpoints for this meta-analysis were
pCR, pCR in estrogen receptor (ER)/progesterone receptor
(PgR)-positive cancer, and pCR in triple-negative breast cancer
(TNBC). The secondary endpoints were OS, DFS and adverse events.
The data extracted included year of publication, study endpoints
such as pCR, pCR in ER/PgR-positive cases, pCR in triple-negative
cases, DFS, OS and any-grade adverse events. Additional data on
demographics or clinical characteristics were also recorded.
Statistical analysis
The incidence and relative risk (RR) for the outcome
measures and standard error (SE) for each study were compared
between Bev and non-Bev. Different studies used different
statistics to summarize time-to-event outcomes, such as OS and DFS.
In such cases, the hazard ratio (HR) and SE of HR were computed
using the suggested methods (20).
To obtain the pooled estimate, inverse variance weights were
computed for each study. Inverse variance weight adds more weight
to more precise studies. After computing incidence, RR, HR, SE and
weight of each outcome for each study, the heterogeneity in the
studies was evaluated. I2 was used to evaluate
heterogeneity in the studies. I2>70% was considered
to reflect substantial heterogeneity. The fixed-effects or
random-effects model was used to compute the combined effect of Bev
compared with non-Bev from different studies. When significant
heterogeneity was present, the random-effects model (DerSimonian
and Laird method) was used for estimating the RR/HR of outcomes in
Bev compared with non-Bev. The combined effect size was summarized
using either overall incidence or RR/HR, along with 95% confidence
interval (CI). All analyses were conducted using Stata software,
v.13.0 (StataCorp LP, College Station, TX, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Study selection
The search through electronic databases using
different combinations of search terms produced 279 articles. The
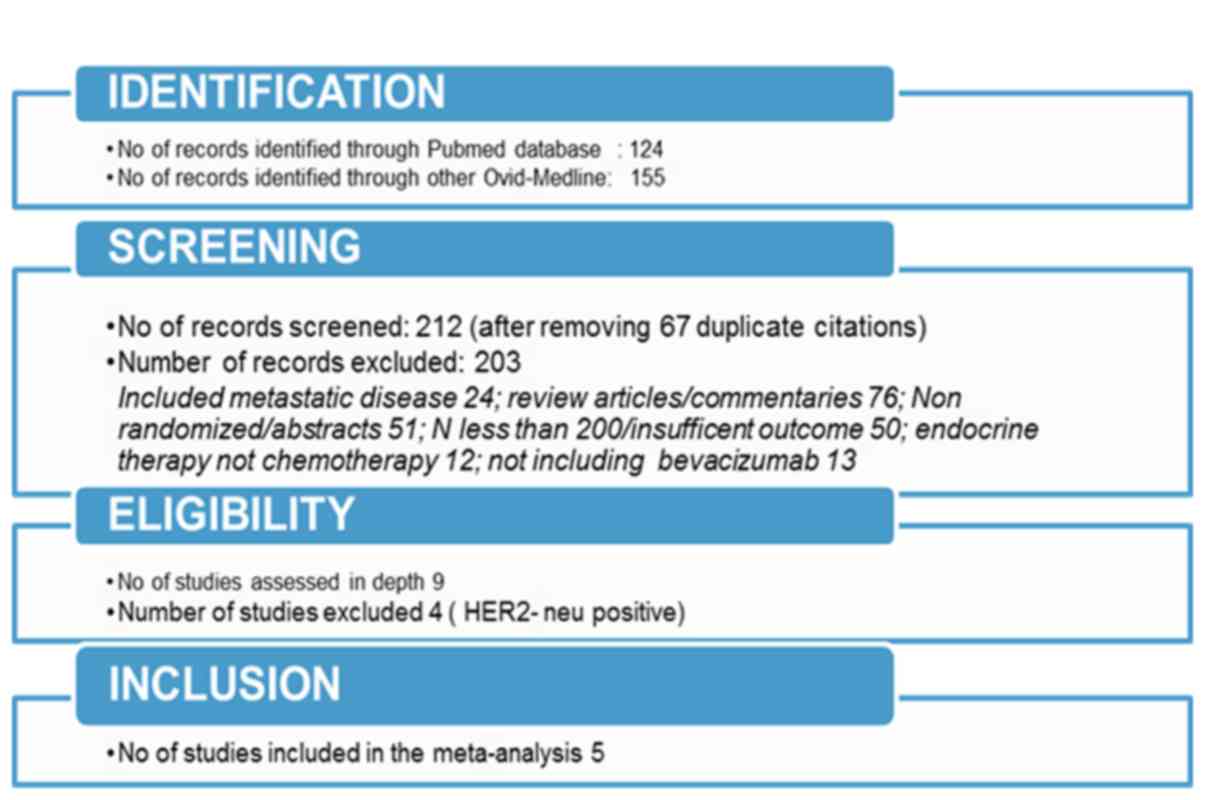
flow chart of the study selection process (Fig. 1) illustrates the identification of
eligible articles at different review stages. A total of 5
randomized studies were ultimately included in this meta-analysis
(5,21–25). A
total of 4,526 patients (2,268 treated with Bev and 2,278 without
Bev) were included in the analysis. The characteristics of the
individual studies are summarized in Table I. The overall incidence of the
outcomes considered in the present study and the pooled effect of
Bev compared with non-Bev on pCR are shown in Table II.
 | Table I.Characteristics of included
studies. |
Table I.
Characteristics of included
studies.
| Authors | Sikov et al,
CALGB 40603 (21) | Earl et al,
ARTemis trial (5) | Bear et al,
NSABP B-40 (22) | von Minckwitz et
al, GeparQuinto (25) | Nahleh et
al, S0800 (24) |
|---|
| Year | 2015 | 2015 | 2012 | 2012 | 2016 |
| Journal | JCO | Lancet
Oncology | NEJM | NEJM |
BCRT |
| Study type | 2×2 factorial
randomized, open-label, phase II trial | Randomized,
open-label, phase III trial | 3×2 factorial
randomized, open-label, phase III trial | Randomized,
open-label, phase III trial | Randomized,
open-label, phase II trial |
| Sample size
(Bev/non-Bev) | 443 (222/221) | 781 (388/393) | 1,166
(591/595) | 1,925
(969/956) | 211 (98/113) |
| Patient
population | Early-stage and
HER2-negative | Early-stage and
HER2-negative | HER2-negative | HER2-negative | Locally advanced
and HER2-negative |
| Inclusion
criteria | Operable,
previously untreated, clinical stage II–III non-inflammatory
invasive breast cancer, with ER and PgR expression ≤10% and
HER2-negative | Early-stage
invasive breast cancer; radiological tumor size >20 mm ±
axillary involvement | Operable breast
cancer | Previously
untreated, unilateral or bilateral, primary invasive breast
carcinoma | Previously
untreated, clinical stage IIB-IIIC HER2-negative breast carcinoma
and known hormone receptor status |
| Age range,
years | 40–59 | 18–50 | 18–49 | 24–78 | 22–75 |
| Recruitment
time | May 2009-August
2012 | May 2009-January
2013 | January 2007-June
2010 | August
2011-December 2012 | May 2010-September
2012 |
| Primary
outcome | pCR of the
breast | pCR of the breast
and axilla | pCR of the
breast | pCR of the
breast | pCR of the breast
and axilla |
| Secondary
outcome | pCR of the breast
and axilla and side effects | pCR of the breast,
DFS, OS and adverse events | pCR of the breast
and axilla and adverse events | pCR of the and
axilla, DFS, OS and adverse events | DFS, OS and adverse
events |
| Treatment
group | Arm (1,2)-ddAC ±
Bev; Arm (3,4)-wPcarbo followed by ddAC + Bev | D-FEC vs. Bev +
D-FEC | Arm (1,2)-Doc ±
Bev; Arm (3,4)-Doc + capecitabine ± Bev; Arm (5,6)-Doc + Gem ±
Bev | Paclitaxel +
liposomal doxorubicin + Bev ± Carbo | Arm (1)-weekly
nab-paclitaxel and Bev followed by ddAC; Arm (2)-nab-paclitaxel
followed by ddAC; Arm (3)-ddAC followed by nab-paclitaxel |
| Median follow-up,
months | 39 | 40 | N/A | 28 | 36 |
 | Table II.Pooled estimate of pCR by
subgroups. |
Table II.
Pooled estimate of pCR by
subgroups.
|
|
|
| 95% CI |
|---|
|
| I2 | Overall incidence
of outcomes investigated |
|
|---|
| pCR | 98.1 | 0.30 | 0.21 | 0.40 |
| In
breast + lymph nodes | 98.1 | 0.27 | 0.17 | 0.36 |
| In
ER/PgR+ cases | 0.0 | 0.50 | 0.48 | 0.52 |
| In
TNBC | 95.3 | 0.53 | 0.44 | 0.62 |
Comparison of pCR between the Bev and
non-Bev groups
The rate of pCR was estimated as 30% (95% CI:
21–40%), irrespective of the treatment groups (pooled estimate).
The pCR range was 18–52% (21–59% in Bev vs. 17–48% in the non-Bev
group), and the overall incidence of pCR was 35% with Bev vs. 26%
without Bev. A relative increase of 26% in the incidence of pCR was
observed in the Bev-treated group compared with the non-Bev group
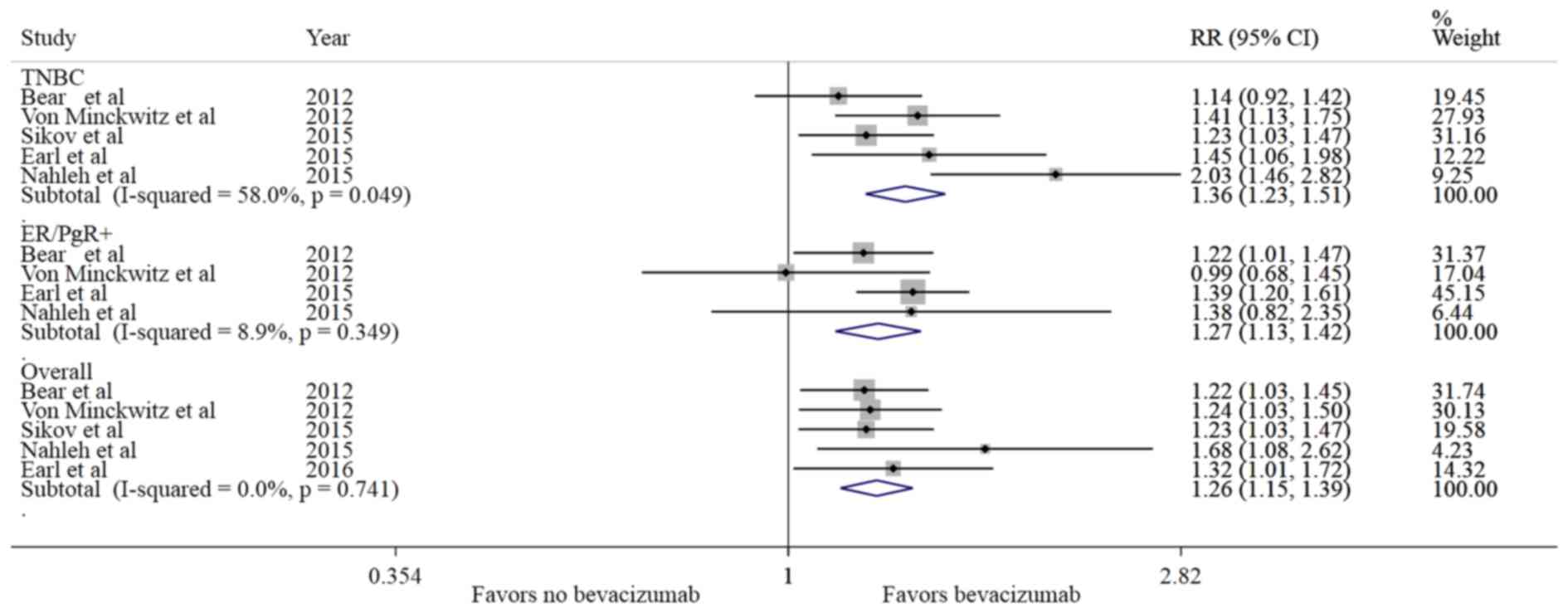
(RR=1.26, 95% CI: 1.15–1.38, P<0.001). As shown in Fig. 2, all the studies favored Bev for pCR
compared with non-Bev, without significant heterogeneity
(I2=0%). The addition of Bev to chemotherapy in the
neoadjuvant setting significantly increased the rate of pCR (breast
and lymph nodes) (RR=1.22, 95% CI: 1.10–1.36, P<0.001) compared
with treatment without Bev. In TNBC, there was a 30% relative
increase in the incidence of pCR (RR=1.30, 95% CI: 1.16–1.45,
P<0.001) in patients treated with Bev vs. the non-Bev group. In
addition, in ER/PgR+ cases, there was a 27% relative increase in
the incidence of pCR (RR=1.27, 95% CI: 1.13–1.42, P<0.001) in
patients treated with Bev compared with the non-Bev group (Table III).
 | Table III.Pooled estimate of pCR in Bev
compared with non-Bev. |
Table III.
Pooled estimate of pCR in Bev
compared with non-Bev.
|
|
|
| 95% CI |
|
|---|
|
| I2 | RR |
| P-value |
|---|
| pCR | 0.0 | 1.26 | 1.15 | 1.38 | <0.001 |
| In breast + lymph
nodes | 0.0 | 1.22 | 1.10 | 1.36 | <0.001 |
| In
ER/PgR+ cases | 8.9 | 1.27 | 1.13 | 1.42 | <0.001 |
| In TNBC | 0.0 | 1.30 | 1.16 | 1.45 | <0.001 |
Comparison of OS and DFS between the
Bev and non-Bev groups
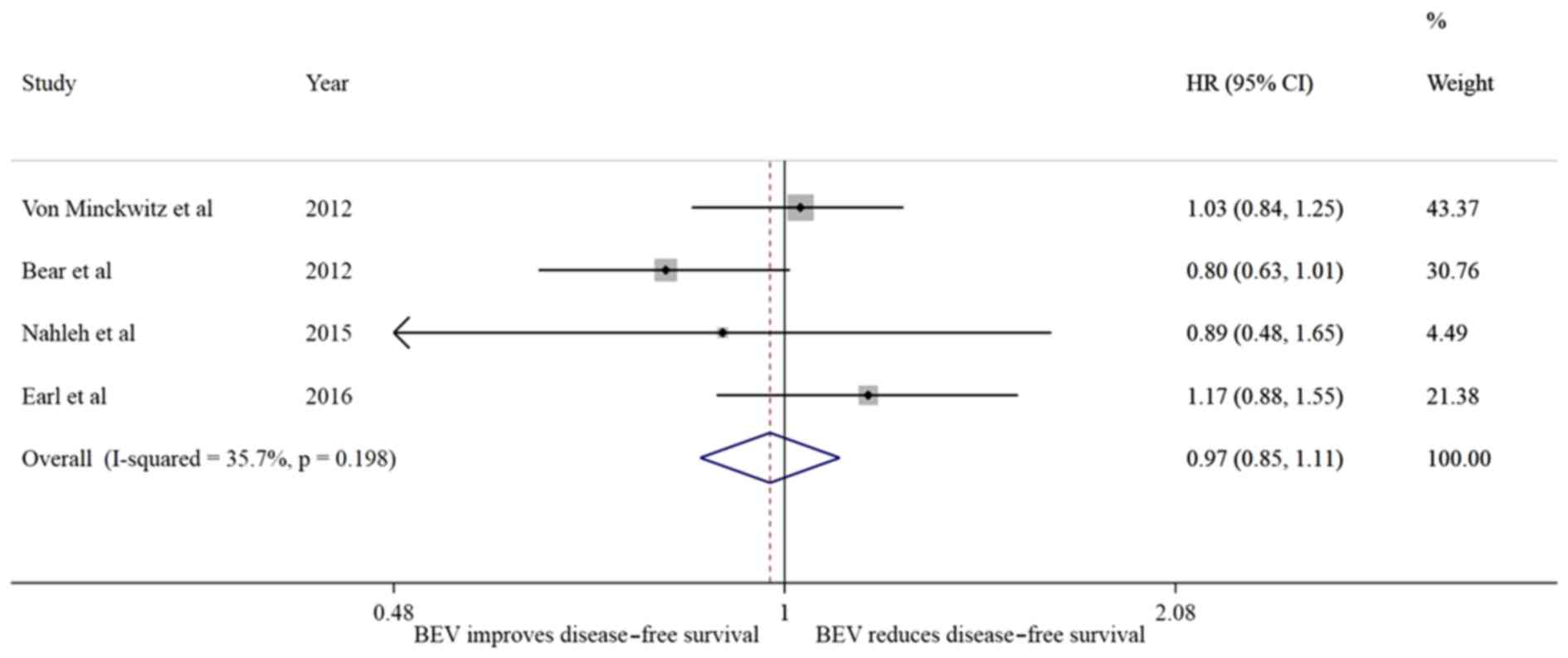
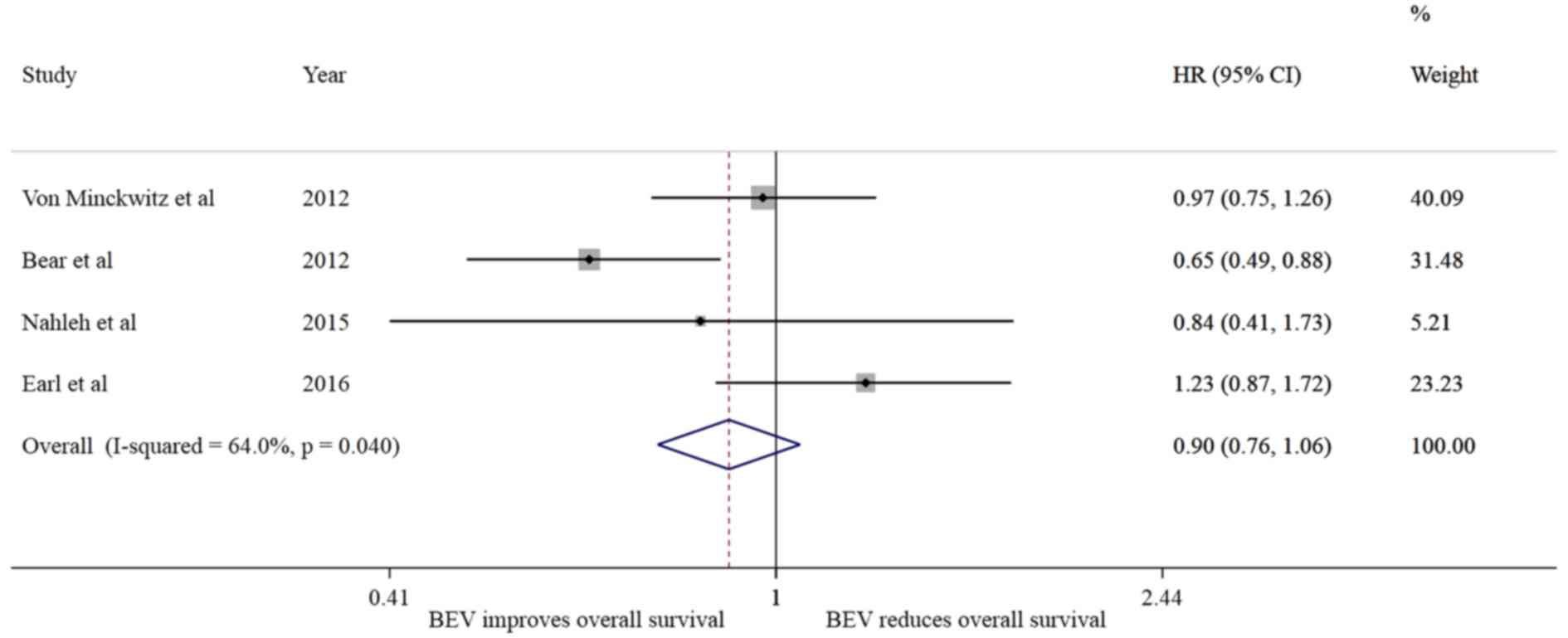
Of the 5 studies analyzed, 4 reported the DFS and OS
status by treatment groups (Figs. 3
and 4). Patients treated with Bev
exhibited no difference in the risk of recurrence
(I2=35.7%; HR=0.97, 95% CI: 0.85–1.11, P=0.684) without
presence of significant heterogeneity (P=0.198); however, a trend
towards reducing the risk of death (I2=64%; HR=0.90; 95%
CI: 0.76–1.06, P=0.194) was noted, without substantial
heterogeneity in the effect (I2=64, P=0.04).
Comparison of adverse events between
the Bev and non-Bev groups
The incidence of grade 3 and 4 adverse events was
compared between treatment groups and the results are summarized in
Table IV. Individual reported
adverse events were compared across studies. Some adverse events
were found to be significantly more common in the Bev group
compared with the non-Bev group, including hypertension (RR=5.36),
mucositis (RR=5.23), peripheral neuropathy (RR=1.75), febrile
neutropenia (RR=1.71), infection (RR=1.68), hand-foot syndrome
(RR=1.57) and neutropenia (RR=1.06).
 | Table IV.Risk of grade3/4 adverse events in
Bev compare with non-Bev. |
Table IV.
Risk of grade3/4 adverse events in
Bev compare with non-Bev.
|
|
|
| 95% CI |
|
|---|
| Adverse events | I2 | RR |
| P-value |
|---|
| Grade 1/2 | 0.0 | 1.00 | 0.94 | 1.06 | 0.915 |
| Grade 3/4 | 93.9 | 1.14 | 0.90 | 1.44 | 0.280 |
| Leukopenia | 0 | 1.02 | 0.74 | 1.41 | 0.882 |
| Neutropenia | 37.5 | 1.06 | 1.01 | 1.11 | 0.024 |
|
Thrombocytopenia | 4 | 1.05 | 0.61 | 1.81 | 0.849 |
| Hemoglobin | 50.9 | 1.32 | 0.70 | 2.50 | 0.392 |
| Febrile
neutropenia | 50.9 | 1.71 | 1.39 | 2.11 | <0.001 |
| Nausea | 0 | 0.91 | 0.65 | 1.30 | 0.613 |
| Vomiting | 73.4 | 0.80 | 0.53 | 1.21 | 0.293 |
| Mucositis | 72.6 | 5.23 | 3.70 | 7.40 | <0.001 |
| Diarrhea | 50.1 | 0.84 | 0.60 | 1.18 | 0.316 |
| Hypertension | 74.1 | 5.36 | 3.51 | 8.19 | <0.001 |
| Peripheral
neuropathy | 3.2 | 1.75 | 1.086 | 2.81 | 0.021 |
| Fatigue | 0 | 1.20 | 0.95 | 1.52 | 0.136 |
| Pain | 22.4 | 1.11 | 0.71 | 1.73 | 0.665 |
| Infection | 25.2 | 1.68 | 1.35 | 2.09 | <0.001 |
| Thrombosis | 0 | 1.43 | 0.91 | 2.24 | 0.119 |
| Hand-foot
syndrome | 0 | 1.57 | 1.04 | 2.38 | 0.034 |
Discussion
To the best of our knowledge, this is the first
meta-analysis addressing the long-term outcomes of Bev in
combination with chemotherapy in the neoadjuvant treatment of
HER2-negative breast cancer. The addition of Bev to chemotherapy
was found to significantly increase the incidence of pCR (breast
and lymph nodes) compared with treatment without Bev in
ER/PgR-positive and triple-negative cases. Although the addition of
Bev to chemotherapy did not translate into a significant difference
in DFS, it exhibited a trend towards improving OS, but without
reaching statistical significance. Despite the heterogeneity in
reporting adverse events among the trials, there was no significant
difference in the incidence of grade 3 and 4 adverse events between
the Bev and non-Bev groups. However, patients treated with Bev more
commonly experienced hypertension, mucositis, peripheral neuropathy
and neutropenia compared with those treated without Bev. These
findings are consistent with the adverse events associated with the
use of Bev, as the most common grade 3/4 adverse events include
hypertension followed by mucositis.
The results of the present study are consistent with
the findings from another recent meta-analysis that included 9
studies and reported the efficacy of Bev with chemotherapy compared
with Bev without chemotherapy in terms of pCR as the primary
endpoint of interest, without assessing long-term survival outcome
(17). However, there were notable
differences between the two studies: Cao et al (17), combined studies with both
HER2-negative and HER2-positive breast cancer and included mostly
preliminary findings, without examining the long-term effects of
Bev. By contrast, the present analysis included completed
randomized trials, excluded HER2-positive breast cancer, and
reported long-term survival data. As regards to efficacy, we also
observed some differences. The overall efficacy of Bev in terms of
pCR in HER2-negative patients was found to be slightly lower in our
analysis, which had a larger sample size, compared with the one
reported by Cao et al (17).
The results of our analysis were consistent with the
reported outcome on pCR in the 5 neoadjuvant trials considered
(5,21–25).
However, there were also certain variations. In our analysis, an
overall relative increase of 26% in the incidence of pCR was
observed in the Bev group compared with the non-Bev group, which is
very similar to the pCR reported in the studies analyzed (5,21–23). One
study (24) that included primarily
higher-risk patients with locally advanced and inflammatory breast
cancer (S0800) reported a slightly higher RR of pCR in the Bev
group compared with the non-Bev group, as opposed to the other 4
studies and the pooled effect obtained in this study. The variation
observed in the S0800 study may be due to its comparatively smaller
size relative to the other 4 studies considered in this
meta-analysis.
A notable difference in our analysis was that the
benefit of Bev treatment in increasing pCR rate was observed in
both the triple-negative (30% increase in relative incidence of
pCR, 11% absolute benefit) as well as in the ER/PgR-positive groups
(26% increase in relative incidence of pCR, 9% absolute benefit).
The ARTemis trial (5) reported a 6%
adjusted absolute increase in the incidence of pCR after addition
of Bev to standard neoadjuvant therapy compared with the non-Bev
group (26 vs. 19% overall (95% CI: 0.1–12.1); P=0.02), but the
efficacy was restricted to the ER-negative/HER-2-negative subgroup
defined as Allred 0–2 (15% absolute improvement) and the ER weakly
positive group (Allred 3–5) (20% absolute improvement), whereas
there was no benefit in the ER strongly positive group (Allred 6–8)
(absolute decrease of 1%). Similarly, in the GeparQuinto study
(25), the addition of Bev
significantly increased the incidence of pCR in TNBC (11.4%
absolute benefit), in contrast to only 0.1% in the hormone
receptor-positive subgroup. The CALGB 40603 study (21), a randomized phase II trial limited to
stage II–III TNBC reported a significant increase in pCR incidence
in the Bev group compared with the non-Bev group (59 vs. 48%,
respectively; P=0.009). The S0800 trial (24) demonstrated a significant increase in
pCR rate with the addition of Bev in both the ER/PgR-positive and
triple-negative groups, but the increase only reached statistical
significance in the TNBC group. The findings of the NSABP B-40
trial (22) were somewhat
contradictory, showing a significant increase in the number of
patients achieving pCR in the hormone-positive group (23.2 vs.
15.1%, P=0.007) but not in the triple-negative group (51.5 vs.
47.1%, P=0.34).
The apparent discordance between the various studies
may be explained by differences in study design and definitions of
hormone receptor-positive disease. An ER/PgR-positive disease was
defined as >10% positive cells for either ER or PgR in
GeparQuinto (25) and CALGB 40603
(21), while a 1% cutoff was used in
S0800 (24) and NSABP B-40 (22), and an Allred score >2 was used in
ARTemis trial to identify ER/PgR-positive patients (5), a definition similar to that of NSABP
B-40 and S0800. It is, therefore, plausible that the increased
proportion of patients who achieved a pCR in the ER/PgR-positive
group in NSABP B-40 and S0800 resulted from patients with low
ER/PgR scores who were excluded by GeparQuinto and CALGB 40603.
Despite these differences, however, our analysis suggests that the
benefits from Bev may not be limited to TNBC cases.
Our meta-analysis failed to demonstrate that the
addition of Bev in the neoadjuvant setting improved DFS. This was
not unexpected, considering the small absolute increment noted in
pCR (11% for TNBC and 9% for ER/PgR-positive cases). This finding
is consistent with the results reported from the 5 considered
studies. The association between pCR and event-free survival (EFS)
has been quite challenging to elucidate (26). Berry and Hudis analyzed the potential
causes for the discordance between pCR and EFS (26). They noted the difficulty to
demonstrate a significant correlation when the treatment effect is
being measured based on a small number of clinical trials or trial
subsets, particularly given the small treatment differences in pCR
rates in the trials and the inherent variability in reassembling
patients into trials and treatment arms. However, the neoadjuvant
approach to future drug development may still have a potentially
significant role, should future phase 3 trials implement specific
novel adaptive designs, such as the use of interim by-treatment
information regarding pCR and EFS and adapting to accumulating
information in the trial, among other strategies (26).
In our analysis, the small change in pCR rates
combined with the different combinations of chemotherapy regimens
used, different disease stages, discontinuation of Bev after
surgery and possible rebound angiogenesis (27), as well as the varied median follow-up
periods, would be plausible explanations for the lack of effect
regarding DFS. However, the overall effect of Bev on OS exhibited a
positive trend favoring Bev compared with non-Bev. This positive
effect on OS was largely attributed to two trials (22,24),
while a lack of effect of Bev on OS was reported in one trial
(5).
The results of the present meta-analysis are
consistent with two large phase III postoperative trials indicating
absence of a DFS and OS advantage from Bev when used in
HER2-negative breast cancer (28,29). The
E5103 trial recruited 4,994 patients with HER2-negative disease and
both hormone receptor-positive and -negative disease (29), while the BEATRICE trial enrolled
2,591 patients with only TNBC (28).
In both trials, Bev was added to chemotherapy. After completing
chemotherapy, the patients were randomly assigned to receive or not
receive single-agent Bev for a total duration of 1 year. Neither
trial showed an improvement in DFS or OS with the addition of 1
year of Bev to the adjuvant chemotherapy. Thus, the addition of
continuous Bev treatment after surgery cannot be fully supported by
the outcome of those trials. On the other hand, the positive trend
in OS favoring Bev compared with chemotherapy without Bev noted in
our analysis requires further investigation. In light of the
NSABP-B40 data, it may be hypothesized that the benefit of Bev is
most pronounced when used in a combined pre- and postoperative
setting. When used as such, Bev, a targeted anti-angiogenic agent,
may be most useful in treating relatively large or more locally
advanced tumors in the breast and lymph nodes, where high
dependence on angiogenesis may be at play. This activity is
reflected by the increased pCR with the use of Bev in the present
meta-analysis, and particularly in trials including locally
advanced and inflammatory tumors, such as the S0800 (24). The continuation of Bev after surgery
may provide an additional benefit by suppressing possible rebound
tumor cell growth (27).
As noted above, there was some heterogeneity
observed for reporting adverse events across the different studies.
In the CALGB 40603 study (21), 12%
of patients administered Bev, with or without carboplatin, stopped
treatment early due to toxicity. Similarly, 14% of patients
receiving Bev in the ARTemis (5)
trial and 12% of patients in the GeparSixto trial (23) stopped treatment early due to the
toxicity. The most common grade 3/4 adverse events included
hypertension followed by mucositis, peripheral neuropathy, febrile
neutropenia, infection and hand-foot syndrome, and the incidence
was increased with Bev compared with non-Bev. Other studies have
also demonstrated that Bev use is associated with increased
incidence of grade 3 and 4 hypertension and mucositis. However, an
increase in grade 3 and 4 left ventricular systolic dysfunction,
thromboembolic events, bleeding and postoperative complications
with Bev was not reported in the individual studies included in our
analysis (5,7,21,23–25).
One of the major limitations in the clinical
applicability of Bev and potentially other similar agents in breast
cancer is the current lack of information regarding specific
prognostic markers correlated with Bev efficacy. Prognostic markers
for Bev therapy have been studied in other solid cancers, and it
has been suggested that improved efficacy of Bev in metastatic
colorectal cancer is correlated with specific endothelial nitric
oxide synthase (eNOS) polymorphisms (30). The greatest improvement in
progression-free survival was associated with the haplotype
homozygous for eNOS VNTR 4bb and eNOS + 894 TT. Various VEGFR1 and
VEGFA single-nucleotide polymorphisms (SNPs) have also been
implicated as prognostic markers for Bev therapy (31,32). The
recent findings of the ANGIOMET study demonstrated that VEGFA SNP
rs3025039 and VEGFR1 SNP rs9582036 were associated with reduced OS
in patients treated with Bev for non-small-cell lung cancer
(32). Lower basal VEGF levels were
also associated with better patient outcomes. This is in agreement
with earlier reports suggesting that high levels of VEGF are
correlated with increased vascular count and density, which may
result in greater tumor growth (33). In the CALGB 40603 trial, the
investigators studied how intrinsic subtype assigned by PAM50 and
other gene signatures affected the impact of Bev on pCR rates in
TNBC (34). A significant benefit of
Bev was noted in the basal-like subtype, yielding a more than
double the odds of achieving pCR. In basal-like tumors, pCR was 64%
with Bev vs. 45% without Bev (odds ratio = 2.15, P=0.0009).
However, for the other subtypes, the rates were 43 and 60%,
respectively (odds ratio = 0.50, P=0.25), indicating a possible
trend toward worse outcome when Bev was added (33). Furthermore, higher levels of
expression of any of the following immune signatures, mRNA
signatures for high proliferative rate, low estrogen signaling,
high TP53 mutation, and an increase in the number of
tumor-infiltrating lymphocytes, were associated with higher pCR
rates. High proliferation rate, low estrogen expression, or high
TP53-mutation signatures, in particular, were predictive of higher
pCR rates overall, and a greater pCR benefit from the addition of
Bev. These findings suggest that, even within TNBC, there are
biologically defined patient subsets that may benefit from this
agent. Further research is necessary, however, to confirm and
validate reliable predictive and prognostic markers of Bev efficacy
for the treatment of breast cancer.
In summary, the present meta-analysis confirms the
benefit of Bev combined with chemotherapy compared with
chemotherapy alone in the neoadjuvant treatment of HER2-negative
breast cancer. This benefit in pCR is quite intriguing, as it does
not translate into a long-term DFS or definitive OS advantage.
Therefore, in the absence of specific predictive or efficacy
markers, the use of non-targeted Bev in the treatment of an
unselected breast cancer patient population cannot be justified at
this time. This is particularly true in view of the adverse effect
profile associated with the use of Bev. Further research is
warranted for the pre-treatment identification of predictive and
prognostic markers to identify the subsets of breast cancer
patients who are most likely to benefit from Bev therapy. However,
the premature dismissal of this effective agent in the treatment
armamentarium of breast cancer is not recommended. Should a breast
cancer patient subset be identified that may benefit from the
addition of Bev, the studies included in this analysis can be
revisited and confirmatory studies conducted using collected stored
tissue samples. Furthermore, predictors of the efficacy of Bev, if
identified in ongoing or future translational studies, may prompt
new trials of this drug in better defined patient subgroups.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Authors contributions
ZN developed the concept and design of the present
study, collected the data and wrote and edited manuscript. GB
collected data and contributed to the writing of the manuscript. AD
developed the statistical method and performed data analysis. MJ
collected data and SN contributed to data collection. AT wrote the
manuscript performed the final revision. All the authors read and
approved the final version of this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
Mariotto A, Lewis DR, Chen HS, Feuer EJ and Cronin KA: SEER Cancer
Statistics Review. pp. 1975–2013. National Cancer Institute;
Bethesda, MD, USA: 2016
|
|
2
|
DeSantis CE, Fedewa SA, Goding Sauer A,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gampenrieder SP, Rinnerthaler G and Greil
R: Neoadjuvant chemotherapy and targeted therapy in breast cancer:
Past, present, and future. J Oncol. 2013:7320472013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fisher B, Brown A, Mamounas E, Wieand S,
Robidoux A, Margolese RG, Cruz AB Jr, Fisher ER, Wickerham DL,
Wolmark N, et al: Effect of preoperative chemotherapy on
local-regional disease in women with operable breast cancer:
Findings from national surgical adjuvant breast and bowel project
B-18. J Clin Oncol. 15:2483–2493. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Earl HM, Hiller L, Dunn JA, Blenkinsop C,
Grybowicz L, Vallier AL, Abraham J, Thomas J, Provenzano E,
Hughes-Davies L, et al: ARTemis Investigators: Efficacy of
neoadjuvant bevacizumab added to docetaxel followed by
fluorouracil, epirubicin, and cyclophosphamide, for women with
HER2-negative early breast cancer (ARTemis): An open-label,
randomised, phase 3 trial. Lancet Oncol. 16:656–666. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huober J, von Minckwitz G, Denkert C,
Tesch H, Weiss E, Zahm DM, Belau A, Khandan F, Hauschild M,
Thomssen C, et al: Effect of neoadjuvant anthracycline-taxane-based
chemotherapy in different biological breast cancer phenotypes:
Overall results from the GeparTrio study. Breast Cancer Res Treat.
124:133–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gerber B, Loibl S, Eidtmann H, Rezai M,
Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C,
et al: German Breast Group Investigators: Neoadjuvant bevacizumab
and anthracycline-taxane-based chemotherapy in 678 triple-negative
primary breast cancers; results from the geparquinto study (GBG
44). Ann Oncol. 24:2978–2984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levasseur N, Clemons M, Hilton J, Addison
C, Robertson S, Ibrahim M and Arnaout A: Neoadjuvant endocrine
therapy and window of opportunity trials: New standards in the
treatment of breast cancer? Minerva Chir. 70:181–193.
2015.PubMed/NCBI
|
|
9
|
Dowsett M: Predictive and prognostic
factors. Breast Cancer Res. 12 Suppl 4:S22010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shinkaruk S, Bayle M, Laïn G and Déléris
G: Vascular endothelial cell growth factor (VEGF), an emerging
target for cancer chemotherapy. Curr Med Chem Anticancer Agents.
3:95–117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrara N, Hillan KJ, Gerber HP and
Novotny W: Discovery and development of bevacizumab, an anti-VEGF
antibody for treating cancer. Nat Rev Drug Discov. 3:391–400. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Summers J, Cohen MH, Keegan P and Pazdur
R: FDA drug approval summary: Bevacizumab plus interferon for
advanced renal cell carcinoma. Oncologist. 15:104–111. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gil-Gil MJ, Mesia C, Rey M and Bruna J:
Bevacizumab for the treatment of glioblastoma. Clin Med Insights
Oncol. 7:123–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen MH, Gootenberg J, Keegan P and
Pazdur R: FDA drug approval summary: Bevacizumab (Avastin) plus
Carboplatin and Paclitaxel as first-line treatment of
advanced/metastatic recurrent nonsquamous non-small cell lung
cancer. Oncologist. 12:713–718. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conti RM, Dusetzina SB, Herbert AC, Berndt
ER, Huskamp HA and Keating NL: The impact of emerging safety and
effectiveness evidence on the use of physician-administered drugs:
The case of bevacizumab for breast cancer. Med Care. 51:622–627.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lenzer J: FDA committee votes to withdraw
bevacizumab for breast cancer. BMJ. doi.org/10.1136/bmj.d4244.
|
|
17
|
Cao L, Yao GY, Liu MF, Chen LJ, Hu XL and
Ye CS: Neoadjuvant bevacizumab plus chemotherapy versus
chemotherapy alone to treat non-metastatic breast cancer: A
meta-analysis of randomised controlled trials. PLoS One.
10:e01454422015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. BMJ.
doi.org/10.1136/bmj.b2535.
|
|
19
|
Hutton B, Moher D and Cameron C: The
PRISMA extension statement. Ann Intern Med. 163:566–567. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sikov WM, Berry DA, Perou CM, Singh B,
Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER,
et al: Impact of the addition of carboplatin and/or bevacizumab to
neoadjuvant once-per-week paclitaxel followed by dose-dense
doxorubicin and cyclophosphamide on pathologic complete response
rates in stage II to III triple-negative breast cancer: CALGB 40603
(Alliance). J Clin Oncol. 33:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bear HD, Tang G, Rastogi P, Geyer CE Jr,
Robidoux A, Atkins JN, Baez-Diaz L, Brufsky AM, Mehta RS,
Fehrenbacher L, et al: Bevacizumab added to neoadjuvant
chemotherapy for breast cancer. N Engl J Med. 366:310–320. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
von Minckwitz G, Schneeweiss A, Loibl S,
Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S,
Gerber B, et al: Neoadjuvant carboplatin in patients with
triple-negative and HER2-positive early breast cancer (GeparSixto;
GBG 66): A randomised phase 2 trial. Lancet Oncol. 15:747–756.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nahleh ZA, Barlow WE, Hayes DF, Schott AF,
Gralow JR, Sikov WM, Perez EA, Chennuru S, Mirshahidi HR, Corso SW,
et al: SWOG S0800 (NCI CDR0000636131): Addition of bevacizumab to
neoadjuvant nab-paclitaxel with dose-dense doxorubicin and
cyclophosphamide improves pathologic complete response (pCR) rates
in inflammatory or locally advanced breast cancer. Breast Cancer
Res Treat. 158:485–495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
von Minckwitz G, Eidtmann H, Rezai M,
Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C,
Kreienberg R, et al: German Breast Group; Arbeitsgemeinschaft
Gynäkologische Onkologie–Breast Study Groups: Neoadjuvant
chemotherapy and bevacizumab for HER2-negative breast cancer. N
Engl J Med. 366:299–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berry DA and Hudis CA: Neoadjuvant therapy
in breast cancer as a basis for drug approval. JAMA Oncol.
1:875–876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mancuso MR, Davis R, Norberg SM, O'Brien
S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et
al: Rapid vascular regrowth in tumors after reversal of VEGF
inhibition. J Clin Invest. 116:2610–2621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cameron D, Brown J, Dent R, Jackisch C,
Mackey J, Pivot X, Steger GG, Suter TM, Toi M, Parmar M, et al:
Adjuvant bevacizumab-containing therapy in triple-negative breast
cancer (BEATRICE): Primary results of a randomised, phase 3 trial.
Lancet Oncol. 14:933–942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miller K, O'Neill AM, Dang CT, et al:
Bevacizumab in the adjuvant treatment of HER2 negative breast
cancer: Final results from Eastern Cooperative Oncology Group
E5103. 2014 ASCO Annual Meeting (abstract 500). J Clin Oncol. 32
Suppl 15:5002014. View Article : Google Scholar
|
|
30
|
Ulivi P, Scarpi E, Passardi A, Marisi G,
Calistri D, Zoli W, Del Re M, Frassineti GL, Tassinari D, Tamberi
S, et al: eNOS polymorphisms as predictors of efficacy of
bevacizumab-based chemotherapy in metastatic colorectal cancer:
Data from a randomized clinical trial. J Transl Med. 13:2582015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lambrechts D, Claes B, Delmar P, Reumers
J, Mazzone M, Yesilyurt BT, Devlieger R, Verslype C, Tejpar S,
Wildiers H, et al: VEGF pathway genetic variants as biomarkers of
treatment outcome with bevacizumab: An analysis of data from the
AViTA and AVOREN randomised trials. Lancet Oncol. 13:724–733. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Massuti Sureda B, Jantus-Lewintre E,
Gonzalez-Larriba JL, Rodriguez Abreu D, Juan OJ, Domine M,
Provencio Pulla M, de Castro J, Camps C and Rosell R: 37PDSNPS in
angiogenic factors as predictive markers for outcome in patients
(P) with advanced non-squamous NSCLC (NS-NSCLC) treated with
carboplatin, paclitaxel (CP), and bavacizumab (BEV). Final results
of angiomet spanish lung cancer group trial. Ann Oncol. 26 Suppl
1:i102015. View Article : Google Scholar
|
|
33
|
Mattern J, Koomägi R and Volm M:
Association of vascular endothelial growth factor expression with
intratumoral microvessel density and tumour cell proliferation in
human epidermoid lung carcinoma. Br J Cancer. 73:931–934. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sikov WM, Barry WT, Hoadley KA, et al:
Impact of intrinsic subtype by PAM50 and other gene signatures on
pathologic complete response (pCR) rates in triple-negative breast
cancer (TNBC) after neoadjuvant chemotherapy (NACT) plus/-
carboplatin (Cb) or bevacizumab (Bev): CALGB 40603/150709
(Allianc). Cancer Res. 75:S4–S05. 2015. View Article : Google Scholar
|