Introduction
Epidermal growth factor-tyrosine kinase inhibitors
(EGFR-TKIs) have been proven to be effective for non-small-cell
lung cancer (NSCLC) with EGFR mutations. With the advent of
EGFR-TKIs, the prognosis of NSCLC has markedly improved, but the
incidence of brain metastases (BM) and leptomeningeal metastases
(LM) is reportedly increasing, with a reported cumulative incidence
of BM of ~46.7% at 3 years (1). Of
note, EGFR-TKIs may also be effective for BM as well as
extracranial disease (2); therefore,
they are considered as one of the most important therapeutic
options. Osimertinib is a third-generation EGFR-TKI, which was
designed for NSCLC patients with T790M mutation, and has also been
reported to be effective for the treatment of BM (3). However, it remains unclear what
treatment strategy would be preferable for BM developing during
salvage cytotoxic chemotherapy after osimertinib failure. We herein
report a case of a successful osimertinib re-challenge for multiple
BM from NSCLC developing during salvage cytotoxic chemotherapy.
Case report
A 73-year-old female patient was diagnosed with
stage IVb lung adenocarcinoma (T1bN2M1b, brain metastases) in April
2013. As there were only two small BM lesions, stereotactic
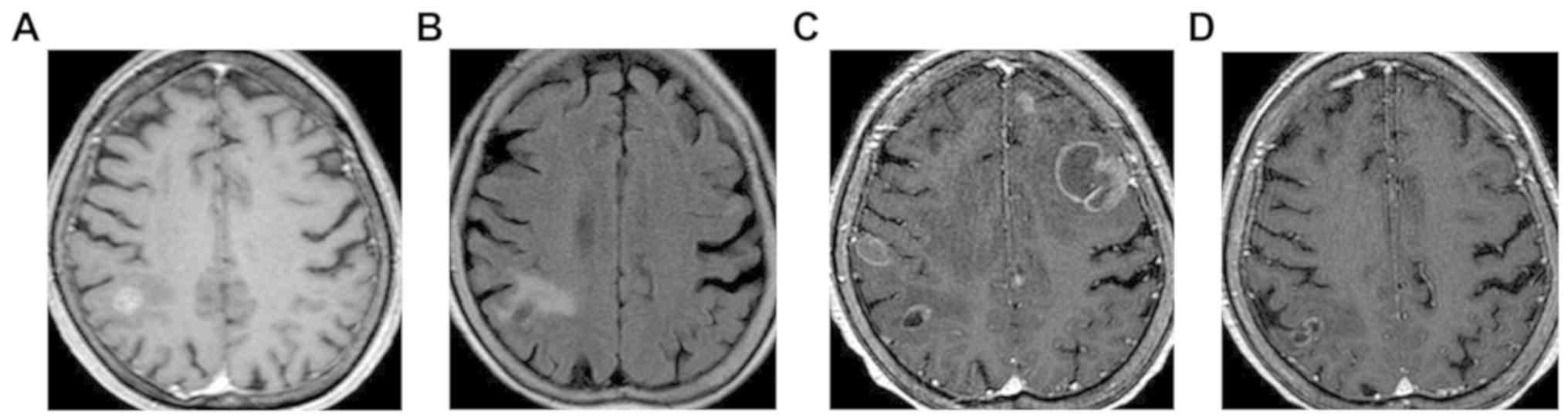
radiosurgery was performed (Fig.
1A). Subsequently, 250 mg gefitinib was administered daily, as
the patient was found to harbor an EGFR gene mutation (exon 19
deletion). After 1.5 years of partial response, multiple lung
metastases developed. As T790M was detected in the specimen
collected by transbronchial lung biopsy, daily treatment with 80 mg
osimertinib was initiated, based on the AURA 3 clinical study
(AstraZeneca, Cambridge, UK; NCT02151981) (4), resulting in rapid and apparent
shrinkage of the primary tumor and multiple lung metastases. Three
years later, the primary tumor enlarged, with the cranial lesion
remaining stable (Fig. 1B). As one
cycle of docetaxel and two cycles of S-1 were ineffective, the
patient was administered pemetrexed as fifth-line chemotherapy. Two
weeks after the initiation of pemetrexed therapy, however, she
developed numbness of the left hand, severe dizziness, and
disturbances of behavior and thought, resulting in worsening of the
performance status (PS) score to 3. Radiological evaluation
revealed the development of multiple BM with severe peritumoral
brain edema (Fig. 1C). Whole-brain
radiotherapy (WBRT) was excluded due to concerns regarding the
exacerbation of the cognitive impairment. Therefore, osimertinib
re-challenge therapy (80 mg/day) was selected. At 2 weeks after
treatment initiation, the neurological symptoms drastically
improved, with a PS score of 1. One month later, brain magnetic
resonance imaging revealed apparent shrinkage of the BM and
subsiding brain edema (Fig. 1D),
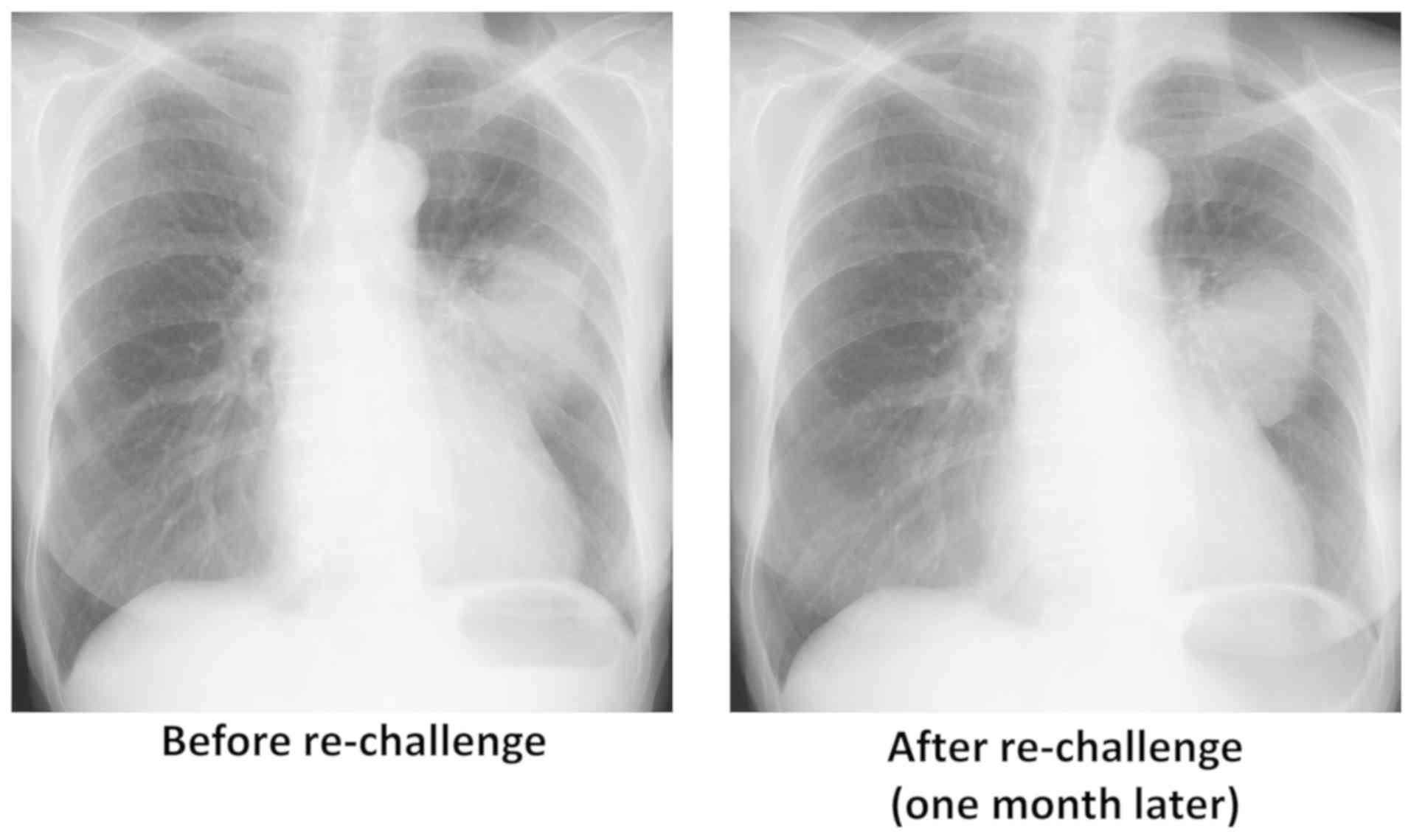
although the primary lung tumor remained stable (Fig. 2). In October 2018, 6 months after
initiating osimertinib re-challenge, the patient continued
osimertinib treatment and BM remained stable.
Discussion
The findings of the present case indicate that
osimertinib re-challenge may be a viable therapeutic option for BM
developing during the course of salvage cytotoxic chemotherapy.
There is currently no established optimal therapeutic strategy for
BM that develop during salvage cytotoxic chemotherapy following
osimertinib failure and disease progression. Radiotherapy, mainly
WBRT, may be effective for BM from EGFR-mutated NSCLC, but it is
associated with increased risk of neurocognitive impairment
(5). In the present case,
osimertinib re-challenge was proven to be effective for BM,
although there was no change in the primary tumor. Generally,
central nervous system progression has been reported as a major
concern in NSCLC patients treated with gefitinib, with a prevalence
of 35.1% (6), which is attributable
to the penetration rate of the blood-brain barrier (7). With regard to osimertinib, the
pre-clinical data indicate favorable penetration into the brain
parenchyma (8), which is supported
by the marked response of the BM to osimertinib in the present
case. Of note, there was a difference in therapeutic efficacy
between the BM and the primary lesion; however, as this is beyond
the scope of the present case report, this observation is not
discussed in detail at present. It is known that there is
heterogeneity among T790M-positive cancer cells (9), and it is hypothesized that the
difference in therapeutic efficacy in this patient may also be
associated with this heterogeneity.
Furthermore, as osimertinib re-challenge acted
rapidly on BM, it may be one of the preferable therapies to be
considered in the future. Generally, the therapeutic strategy for
BM should be decided taking into consideration the activity of
extracranial disease and the risk of WBRT-induced cognitive
impairment (10). In the present
case, osimertinib re-challenge was selected as the patient was
elderly and already exhibited signs of cognitive impairment. Due to
the rapid and dramatic improvement of the patient's PS within 2
weeks after the initiation of osimertinib re-challenge, there was
no need to add radiation to the treatment. Koba et al
reported two cases of BM from T790M-positive NSCLC: A rapid
response was observed 2 weeks later, and WBRT was therefore deemed
unnecessary (11). Taking this
report together with ours into consideration, osimertinib
treatment, even as re-challenge, may exert a rapid and marked
effect on BM. Therefore, osimertinib re-challenge may be valuable
for WBRT candidates with a concern for potential development of
cognitive impairment.
In conclusion, we herein report a case of successful
osimertinib re-challenge for BM from lung adenocarcinoma developing
during salvage cytotoxic chemotherapy. Although the optimal
therapeutic strategy for BM in NSCLC patients previously treated
with osimertinib has yet to be determined, the results in the
present case suggest that osimertinib re-challenge is a viable
treatment option. Accumulation of clinical information in patients
with similar treatment status is required to confirm our
results.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AS and HS participated in the conception and design
of the study, analyzed and interpreted the data and wrote the
manuscript. AS, SI, TOd and TOg evaluated the patient and
participated in the therapy. SI, HS and TOg revised the manuscript
for intellectual content. TI and KO evaluated the radiological
images or pathological specimens. All authors have read and
approved the final draft of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of the case details and any associated images.
Competing interests
Dr Sekine and Dr Ikeda have received lecture fees
from AstraZeneca, Boehringer Ingelheim and Chugai Pharmaceuticals.
Dr Ogura has received a lecture fee from Boehringer Ingelheim. The
remaining authors have stated that they have no conflicts of
interest to disclose.
References
|
1
|
Rangachari D, Yamaguchi N, VanderLaan PA,
Folch E, Mahadevan A, Floyd SR, Uhlmann EJ, Wong ET, Dahlberg SE,
Huberman MS, et al: Brain metastases in patients with EGFR-mutated
or ALK-rearranged non-small-cell lung cancers. Lung Cancer.
88:108–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iuchi T, Shingyoji M, Sakaida T, Hatano K,
Nagano O, Itakura M, Kageyama H, Yokoi S, Hasegawa Y, Kawasaki K,
et al: Phase II trial of gefitinib alone without radiation therapy
for Japanese patients with brain metastases from EGFR-mutant lung
adenocarcinoma. Lung Cancer. 82:282–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahn MJ, Tsai CM, Yang JC, Shepherd FA,
Satouchi M, Kim DW, Bazhenova L, Hirashima T, Rukazenkov Y,
Cantarini M, et al: AZD9291 activity in patients with EGFR-mutant
advanced non-small cell lung cancer (NSCLC) and brain metastases:
Data from Phase II studies. Eur J Cancer. 51:S625–S626. 2015.
View Article : Google Scholar
|
|
4
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et al
AURA3 investigators, : Osimertinib or platinum-pemetrexed in EGFR
T790M-positive lung cancer. N Engl J Med. 376:629–640. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tallet AV, Azria D, Barlesi F, Spano JP,
Carpentier AF, Gonçalves A and Metellus P: Neurocognitive function
impairment after whole brain radiotherapy for brain metastases:
Actual assessment. Radiat Oncol. 7:772012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li MX, He H, Ruan ZH, Zhu YX, Li RQ, He X,
Lan BH, Zhang ZM, Liu GD, Xiao HL, et al: Central nervous system
progression in advanced non-small cell lung cancer patients with
EGFR mutations in response to first-line treatment with two
EGFR-TKIs, gefitinib and erlotinib: A comparative study. BMC
Cancer. 17:2452017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Togashi Y, Masago K, Masuda S, Mizuno T,
Fukudo M, Ikemi Y, Sakamori Y, Nagai H, Kim YH, Katsura T, et al:
Cerebrospinal fluid concentration of gefitinib and erlotinib in
patients with non-small cell lung cancer. Cancer Chemother
Pharmacol. 70:399–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ballard P, Yates JW, Yang Z, Kim DW, Yang
JC, Cantarini M, Pickup K, Jordan A, Hickey M, Grist M, et al:
Preclinical comparison of osimertinib with other EGFR-TKIs in
EGFR-mutant NSCLC brain metastases models, and early evidence of
clinical brain metastases activity. Clin Cancer Res. 22:5130–5140.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hata AN, Niederst MJ, Archibald HL,
Gomez-Caraballo M, Siddiqui FM, Mulvey HE, Maruvka YE, Ji F, Bhang
HE, Krishnamurthy Radhakrishna V, et al: Tumor cells can follow
distinct evolutionary paths to become resistant to epidermal growth
factor receptor inhibition. Nat Med. 22:262–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sekine A and Satoh H: Paradigm shift of
therapeutic management of brain metastases in EGFR-mutant non-small
cell lung cancer in the era of targeted therapy. Med Oncol.
34:1212017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koba T, Kijima T, Takimoto T, Hirata H,
Naito Y, Hamaguchi M, Otsuka T, Kuroyama M, Nagatomo I, Takeda Y,
et al: Rapid intracranial response to osimertinib, without
radiotherapy, in nonsmall cell lung cancer patients harboring the
EGFR T790M mutation: Two Case Reports. Medicine (Baltimore).
96:e60872017. View Article : Google Scholar : PubMed/NCBI
|