Introduction
Chordoma is a rare tumor that originates from the
notochord, with an incidence of <0.1/100,000 (1). Half of chordomas involve the sacral
region, followed by the skull base (35%) and the vertebral column
(15%) (2). Surgery is still
considered to be the standard cure management for sacral chordoma,
whereas radiotherapy has been used for radical treatment in
inoperable patients. However, carbon ion radiotherapy (CIRT) has
recently emerged as a promising treatment for unresectable sacral
chordoma in Japan (3). Heavy ions,
such as carbon ions, have less lateral scattering than that of
photons, which leads to further improvement in the dose
distribution to the target area (4).
A distinguishing feature of carbon ions versus photons is the
release of low-level radiation along their travel paths except for
the maximum energy at the end of their range (Bragg peak) (4). Thus, CIRT can selectively irradiate
tumors by sparing the surrounding normal tissues (4,5).
Furthermore, the relative biological effectiveness (RBE) in the
tumor region is higher for heavy ions than for photons (5). Previous reports have demonstrated that
patients who received marginal or intralesional excision of sacral
chordoma often have worse prognoses, and adequate margins are only
achieved in roughly 50% of cases. In addition, even for widely
resected sacral chordoma, the 5-year local control rate has been
found to be 80% (6–10). On the other hand, Imai et al
reported a 5-year local control rate of sacral chordoma treated by
CIRT of 88%, although >80% of tumors in the study were located
higher than the S2 level (3).
Additionally, CIRT has fewer short-term complications than does
surgical resection for sacral chordoma (3,11).
However, when sacral chordoma is close to the intestine, spacers
are often placed between the tumor and the intestine to reduce the
risk of gastrointestinal complications (4). In Japan, CIRT has increasingly been
used for treatment of sacral chordoma. Here we present two cases of
rectotumoral fistula formation that occurred >5 years after CIRT
for sacral chordoma. We obtained written informed consent from the
two patients.
Case report
Case 1
A 73-year-old man was referred to our outpatient
clinic for sacral tumor detected on magnetic resonance imaging
(MRI). The tumor was pathologically confirmed by biopsy as a
chordoma. Since the tumor invaded the upper level of the sacrum
(S1-5) and resection of the tumor would cause severe impairments,
the patient decided to receive CIRT instead of surgery.
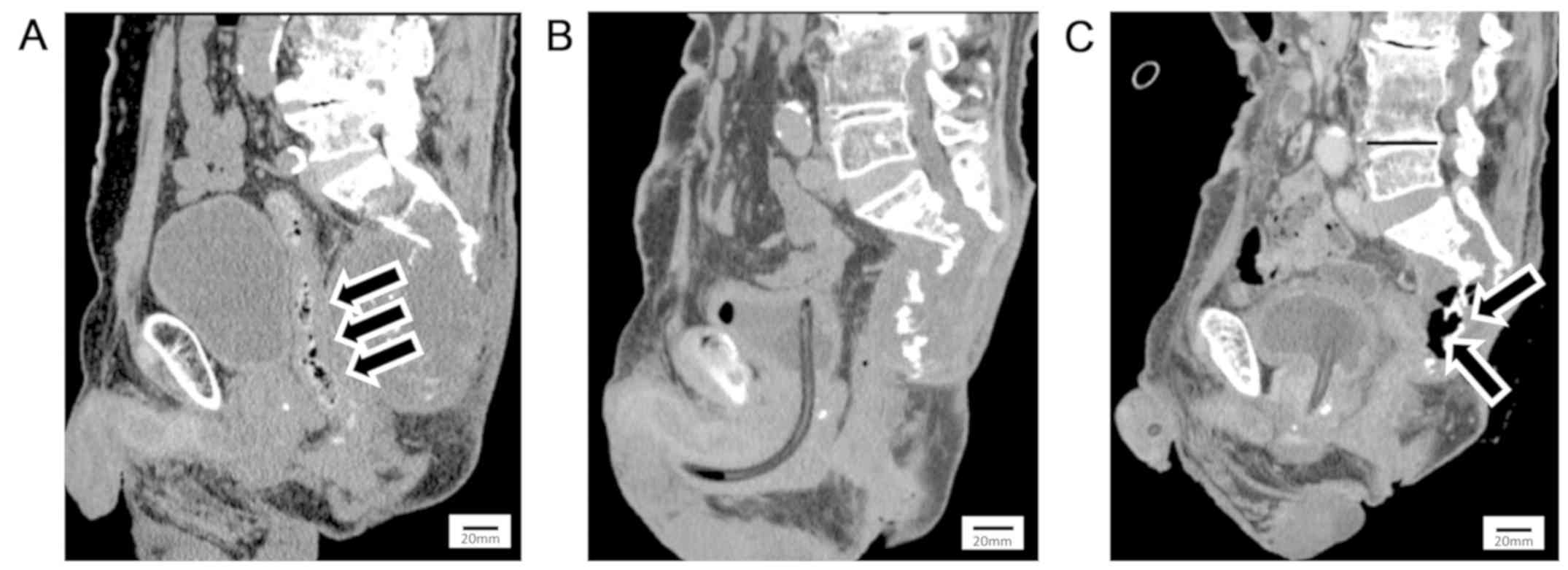
Since the tumor was contiguous with the rectum
(Fig. 1A), we tried to place a
spacer between the tumor and rectum to prevent radiation
enterocolitis. However, we could not place the spacer because of
adhesion of the tumor to the surroundings, so we performed a
colostomy before CIRT. We performed CIRT at a total dose of 70.4 Gy
(RBE) in 16 fractions. Colostomy closure was not performed because
of a neurogenic rectal disturbance that occurred before CIRT. The
tumor shrank markedly and partially ossified 6 years after CIRT, as
shown by computed tomography (CT) (Fig.
1B) and MRI. Seven years after CIRT, the patient visited a
general practitioner because of a high-grade fever and worsening
sacral pain. A colonoscopy revealed rectotumoral fistula formation.
After antibiotic treatment failed to reduce the high fever, he was
referred to our outpatient clinic. Contrast-enhanced CT showed a
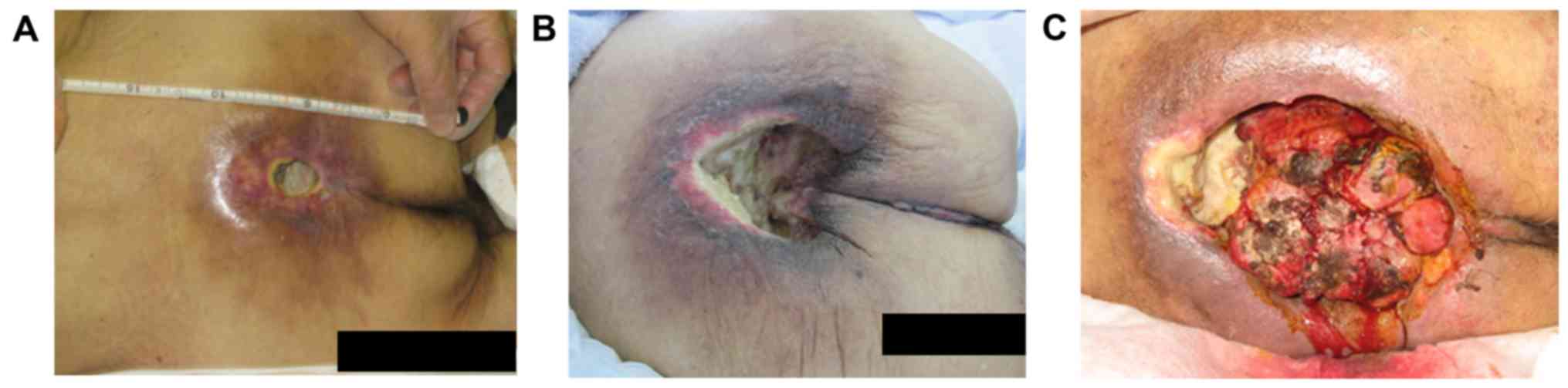
rectotumoral fistula and emphysema inside the tumor (Fig. 1C). Physical examination showed skin
flare that was centrally ulcerated around the sacrum (Fig. 2A), and a tumor was observed in the
dimple of ulceration. A blood test showed that his white blood cell
(WBC) count and C-reactive protein (CRP) were elevated: WBC,
1.12×104/µl; CRP, 13.1 mg/dl. He was diagnosed as having
sepsis from the rectotumoral fistula with tumor recurrence and
received continuous intravenous antibiotic treatment. Resection of
the fistula and residual rectum with anal closure and necrotic
sacral bone excision were performed to control the infection 1
month after admission. During the procedures, a tumor biopsy was
performed. However, after the operation, the ulceration enlarged
and the tumor kept growing (Fig.
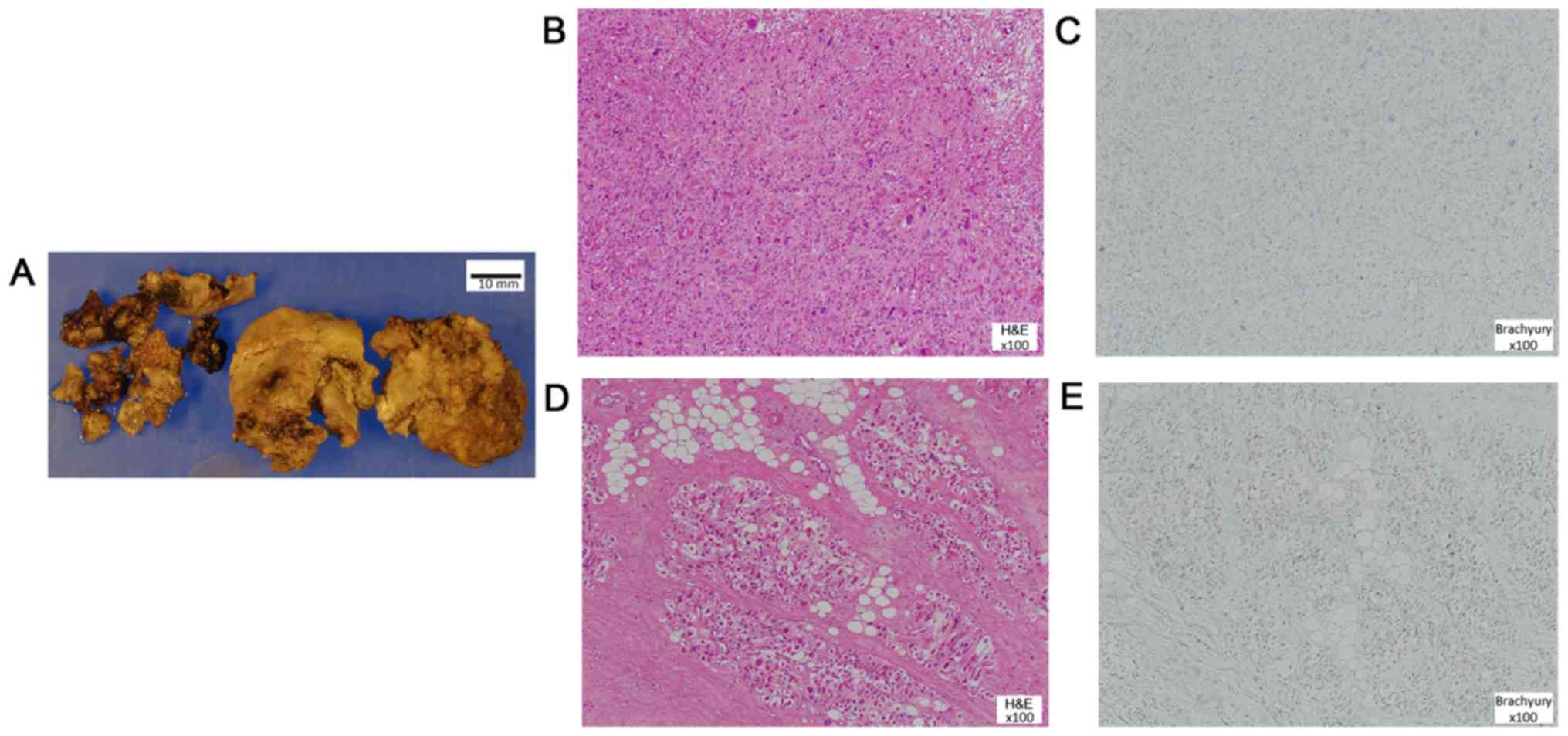
2B). Pathological examination revealed that most components
appeared as undifferentiated pleomorphic sarcoma and some parts
still resembled a chordoma, however, brachyury staining, which is
diagnostic marker for chordoma, was negative. The tumor showed no
identifiable line of differentiation based on the
immunohistochemical negativity for pan-cytokeratin, epithelial
membrane antigen (EMA), desmin, α-smooth muscle actin (α-SMA),
S100, and CD34; all of which were negative in the morphologically
chordoma-like areas as well. The specimen was considered as either
a post-CIRT sarcoma or a recurrent dedifferentiated chordoma
(Fig. 3). Despite trans-arterial
embolization (TAE) and chemical debridement using Mohs paste, the
tumor kept growing and bleeding. Finally, he died 9 years after
CIRT because of bleeding from the tumor (Fig. 2C).
Case 2
A 55-year-old man was referred to our outpatient
clinic for an expanding tumor, which was previously diagnosed as a
benign tumor by biopsy when he received surgery for rectal cancer 7
years ago. We performed a biopsy again, and the specimen was
pathologically confirmed as chordoma. Surgical resection had a risk
of inducing severe neurological deficit because the tumor invaded
the upper level of the sacrum (S1-5). Thus, he rejected the surgery
and accepted CIRT instead. The total dose was 70.4 GyE in 16
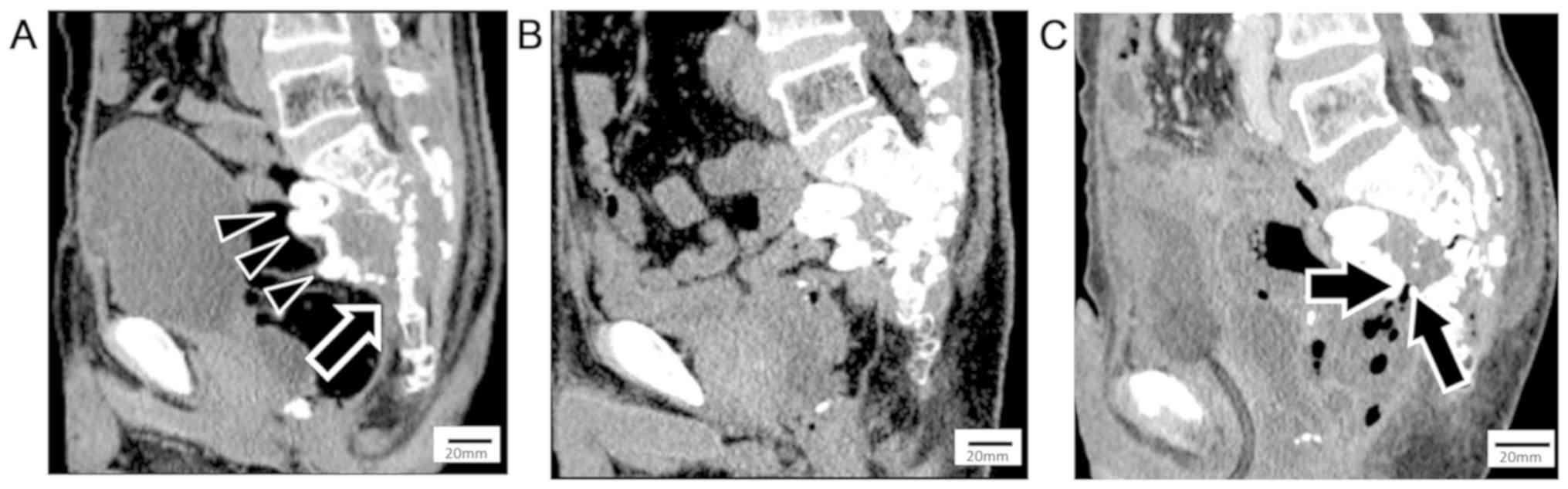
fractions. A 15×20-cm polytetrafluoroethylene spacer was placed
between the rectum and tumor before CIRT to prevent radiation
enterocolitis. The tumor in front of the coccyx could not be
covered because of adhesion of the rectal surroundings caused by
previous surgery (Fig. 4A). The
tumor had shrunk markedly and become partially ossified 8 years
after CIRT (Fig. 4B). However,
follow-up MRI and CT revealed tumor regrowth and lung metastasis 10
years after CIRT. Surgery had not been performed because the tumor
had invaded the pelvic structures, and radiotherapy could not be
delivered because of the previous CIRT. He visited our hospital
because of a high fever 1 month after diagnosis of the tumor
regrowth. A blood test revealed high WBC count
(1.12×104/µl) and CRP (20.0 mg/dl), and a blood culture
was positive for proteus vulgaris. Contrast-enhanced CT revealed
that the rectotumoral fistula at the site of the spacer was
uncovered and emphysema was inside the tumor (Fig. 4C). He was diagnosed as having sepsis
from the rectotumoral fistula that was difficult to remove and
received intravenous antibiotic treatment. He died of uncontrolled
sepsis 7 months after admission despite continuous antibiotic
treatment.
Discussion
There are few reports about complications of CIRT
for sacral chordoma. Neuropathic pain (15–16%) and skin toxicity
(5–22%) have been reported (3,11). Imai
et al reported 188 cases analysis of CIRT for unresectable
sacral chordoma. In that study, the median follow-up period was 62
month (6.8–147.5) and 70% of patients were followed for over 5
years or until death. The 5-year local control rates were 77.2%
(3,12). The local recurrence rate is not high.
Among the locally recurrent cases there are no previous report
about fistula formation. Thus, it is quite rare complication, and
to our best knowledge, this is the first report of rectotumoral
fistula formation after CIRT for sacral chordoma.
We considered two possible explanations for the
fistula formation: First, radiation enterocolitis after CIRT might
have caused formation of the fistula over a long period of time
(13). Wallner et al reported
that 8 (0.3%) of 2464 patients developed a radiation-related
fistula after prostate brachytherapy (13). The average volume of the rectum
receiving 100% of the prescription dose was moderately higher for
the fistula patients than for the non-fistula patients. Time to
fistulation ranged from 23 to 66 months (median, 25 months)
(13). Thus, irradiation of the
gastrointestinal tract around the tumor has a risk for fistula
formation. Although both of our patients were operated on for
spacer protection, coverage of the rectum was not achieved, which
led to fistulation. Therefore, it is important to be aware that
when sufficient protection during CIRT cannot be provided, careful
follow-up to monitor potential fistulation is necessary. Second,
tumor regrowth can compress and invade the rectum and enforce
fistula formation. Imai et al reported that the local
recurrence rate for 5 years was 22.8% (12). However, the long-term recurrence rate
remains unknown. In our cases, the patients experienced tumor
regrowth 7 years (Case 1) and 10 years (Case 2) after CIRT and
increased rapidly. Late tumor regrowth might affect the long
latency of fistula formation.
We confirmed dedifferentiated morphology similar to
undifferentiated pleomorphic sarcoma by biopsy 7 years after CIRT
in Case 1. Huvos et al proposed the following criteria for
diagnosis of post-radiation sarcoma: i) The patient received
irradiation, ii) the neoplasm occurred in the radiation field, iii)
a latent period of some years had elapsed, and iv) there was
histological or roentgenographic evidence for the pre-existing
osseous condition, if present, in addition to microscopic proof of
a sarcoma (14). The risk of
post-radiation sarcoma has been found to be 0.06% at a mean latency
of 15 years (3–50 years) (15). It
is difficult to determine whether our cases involved
dedifferentiated chordomas or post-CIRT sarcomas. However,
according to Huvos' criteria, we concluded that it was post-CIRT
sarcoma.
The limitation of our study is little pathological
evidences to show sarcoma formation from chorodoma. In Case 1,
unfortunately, the pathology preparation slide before CIRT was
lost. However, needle biopsy under CT guide was performed. The
pathological report was slow growing chordoma. We confirmed the
diagnosis as chordoma with the result of this needle biopsy and the
typical radiographic feature of MRI and CT. The radiographic
feature represent no evidence of high grade sarcoma before CIRT.
After CIRT, some parts of tumor still resembled a chorodma.
However, brachyury staining was negative. In Case 2, we also could
not find the pathological preparation slide nor paraffin block of
initial open biopsy specimen in case 2. However, this case was
irradiated in National Institute of Radiological Sciences in Chiba,
where all cases were pathologically and radiographically reviewed
by the board members. This case was also centrally reviewed and
diagnosed as typical chordoma. We did not perform biopsy for
recurrent tumor because of the sepsis and malnourishment.
It is important to prevent fistula formation at the
tumor because treatments of the wound within the radiation area of
CIRT are very difficult. Unfortunately, when a tumor is found that
shows regrowth, resection of the tumor including the residual
rectum should be considered.
In conclusion, we experienced two cases of
rectotumoral fistula formation that occurred >5 years after
CIRT. It is important to be aware of the possibility of rectal
complications and to investigate rectal conditions after CIRT
during long-term follow-up. If rectal complications are revealed,
resection of the rectum should be considered. Additionally, tumor
regrowth can worsen rectal complications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available to maintain the privacy of
the patients but are available from the corresponding author on
reasonable request.
Authors' contributions
YU collected the patient data and was a principal
contributor to the writing of the manuscript. NA contributed to the
design and conception of the present study, and corrected the
manuscript. HO additionally contributed to the design and
conception of the present study, and reviewed the manuscript. SN
and EK performed the histological examination of chordoma
specimens. RI treated the patient by carbon ion radiotherapy in
Case 2 and contributed to data collection. YD and TO treated the
patient by carbon ion radiotherapy in Case 1 and contributed to
data collection. NN contributed to the data collection. All authors
contributed to the interpretation, discussion and critical review
of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Osaka
International Cancer Institutional Review Board (Osaka, Japan).
Patient consent for publication
The patients provided informed consent regarding
medical information and the publication of the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu E, Koffer PP, DiPetrillo TA and
Kinsella TJ: Incidence, treatment, and survival patterns for sacral
chordoma in the United States, 1974–2011. Front Oncol. 6:2032016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chugh R, Tawbi H, Lucas DR, Biermann JS,
Schuetze SM and Baker LH: Chordoma: The nonsarcoma primary bone
tumor. Oncologist. 12:1344–1350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Imai R, Kamada T, Sugahara S, Tsuji H and
Tsujii H: Carbon ion radiotherapy for sacral chordoma. Br J Radiol.
84:S48–S54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lorenzo C, Andrea P, Barbara V, Denis P,
Rosaria FM, Piero F, Viviana V, Alberto I, Mario C, Brugnatelli S,
et al: Surgical spacer placement prior carbon ion radiotherapy
(CIRT): An effective feasible strategy to improve the treatment for
sacral chordoma. World J Surg Oncol. 14:2112016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamada T, Tsujii H, Blakely EA, Debus J,
De Neve W, Durante M, Jäkel O, Mayer R, Orecchia R, Pötter R, et
al: Carbon ion radiotherapy in Japan: An assessment of 20 years of
clinical experience. Lancet Oncol. 16:e93–e100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varga PP, Szövérfi Z, Fisher CG, Boriani
S, Gokaslan ZL, Dekutoski MB, Chou D, Quraishi NA, Reynolds JJ,
Luzzati A, et al: Surgical treatment of sacral chordoma: Prognostic
variables for local recurrence and overall survival. Eur Spine J.
24:1092–1101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Radaelli S, Stacchiotti S, Ruggieri P,
Donati D, Casali PG, Palmerini E, Collini P, Gambarotti M, Porcu L,
Boriani S, et al: Sacral chordoma: Long-term outcome of a large
series of patients surgically treated at two reference centers.
Spine. 41:1049–1057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Y, Niu X, Li Y, Liu W and Xu H:
Recurrence and survival factors analysis of 171 cases of sacral
chordoma in a single institute. Eur Spine J. 26:1910–1916. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stacchiotti S and Sommer J; Chordoma
Global Consensus Group, : Building a global consensus approach to
chordoma: A position paper from the medical and patient community.
Lancet Oncol. 16:e71–e83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asavamongkolkul A and Waikakul S: Wide
resection of sacral chordoma via a posterior approach. Int Orthop.
36:607–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demizu Y, Jin D, Sulaiman NS, Nagano F,
Terashima K, Tokumaru S, Akagi T, Fujii O, Daimon T, Sasaki R, et
al: Particle therapy using protons or carbon ions for unresectable
or incompletely resected bone and soft tissue sarcomas of the
pelvis. Int J Radiat Oncol Biol Phys. 98:367–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imai R, Kamada T, Araki N, Abe S, Iwamoto
Y, Ozaki T, Kanehira C, Kaya M, Takahashi K, Chuman H, et al
Working Group for Bone and Soft Tissue Sarcomas, : Carbon ion
radiation therapy for unresectable sacral chordoma: An analysis of
188 cases. Int J Radiat Oncol Biol Phys. 95:322–327. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wallner K, Sutlief S, Bergsagel C and
Merrick GS: Severe rectal complications after prostate
brachytherapy. Radiother Oncol. 114:272–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huvos AG, Woodard HQ, Cahan WG,
Higinbotham NL, Stewart FW, Butler A and Bretsky SS: Postradiation
osteogenic sarcoma of bone and soft tissues. A clinicopathologic
study of 66 patients. Cancer. 55:1244–1255. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mavrogenis AF, Pala E, Guerra G and
Ruggieri P: Post-radiation sarcomas. Clinical outcome of 52
Patients. J Surg Oncol. 105:570–576. 2012. View Article : Google Scholar : PubMed/NCBI
|