Introduction
Radiotherapy has been shown to improve local control
for rectal cancer (1,2). The two commonly used pelvic
radiotherapy regimens are either short-course (SC) radiotherapy
defined as 25 Gy over 5 consecutive days (3–5) or
long-course (LC) chemoradiotherapy defined as 45–54 Gy over 5–6
weeks with concurrent antimetabolite chemotherapy (6–8). The
value of adding concomitant chemotherapy has been evaluated only in
the long-course radiotherapy, showing improved local control
(9,10). Both LC chemoradiotherapy and SC
radiotherapy are acceptable options according to current NCCN
guidelines.
Two randomized trials that compared LC
chemoradiotherapy to SC radiotherapy showed a lack of difference in
disease-free survival (DFS) and overall survival (OS) (11,12).
Only one randomized trial compared LC radiotherapy without
chemotherapy to SC radiotherapy showing no difference in overall
survival (13). However, this trial
included patients with upper rectal tumors (ranging from 17 to 28%
in the different trial groups) usually not treated with
preoperative radiotherapy, and more importantly did not report
clinical stage at diagnosis prior to randomization, making the
results difficult to interpret or apply clinically.
In order to assess differences in efficacy between
LC and SC radiotherapy in locally advanced rectal adenocarcinoma,
we used a US-based nationwide oncology dataset to compare these two
regimens without concomitant use of chemotherapy.
Materials and methods
Data source and patient
population
Our cohort was derived from the National Cancer
Database (NCDB), a hospital-based cancer registry, from 2006 to
2013. The NCDB captures data on 70% of cancer diagnoses in the
United States from >1,400 hospitals with cancer programs
accredited by the American College of Surgeons' Commission on
Cancer and American Cancer Society (14). The cohort included all individuals
with clinical stage II (T3-4N0M0) or III (TanyN1-2M0) rectal
adenocarcinoma who received neoadjuvant radiotherapy followed by
surgery, and did not receive neoadjuvant or adjuvant chemotherapy.
The present study was approved by the Institutional Review Board at
the University of Pennsylvania (Philadelphia, PA, USA).
Variables definition
The primary exposure of interest was radiotherapy
regimen, defined as either SC (25 Gy in 5 Gy fractions) or LC (45
or 50.4 Gy in 1.8 Gy fractions). Covariates included treatment
intensity, age, sex, race, patient comorbidities score
(Charlson-Deyo comorbidity condition, CDCC) (15,16), and
tumor grade. Race and ethnicity were used to create a composite
variable categorized as Caucasian, African-American or
other/unknown. Tumor grade was defined as well-differentiated,
moderately differentiated, poorly differentiated or
undifferentiated.
Outcomes definition
The primary outcome was OS, measured from the time
of cancer diagnosis until death of any cause or last follow-up.
Statistical analysis
Patients were grouped according to radiotherapy
regimen, defined as either SC or LC. Baseline characteristics in
each risk group were compared using chi-square test for categorical
variables and Student's t-test for continuous variables. OS was
measured from date of diagnosis. Difference in OS was compared
between the groups, using Cox proportional hazards. The Cox model
was adjusted to age, sex, race, CDCC and clinical stage. All
statistical analyses were performed using Stata/IC software 13.0
(StataCorp LP, College Station, TX, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
We identified 458 individuals with stage II or III
rectal adenocarcinoma that were treated with radiotherapy followed
by surgery, without the use of neither neoadjuvant nor adjuvant
chemotherapy. Patients received either SC radiotherapy (25 Gy,
N=83) or LC radiotherapy (45/50.4 Gy, N=375). Patient
characteristics are presented in Table
I. The median follow-up time were 21.7 months (IQR 8.3–37.2)
and 41.3 months (IQR 23.3–66.0) for the SC and LC groups,
respectively. Patients receiving SC were older than those receiving
LC (74 vs. 66 years, respectively, P<0.001). All other
characteristics were not statistically different between the two
groups, although the LC group had numerically more stage III
patients compared to the SC group (44.8 vs. 33.7%, respectively).
Median interval between diagnosis and surgery was 58 days (IQR
40–87) and 133 days (IQR 111–161) in the SC and LC groups,
respectively.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| Radiotherapy
protocol |
|---|
|
|
|
|---|
| Characteristics | Short-course
(n=83) | Long-course
(n=375) |
|---|
| Age, median
(IQR) | 74 (65–81) | 66 (56–77) |
| Sex, % male (n) | 54.2 (45) | 58.4 (219) |
| Race, % (n) |
|
|
|
Caucasian | 89.2 (74) | 85.6 (321) |
|
African-American | 4.8
(4) | 8.3
(31) |
|
Other | 6.0
(5) | 6.1
(23) |
| CDCC, % (n) |
|
|
| 0 | 67.5 (56) | 73.9 (277) |
| 1 | 27.7 (23) | 18.4 (69) |
| ≥2 | 4.8
(4) | 7.7
(29) |
| Grade, % (n) |
|
|
| Well | 6.0
(5) | 7.5 (28) |
|
Moderate | 59.0 (49) | 60.8 (228) |
| Poor | 22.9 (19) | 14.7 (55) |
|
Undifferentiated | 1.2
(1) | 1.3
(5) |
|
Other | 10.8 (9) | 15.7 (59) |
| Clinical stage, %
(n) |
|
|
| II | 66.3 (55) | 55.2 (207) |
| III | 33.7 (28) | 44.8 (168) |
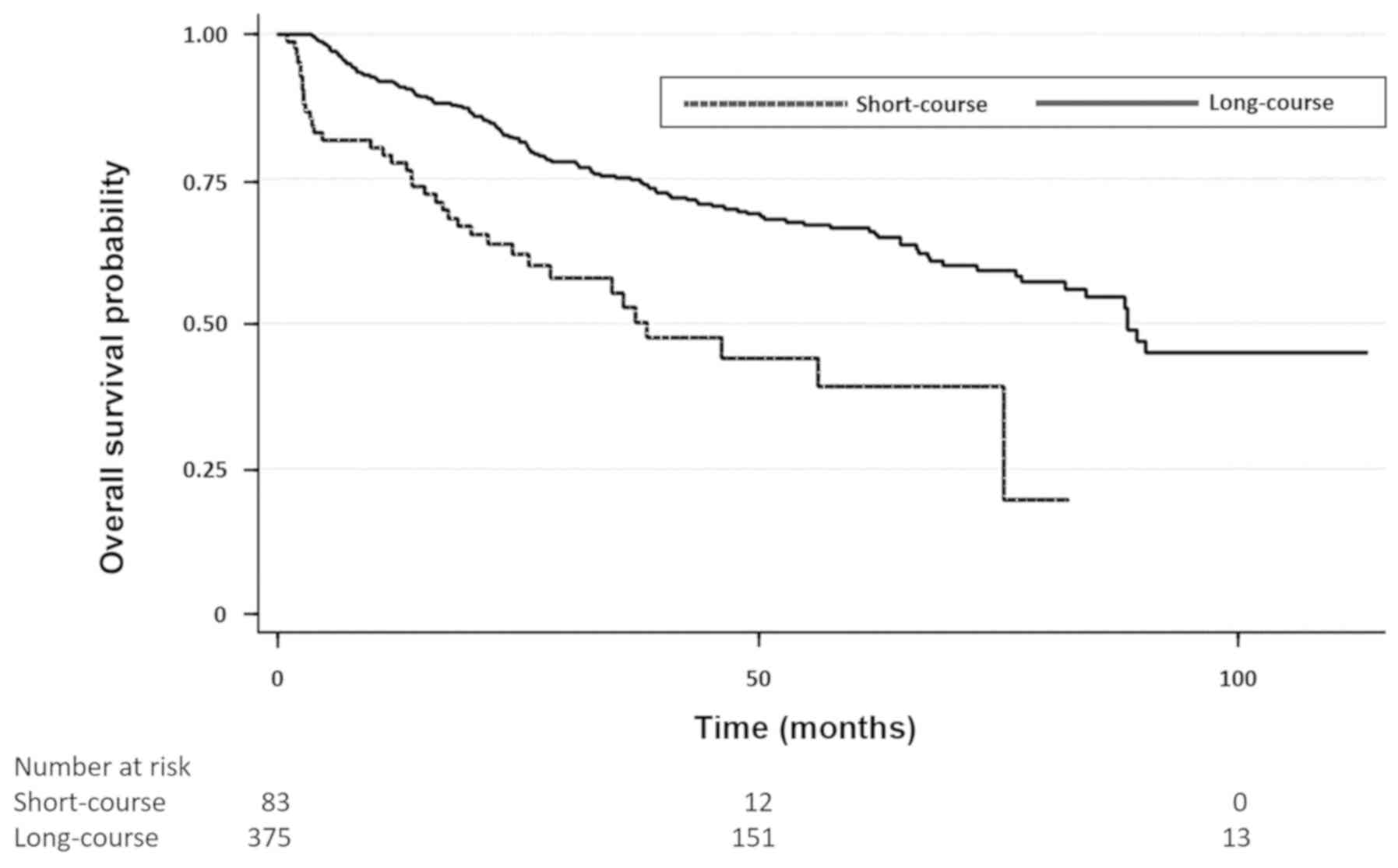
OS according to radiotherapy dose was improved in
the LC group compared with the SC group. The unadjusted and
adjusted HRs for OS were 0.42 (0.29–0.61, P<0.001) and 0.50
(0.34–0.73, P<0.001), respectively (Fig. 1). This difference in OS was
maintained after stratifying patients according to clinical stage.
For clinical stage II patients (n=262) the unadjusted and adjusted
HRs for OS were 0.50 (0.30–0.82, P=0.007) and 0.60 (0.36–0.99,
P=0.05), respectively. For clinical stage III patients (n=196) the
unadjusted and adjusted HRs for overall survival were 0.30
(0.18–0.53, P<0.001) and 0.39 (0.22–0.70, P=0.002),
respectively.
Mortality rates post-surgery were higher in the SC
group compared with the LC group. Thirty-day mortality rates for
the SC and LC were 12.2% (n=10) and 2.4% (n=9), respectively.
Ninety-day mortality rates for the SC and LC were 18.5% (n=15) and
5.4% (n=20), respectively.
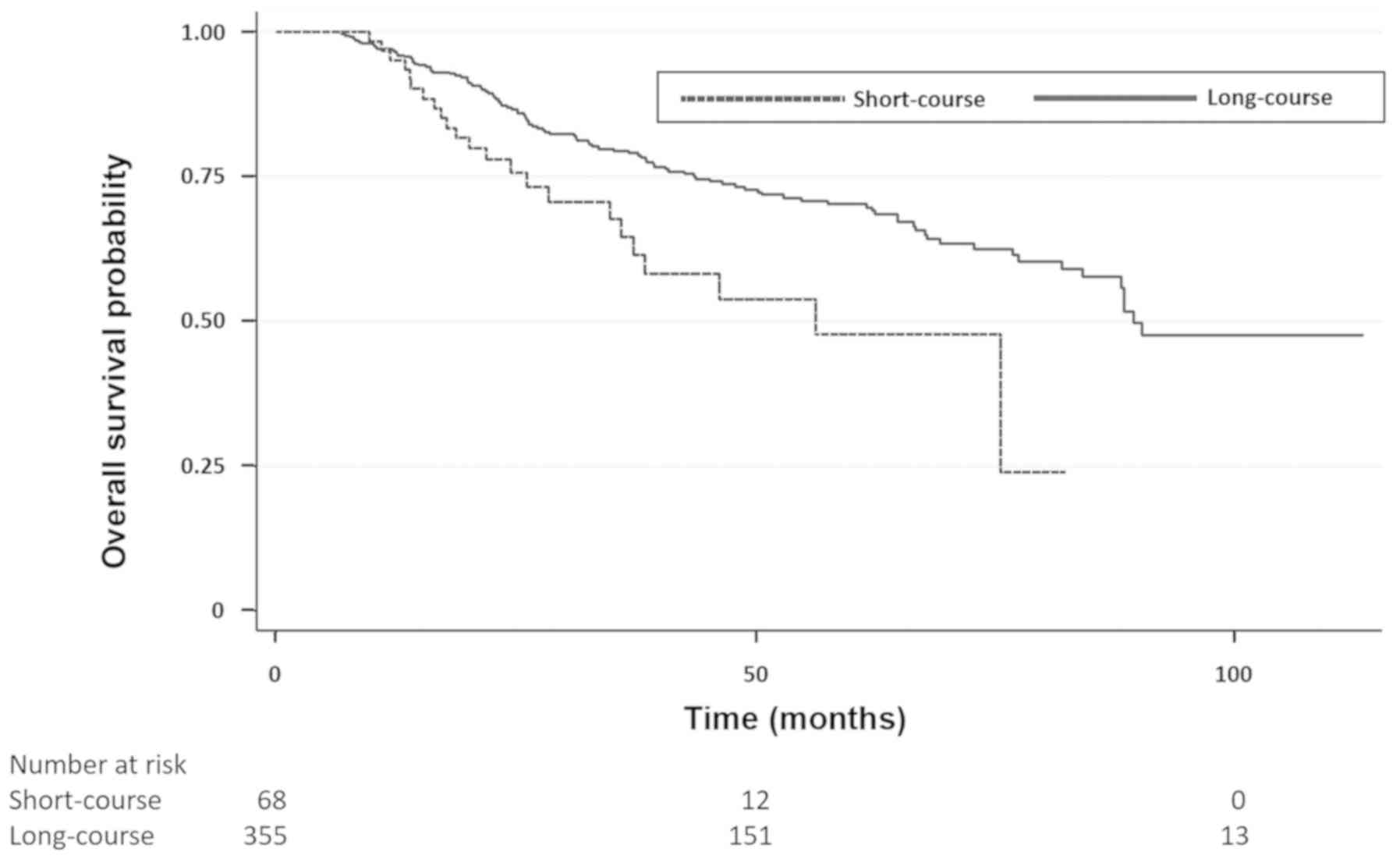
After excluding 35 patients that died within 90-days
of surgical resection, OS according to radiotherapy dose was
improved in the LC group compared with the SC group. The unadjusted
and adjusted HRs for OS were 0.52 (0.33–0.82, P=0.005) and 0.62
(0.39–0.99, P=0.05), respectively (Fig.
2). Median OS was 25.3 months (IQR 16.9–41.6) for the SC group
compared to 43.5 months (IQR 25.6–67.9) for the LC group. For
clinical stage II patients (n=241) the unadjusted and adjusted HRs
for OS were 0.51 (0.28–0.94, P=0.03) and 0.60 (0.33–1.10, P=0.1),
respectively. For clinical stage III patients (n=182) the
unadjusted and adjusted HRs for OS were 0.46 (0.23–0.95, P=0.04)
and 0.63 (0.30–1.34, P=0.23), respectively.
Discussion
In this study we demonstrated that LC radiotherapy
is associated with improved OS compared with SC radiotherapy in
patients with locally advanced rectal adenocarcinoma not receiving
systemic chemotherapy. In a further analysis we excluded early
mortality cases, attributed to patients' poor performance status
and comorbidities. Although these cases were more frequent in the
SC group, possibly due to older age, the association between LC
radiotherapy and improved OS was maintained, and the numerical
difference in median OS in favor of the LC group was approximately
18 months.
Preoperative LC chemoradiotherapy was compared with
SC radiotherapy in two previous phase III studies, a Polish study
and a Trans-Tasman Radiation Oncology Group study (11,12).
Both studies showed no difference in OS between the two groups when
comparing LC radiotherapy including concomitant chemotherapy of
daily infusional 5-fluorouracil to SC radiotherapy. The association
of improved OS with LC radiotherapy in our study may be explained
by two factors. First, in the Polish study, adjuvant chemotherapy
use was more frequent in the SC radiotherapy vs. the LC
chemoradiotherapy arm, 46.4 vs. 30.1%, respectively, biasing the
results in favor of the SC arm (11). Second, even in the presence of
similar rates of adjuvant chemotherapy, as in the Australian study
(12), adjuvant chemotherapy by
itself may mask the difference in favor of LC over SC radiotherapy.
Of note, the biological effective dose (BED) of LC radiotherapy is
significantly higher than that of SC radiotherapy (59.5 vs. 37.5 Gy
assuming alpha-beta of 10 and ignoring time factors), in keeping
with our findings demonstrating a superiority for LC over SC
radiotherapy. The current study suggests that in patients who
cannot tolerate systemic chemotherapy, LC should be preferred over
SC radiotherapy.
Strengths of our study include the large size of the
cohort used for analysis, the detailed available information for
clinical staging, the ability to clearly define patients who
received preoperative radiotherapy followed by surgery, and the
adjustments performed for the common confounders (i.e., age, sex,
race, comorbidities and clinical stage). All these have enabled the
direct comparison between LC and SC radiotherapy, focusing on the
effect of radiation dose and intensity on OS.
This study had several important limitations. First,
our dataset also lacks cancer recurrence and cancer-specific
survival data. However, NCDB includes OS data, which is a more
clinically relevant parameter. Second, other confounding factors
affecting OS cannot be ruled out. Third, since the time from
diagnosis to surgery was shorter in the SC compared with the LC
group, there was a small component of an immortal time bias.
However, this difference is negligible (approximately 14%) compared
with the difference in median overall survival. Fourth, details of
radiation technique are lacking. Fifth, NCDB lacks data regarding
toxicity and side effects in patients that received LC or SC
radiotherapy. Finally, the reasons for omitting
neoadjuvant/adjuvant chemotherapy in both LC and SC groups cannot
be fully elucidated, which may potentially bias overall survival.
However, we adjusted our analysis for the main factors affecting
treatment decision, i.e., age, comorbidities and clinical
stage.
In summary, our findings suggest that LC
radiotherapy is associated with an improved OS compared with SC
radiotherapy in locally advanced rectal adenocarcinoma. To the best
our knowledge, this is the first report in the literature
suggesting an advantage for LC radiotherapy. This possible
advantage is clinically relevant mainly in patients who cannot
tolerate adjuvant chemotherapy, and should therefore be taken into
account in the decision-making process in this setting.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to the University's
licensing agreement with NCDB which prohibits the distribution of
this data to any individual not explicitly listed in the originally
agreed proposal.
Authors' contributions
OM, YRL, YXY, ENB, KAR, TG, NH, DA, BG and ESS
contributed to the study design, data analysis and drafting the
manuscript. RM contributed to the acquisition of data, statistical
analysis and drafting the manuscript. BB contributed to the study
design, acquisition of data, statistical analysis and drafting the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board at the University of Pennsylvania (Philadelphia, PA,
USA).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflict of
interest.
References
|
1
|
Cammà C, Giunta M, Fiorica F, Pagliaro L,
Craxì A and Cottone M: Preoperative radiotherapy for resectable
rectal cancer: A meta-analysis. JAMA. 284:1008–1015. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colorectal Cancer Collaborative Group, :
Adjuvant radiotherapy for rectal cancer: A systematic overview of
8,507 patients from 22 randomised trials. Lancet. 358:1291–1304.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Birgisson H, Påhlman L, Gunnarsson U and
Glimelius B; Swedish Rectal Cancer Trial Group, : Adverse effects
of preoperative radiation therapy for rectal cancer: Long-term
follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol.
23:8697–8705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marijnen CA, Kapiteijn E, van de Velde CJ,
Martijn H, Steup WH, Wiggers T, Kranenbarg EK and Leer JW;
Cooperative Investigators of the Dutch Colorectal Cancer Group, :
Acute side effects and complications after short-term preoperative
radiotherapy combined with total mesorectal excision in primary
rectal cancer: Report of a multicenter randomized trial. J Clin
Oncol. 20:817–825. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sebag-Montefiore D, Stephens RJ, Steele R,
Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint
AS, et al: Preoperative radiotherapy versus selective postoperative
chemoradiotherapy in patients with rectal cancer (MRC CR07 and
NCIC-CTG C016): A multicentre, randomised trial. Lancet.
373:811–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park JH, Yoon SM, Yu CS, Kim JH, Kim TW
and Kim JC: Randomized phase 3 trial comparing preoperative and
postoperative chemoradiotherapy with capecitabine for locally
advanced rectal cancer. Cancer. 117:3703–3712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roh MS, Colangelo LH, O'Connell MJ,
Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L,
Ursiny CS, Petrelli NJ, et al: Preoperative multimodality therapy
improves disease-free survival in patients with carcinoma of the
rectum: NSABP R-03. J Clin Oncol. 27:5124–5130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sauer R, Liersch T, Merkel S, Fietkau R,
Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann
H, et al: Preoperative versus postoperative chemoradiotherapy for
locally advanced rectal cancer: Results of the German
CAO/ARO/AIO-94 randomized phase III trial after a median follow-up
of 11 years. J Clin Oncol. 30:1926–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC; EORTC Radiotherapy Group Trial 22921, : Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McCarthy K, Pearson K, Fulton R and Hewitt
J: Pre-operative chemoradiation for non-metastatic locally advanced
rectal cancer. Cochrane Database Syst Rev.
12:CD0083682012.PubMed/NCBI
|
|
11
|
Bujko K, Nowacki MP, Nasierowska-Guttmejer
A, Michalski W, Bebenek M and Kryj M: Long-term results of a
randomized trial comparing preoperative short-course radiotherapy
with preoperative conventionally fractionated chemoradiation for
rectal cancer. Br J Surg. 93:1215–1223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ngan SY, Burmeister B, Fisher RJ, Solomon
M, Goldstein D, Joseph D, Ackland SP, Schache D, McClure B,
McLachlan SA, et al: Randomized trial of short-course radiotherapy
versus long-course chemoradiation comparing rates of local
recurrence in patients with T3 rectal cancer: Trans-Tasman
Radiation Oncology Group trial 01.04. J Clin Oncol. 30:3827–3833.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erlandsson J, Holm T, Pettersson D,
Berglund Å, Cedermark B, Radu C, Johansson H, Machado M, Hjern F,
Hallböök O, et al: Optimal fractionation of preoperative
radiotherapy and timing to surgery for rectal cancer (Stockholm
III): A multicentre, randomised, non-blinded, phase 3,
non-inferiority trial. Lancet Oncol. 18:336–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Winchester DP, Stewart AK, Bura C and
Jones RS: The National Cancer Data Base: A clinical surveillance
and quality improvement tool. J Surg Oncol. 85:1–3. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deyo RA, Cherkin DC and Ciol MA: Adapting
a clinical comorbidity index for use with ICD-9-CM administrative
databases. J Clin Epidemiol. 45:613–619. 1992. View Article : Google Scholar : PubMed/NCBI
|