Introduction
5 to 40% of patients treated for differentiated
thyroid cancer (DTC) can be affected by persistent or recurrent
disease (1). Treatment with
radioiodine alone, in a significant number of cases, is able to
completely eliminate tumor recurrence. Nevertheless, up to 30% of
tumors will not show 131I uptake. In these cases, a
surgical management of the lesion is required (2). During revision surgery, a significant
number of intraoperative complications can occur, especially in the
central compartment of the neck. Alteration of the normal anatomy,
fibrosis and scar tissue formation in the neck makes identification
and preservation of the recurrent laryngeal nerves and parathyroid
glands difficult (3). Another aspect
to consider during revision surgery is the achievement of oncologic
radicality. Recurrent laryngeal nerves and parathyroids may be
encased in fibrotic tissue, making them indistinguishable from
pathologic tissue. There is a considerable high risk of leaving
residual neoplasm in the field (4).
In order to simplify localization and excision of
neoplastic foci during revision surgery for DTC recurrence, we have
employed a new technique of preoperative ultrasound-guided
tattooing (US-tattoo) for the detection of the structures to be
removed, likewise to the US-tattoo technique routinely applied in
patients affected by breast cancer for more than 15 years (5). To date this procedure is supported by
only four 4 studies reporting encouraging results (2,3,6,7). The
first 13 patients have been reported in our previous article where
the preliminary results have been reported in terms of success rate
and complications (7).
In this study, we report our overall experience with
the technique of fine-needle injection of charcoal pigment under US
guidance for localization, and consequent surgical excision of DTC
lymphatic metastasis in the neck.
Materials and methods
This was a retrospective analysis, performed in the
Head and Neck Department, Ear Nose and Throat (ENT) Unit of Santa
Maria delle Croci Hospital, Ravenna, Italy. All procedures
performed in this study involving human participants were in
accordance with the ethical standards of the institutional and/or
national research committee, and with the 1964 Helsinki declaration
and its later amendments or comparable ethical standards. The study
was designed and conducted in compliance with the principles of
Good Clincal Practice regulations and the Helsinki declaration.
Written informed consent was obtained from patients before
inclusion in this study.
From April 2008 to January 2016, a prospective study
was conducted on patients who previously underwent surgical
treatment for DTC, and thereafter presented to our centre with a
suspected DTC recurrence in the neck. The initial operation and
pTNM classification are shown in Table
I. Vocal folds motility by videofiberoptic endoscopic
assessment and parathyroid function was preoperatively verified.
Radioiodine treatment was administered after surgical treatment in
all patients population, except for one.
 | Table I.Patients and tumor
characteristics. |
Table I.
Patients and tumor
characteristics.
| Case no. | Sex | Age, years | 1st operation | Pathology | pTNM | 131I | REC | FNA and Tg | 2nd operation | Node found | COMPL. |
|---|
| 1 | F | 35 | Total
Thyroidectomy | Pap | pT2Nx | Y | 26 | Y | Lev III, IV, VI | Y 4+/5 Level VI 4/15
level III, IV | N |
| 2 | M | 39 | Total
Thyroidectomy | Pap | pT2Nx | Y | 23 | Y | Lev VI | Y 3+/5 Level VI |
Hypoparathyroidism |
| 3 | F | 70 | Total
Thyroidectomy | Pap | pT2Nx | Y | 28 | Y | Lev VI | Y 2+/8 Level VI | N |
| 4 | F | 31 | Total
Thyroidectomy | Pap | pT2Nx | Y | 48 | Y | Lev III, IV, V,
VI | Y 2+/8 Level VI 3/30
Level III–V | N |
| 5 | F | 26 | Total
Thyroidectomy | Pap | pT2Nx | Y | 13 | Y | Lev VI | Y 1+/6 Level VI | N |
| 6 | F | 25 | Total Thyroidectomy +
Lev VI | Pap | pT1N0 | N | 3 | NS | Single node | Y + parathyroid
cyst | N |
| 7 | F | 36 | Total Thyroidectomy
+ Lev VI | Pap | pT4N1a | Y | 18 | Y | Single node | Y | N |
| 8 | F | 42 | Total Thyroidectomy
+ Lev VI | Pap | pT4N1a | Y | 4 | Y | Single node Fatty
tissue | Y 1+/1 Level
VI | N |
| 9 | F | 49 | Total Thyroidectomy
+ Lev VI | Pap | pT1N0 | Y | 22 | Y | Lev III, IV | Y 1+/3 Level VI
2/23 Level III, IV | N |
| 10 | F | 35 | Total Thyroidectomy
+ Lev VI | Pap | pT3N1a | Y | 14 | Y | Lev II, III,
IV | Y | N |
| 11 | F | 42 | Total Thyroidectomy
+ Lev VI | Pap | pT3N1a | Y | 8 | Y | Lev II, III,
IV | Y (2nd rev. Surg.)
2/14 Level III, V | N |
| 12 | M | 65 | Total Thyroidectomy
+ Lev VI | Pap | pT3N1a | Y | 15 | NS | Single node | Y | N |
| 13 | F | 46 | Total Thyroidectomy
+ Lev III+ IV + VI | Pap | pT4N1b | Y | 12 | Y | Single node + fatty
tissue | Y 1+/3 Level
VI | Nerve paresis |
| 14 | F | 59 | Total + right Lev.
II–V + VI | Pap | pT3N1b | Y | 10 | Y | Single node +
fibrous tissue | Y | Nerve paresis |
| 15 | F | 69 | Total + left Lev.
III–IV + VI | Pap | pT1N1a | Y | 35 | Y | Single node +
fibrous tissue | Y | N |
| 16 | F | 56 | Total
Thyroidectomy | Pap | pT1Nx | Y | 52 | NS | Fatty tissue with 2
nodes | Y | Nerve paresis |
| 17 | F | 38 | Total + VI | Pap | pT3N1a | Y | 9 | Y | Group of 3 nodes,
Lev. II–IV bilateral | Y 1+/3 L. VI (7+/18
right) (4+/14 left) | N |
| 18 | M | 36 | Total + right Lev.
II–IV + VI | Pap | pT3N1b | Y | 16 | Y | Group of 3
nodes | N | N |
| 19 | F | 73 | Total + VI | Pap | pT3N1a | Y | 4 | Y | Single node, fatty
tissue | Y 1+/3 left VI | N |
| 20 | F | 41 | Total + VI | Pap | pT1N0 | Y | 84 | NS | Group of 3 nodes
and fatty tissue | N | Hypo-PTH |
| 21 | M | 47 | Total + right and
left Lev. II–V + VI | Pap | pT3N1b | Y | 16 | Y | Single node +
fibrous tissue | Y | N |
| 22 | F | 41 | Total + left Lev.
II–V + VI | Pap | pT3N1b | Y | 8 | Y | Lev. II–IV Dx (node
marked lev. IV dorsal to jugular vein) + Lev. VI | Y 4+/24 L. IV–V-m.
node (2+/27 left II) (3+/24 left III) (5+/9 left VI) | N |
| 23 | F | 60 | Total + VI | Pap | pT3N0 | Y | 9 | Y | Lev. II–V (node
marked between lev. V and VI) | Y (1+/30 left) | N |
| 24 | M | 35 | Total + VI | Pap | pT3N1a | Y | 16 | Y | Lev. II–V Sin (node
marked lev. IV) | Y (1+/25 left) | N |
| 25 | F | 58 | Total + VI | Pap | pT1N0 | Y | 8 | Y | Lev. III, IV
Dx | Y (1+/8 left) | N |
| 26 | M | 39 | Total + left Lev.
III–V + VI | Pap | pT3N1a | Y | 6 | Y 1+/11 left V
(3+/14 left II) | Lev. II, rev.
V | Y | N |
| 27 | M | 56 | Total + VI | Pap | pT3N1a | Y | 14 | Y | Lev. II–V (node
marked lev. III) | Y 1+/10 L. III-m.
node (1+/29 left II) (0/12 left IV) (2+/9 left V) | N N |
Recurrence or suspicious lesions discovered during
follow-up, by means of US and TSH-stimulated Tg determination were
investigated by cytology (FNA), in association with Tg
determination in the washout of fine needle aspiration (FNAB-Tg),
following the TI-RADS criteria (8).
Recurrent lesions have been detected both in central and lateral
compartment of the neck. In order to perform a super-selective neck
dissection around the metastatic node, suspicious metastatic
lymph-nodes in the lateral groups are also marked (2,3,6). Informed consent was obtained from all
patients.
A suspension of active charcoal 80 mg/2 ml (4%),
commonly used for tattooing breast cancer lesions, was used
(composition: active charcoal 80 mg + Polysorbate-80 80 mg + water
for injectable preparations to reach 2 ml). Before aspiration and
injection, the charcoal vial is heated by keeping it between the
operator hands for a few minutes. This is done to prevent blockage
of the needle tip by charcoal particles. The lesion is identified
under US guidance; US imaging is performed using a machine with
multi-frequency probe (7–12 MHz Esaote MyLab 70).
After identification, a 23-gauge needle is inserted
near the suspected lesion, since we prefer to avoid injecting
inside the lesion so as not to compromise histological examination.
At this point we can proceed with injection of 0.5–2 ml of charcoal
by means of a 5 ml syringe. The amount to be injected depends on
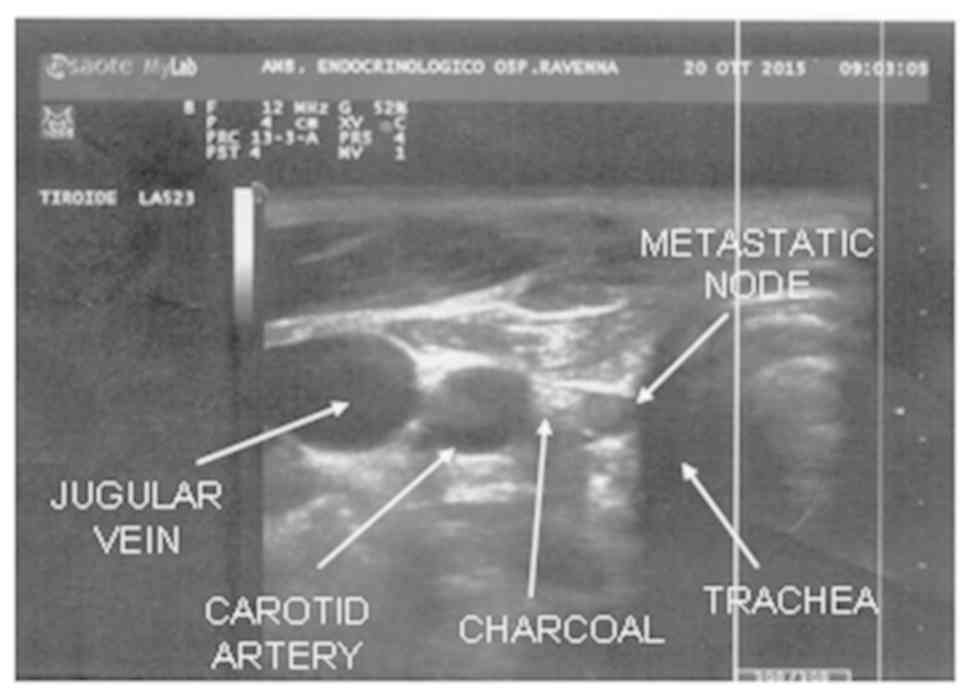
the depth of the lesion. In proximity of the suspected neoplasm, a
store of colouring which we define as charcoal ‘puddle’ takes form
(Fig. 1). Extraction of the needle
is accompanied by an injection at constant pressure of the charcoal
in order to leave a trace of colouring along the way of the needle
till the skin, where a tattoo resembling a little nevus will
persist for some weeks. This last step provided a valid indication
about the direction to follow during surgical operation, in order
to reach the suspected metastatic lesion.
This procedure can be performed from just a few days
to some weeks before surgery, as it has been demonstrated that
charcoal remains in place for at least 3 months after injection
(9,10). Charcoal injection is performed in the
Endocrinology unit as an ambulatory setting. The patient can return
home after at least 20 min of observation. The patient is advised
to rest, avoid in particular bending the neck and the head, and
raising heavy loads after the procedure. In case of soreness or
pain in the area of needle insertion, the use of a common analgesic
is recommended. Only a descriptive analysis has been performed.
Results
A total number of 27 patients (20 females and 7
males) with an average age of 46 years (range, 25–73 years)
presented to our centre (1 of them was initially treated in another
hospital) with a suspected DTC recurrence in the neck (Table I). The average time of recurrence was
19 months (range, 3–84 months). Preoperative injection was well
tolerated in all cases with patients complaining of the same mild
discomfort of a FNA, experienced previously by all of them. No
complications related to the procedure of US-tattooing were
observed.
In the last 93 months (April 2008-January 2016) we
have re-operated 27 patients with suspected metastatic lesion of
DTC using the technique of US-tattoo localization. The primary
cancer was always papillary carcinoma (with Warthin like aspects in
1 case, no. 22). Immediately prior to surgery in the operating
room, we perform another US examination to have a better conception
of the metastatic lesion and to define its anatomical relation. All
the operations were performed by the same expert surgeon (FS and
ADV). To recognize neural structures and assess their functionality
we made use during surgery of Bovie Neuro-Pulse™ surgical nerve
locator.
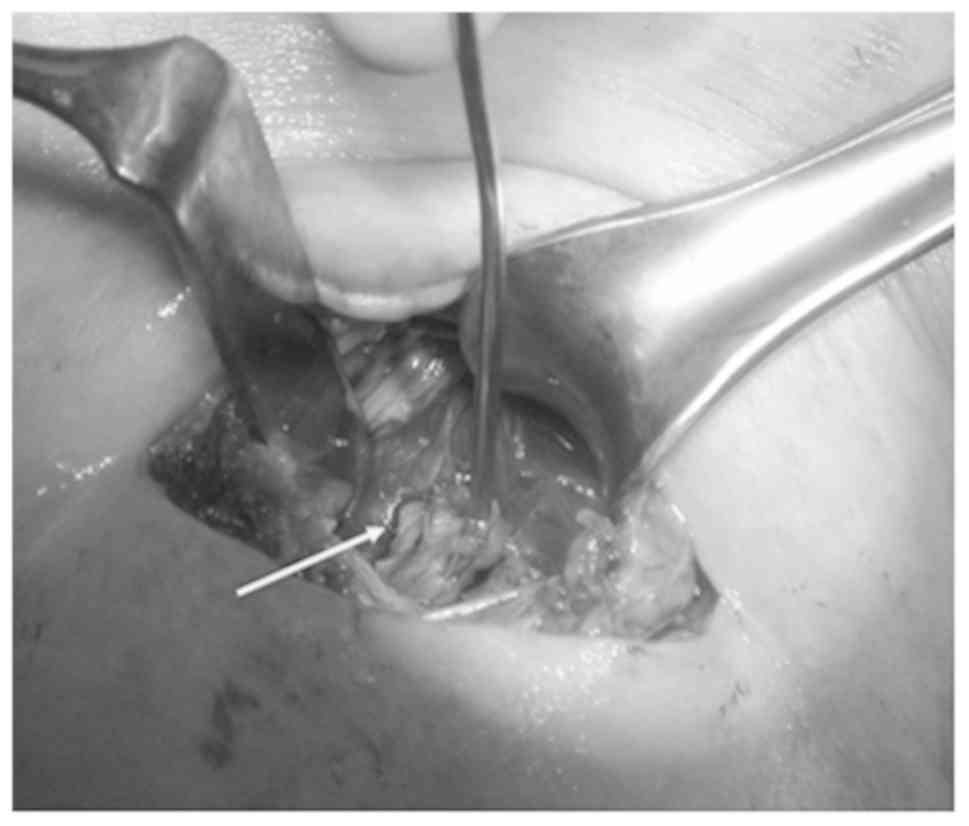
For the 16 cases the target was a suspected level VI
node (nos. 2, 3, 5–8, 12–16, 18–21) (Table I; Fig.
2).
The first operation was a total thyroidectomy in 4
cases, thyroidectomy with dissection of level VI in 7 cases, and
thyroidectomy with dissection of level VI in associated with
selective neck dissection in 5 cases (Table I). The re-operation for these
patients consisted of level VI dissection (comprehensive of the
marked node) in 3 cases, revision of level VI dissection in 13
cases; number of nodes removed in central or lateral compartment is
reported in Table I. For patients
firstly not undergone central compartment lymphadenectomy, the use
of charcoal tattoo is of great importance to assure inclusion of
the suspected node in the dissection.
In patients already submitted to central compartment
dissection, the operation consisted of removal of the
charcoal-localized node possibly associated with fatty or
connective tissue (suspected of containing other lymphatic nodes)
surrounding the targeted lesion. In four patients, cytology was not
suggestive of a definitive diagnosis. However, on the basis of US
aspects and TG determination, we proceeded to perform revision
surgery and metastatic lesion was found in 2 cases (nos. 12 and
16), in the other one a metastatic lesion was found associated with
parathyroid cyst (no. 6) and finally the suspected lesion, which is
of small dimension (3 × 5 mm), were not confirmed at histological
examination, even if a group of 3 nodes were removed (no. 20).
The last was the unique case in our experience we
did not observe any trace of charcoal in proximity of the suspected
lesion during the surgical operation. We found colouring traces
just in the first layer of dissection (skin, subcutaneous and scar
tissue), which is of poor usefulness for our surgical aim. Similar
to what occurred in case no. 20, in patient no. 18 the metastatic
node was not found despite the removal of three nodes around the
coal deposit (Table I). For these
patients, considering the small size of the lesion together with
objective surgical difficulties, we decided for a further
radioiodine treatment followed by close controls and reoperation in
case of volumetric increase of the lesion.
After revision surgery in the central compartment,
we observed minor complications in 5 cases: 2 patients suffered
temporary hypoparathyroidism (solved with medical therapy) and 3
experienced temporary recurrent nerve paralysis. In a subset of 9
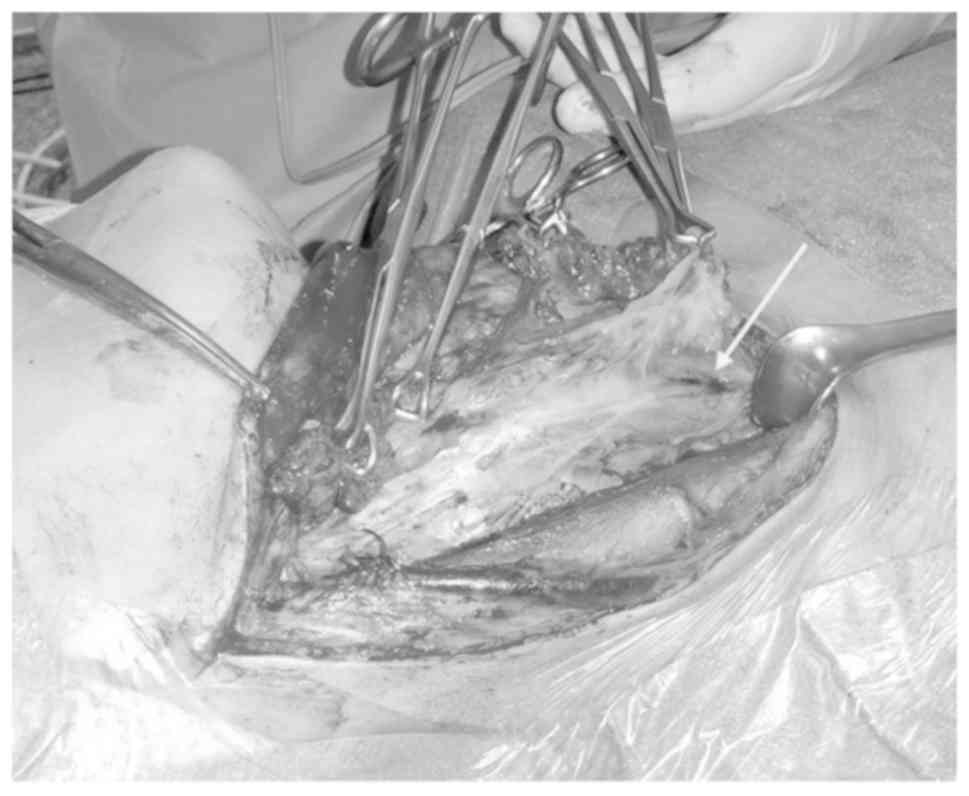
patients (patients nos. 1, 9–11 and 23–27) we marked exclusively
lesions in lateral compartment of the neck. In one case (no. 26) 2
distinct marks were performed and in 1 of these, in the level II
area, we observed a widespread diffusion of charcoal. In all other
cases we could follow the trace created during extraction of the
needle, and found the charcoal injected quite localized on the
suspected lesion and therefore useful for surgical manoeuvres
(Fig. 3).
The histological examination has given a favourable
outcome, confirming the removal of metastatic lesion in all cases.
No complications related to lateral neck dissections were
registered.
In case no. 4 and no. 22, we performed a double
marking of a lesion localized in VI area and another respectively
in level III and IV (dorsal to jugular vein). Removal of metastatic
lesion was obtained in both cases with no registration of surgical
complications. Considering overall results, metastatic lesions were
found in 25 out of 27 patients (92%) (Table I). Follow up with US and Tg
determination of at least 6 months confirmed the positive outcome
of surgery.
Discussion
DTC is the most common endocrine tumour. Despite the
increase in the number of cases in recent years, its course is
favourable with a survival rate of 10 years till 90%, and its
incidence in all deaths for neoplastic diseases is at only 0,5%
(11). Lymphatic metastasis of DTC
has been considered, until a short time ago of negligible
importance for the prognosis. Various studies have reported
percentages of survival rates of 10 years till 95% for papillary
carcinoma, and till 70% for the follicular variant (12–16).
Recently, however, major attention is placed on the concept of
‘disease free survival’ considering, in spite of high values of
survival, the not negligible incidences of disease recurrence
(15–35% also to 20 years), with a meaningful impact on the quality
of life (due to the necessity of surgical reoperations and/or of
elevated doses of radioiodine) (17,18).
The incidence of DTC recurrence after thyroidectomy
has increased with the routine employment of ultrasound (US) and
thyroglobulin (Tg). Revision surgery in the neck for
recurrent/persistent DTC is associated with increased morbidity
compared with primary surgery because of the presence of scar
tissue and disruption of the normal fascial planes and anatomy.
This may result in a greater risk of injury to nerves and other
important anatomical structures (19). These concepts are valid especially
for area VI surgical revision. In spite of every therapeutic
attitude regarding the N0 necks in DTC, re-operations in the
central compartment may be necessary, even when it has been
performed during the first operation. In fact, radicality is very
difficult to obtain, especially for nodes situated on the superior
edge of mediastinal space. Risk of damaging laryngeal nerves can
reach 20% (ranging from 0.7 to 4.5% during first operation) and
risk of hypoparathyroidism can increase to 30% (ranging from 8 to
13% in primary procedures) (20,21).
Even though our study consists of a small number of
treated patients, we observed a transitory hypoparathyroidism in 2
cases (11% if we consider only 18 cases of area 6 revision) and a
transitory vocal cord paresis in 3 (16% referring exclusively to
area 6 revision). No damages of important anatomical structures
were registered during lateral neck dissection. Greater morbidity
during central compartment revision is related to difficulties in
identification and preservation of recurrent laryngeal nerves and
parathyroid glands. To prevent recurrent nerve injuries, it is
possible to make use of intraoperative neurological monitoring
and/or rely on some surgical tricks such as identification of each
nerve low in the tracheoesophageal groove, distant from the thyroid
bed and the use of meticulous surgical dissection from inferior to
superior (4). In order to preserve
the function of the parathyroid glands, devascularisation should be
prevented. The inferior thyroid artery should be respected
(4).
The gold standard for revision surgery should be a
reliable procedure to guide revision surgery. To date the most
common intraoperative procedure is the use of a gamma probe.
Efficacy of this technique however can be limited by false negative
and false positive findings. It is only useful in cases of
radioiodine-avid foci (22–24). Other modalities such as
intraoperative US exploration hook needle insertion or tattooing
using blue dye have also been described. However, their efficacy,
safety and feasibility are not well demonstrated (25–27).
Other radio guided methods as well as other radiotracers such us
FDG were recently developed for the intraoperative guidance of
non-radioiodine avid cancer, but cost, availability and
signal-to-noise issues may limit their widespread use (28–30).
Despite all these procedures, revision surgery of
central compartment dissection remains difficult even in expert
hands. Given this context, the US-tattoo localization technique can
represent an instrument of considerable usefulness (2,3,6,7).
Active charcoal for its physical and pharmacological
properties, which ensure limited diffusion and good stability, is
the ideal substance for marking of target in soft tissues. In our
experience this technique has been of significant utility in
identifying metastatic lesions during revision surgery in a great
majority of cases (93%). In a total of 30 procedures, we registered
only in 1 case an unexplained absence of coal and in a further one,
an excessive widespread in the surgical field. Furthermore, this
technique, executable even several week before surgery, is easy to
implement and at a very low cost. Finally, another aspect of great
importance is the good tolerability and the complete absence of
complications related to the procedure.
In conclusion, based on our experience we can
suggest that US-tattoo localization of lymphatic neck metastasis of
DTC is a safe technique, of low cost and extremely useful in
facilitating surgical procedures, especially in difficult revision
surgery of the neck. This technique allows risk reduction of
iatrogenic complications, especially of the parathyroid glands and
recurrent laryngeal nerves during area VI revision surgery.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FS and ADV conceived and designed the study,
acquired, analysed and interpreted the data, revised the
manuscript, gave final approval of the version to be published and
agreed to be accountable for all aspects of the work. FB, GM, SSR,
CC, FR, CV and MP conceived and designed the study, drafted the
manuscript, analysed the data, gave final approval of the version
to be published and agreed to be accountable for all aspects of the
work
Ethics approval and consent to
participate
Due to the retrospective nature of the present
study, the requirement for ethical approval was waived. Written
informed consent was obtained from all individual participants
included in the study.
Patient consent for publication
Written informed consent was obtained from all
individual participants included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schlumberger MJ: Papillary and follicular
thyroid carcinoma. N Engl J Med. 338:297–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartl DM, Chami L, Al Ghuzlan A,
Leboulleux S, Baudin E, Schlumberger M and Travagli JP: Charcoal
suspension tattoo localization for differentiated thyroid cancer
recurrence. Ann Surg Oncol. 16:2602–2608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang TW, Shin JH, Han BK, Ko EY, Kang SS,
Hahn SY, Kim JS and Oh YL: Preoperative ultrasound-guided tattooing
localization of recurrences after thyroidectomy: Safety and
effectiveness. Ann Surg Oncol. 16:1655–1659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim MK, Mandel SH, Baloch Z, Livolsi VA,
Langer JE, Didonato L, Fish S and Weber RS: Morbidity following
central compartment reoperation for recurrent or persistent thyroid
cancer. Arch Otolaryngol Head Neck Surg. 130:1214–1216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathieu MC, Bonhomme-Faivre L, Rouzier R,
Seiller M, Barreau-Pouhaer L and Travagli JP: Tattooing breast
cancers treated with neoadjuvant chemotherapy. Ann Surg Oncol.
14:2233–2238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chami L, Hartl D, Leboulleux S, Baudin E,
Lumbroso J, Schlumberger M and Travagli JP: Preoperative
localization of neck recurrences from thyroid cancer: Charcoal
tattooing under ultrasound guidance. Thyroid. 25:341–346. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soprani F, Bondi F, Puccetti M and
Armaroli V: Charcoal tattoo localization for differentiated thyroid
cancer recurrence in the central compartment of the neck. Acta
Otorhinolaryngol Ital. 32:87–92. 2012.PubMed/NCBI
|
|
8
|
Wang Y, Lei K-R, He Y-P, Li XL, Ren WW,
Zhao CK, Bo XW, Wang D, Sun CY and Xu HX: Malignancy risk
stratification of thyroid nodules: Comparisons of four ultrasound
Thyroid Imaging Reporting and Data Systems in surgically resected
nodules. Sci Rep. 7:115602017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonhomme-Faivre L, Depraetere P, Savelli
MP, Amdidouche D, Bizi E, Seiller M and Orbach-Arbouys S: Charcoal
suspension for tumor labelling modifies macrophage activity in
mice. Life Sci. 66:817–827. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biffoni M, Scipioni P and Macrina N:
Surgical treatment of differentiated thyroid cancer recurrence.
L'Endocrinologo. 10:143–148. 2009. View Article : Google Scholar
|
|
11
|
Moley JF and Wells SA:
Compartment-mediated dissection for papillary thyroid cancer.
Langenbecks Arch Surg. 384:9–15. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lundgren CI, Hall P, Dickman PW and
Zedenius J: Clinically significant prognostic factors for
differentiated thyroid carcinoma: A population-based, nested
case-control study. Cancer. 106:524–531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaha A: Treatment of thyroid cancer based
on risk groups. J Surg Oncol. 94:683–691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kupferman ME, Patterson M, Mandel SJ,
LiVolsi V and Weber RS: Patterns of lateral neck metastasis in
papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg.
130:857–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hassanain M and Wexler M: Conservative
management of well-differentiated thyroid cancer. Can J Surg.
53:109–118. 2010.PubMed/NCBI
|
|
16
|
Shaha AR, Shah JP and Loree TR: Patterns
of nodal and distant metastasis based on histologic varieties in
differentiated carcinoma of the thyroid. Am J Surg. 172:692–694.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hay ID, Bergstralh EJ, Grant CS, McIver B,
Thompson GB, van Heerden JA and Goellner JR: Impact of primary
surgery on outcome in 300 patients with pathologic
tumor-node-metastasis stage III papillary thyroid carcinoma treated
at one institution from 1940 through 1989. Surgery. 126:1173–1181;
discussion 1181–1182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pai SI and Tufano RP: Reoperation for
recurrent/persistent well-differentiated thyroid cancer.
Otolaryngol Clin North Am. 43353–363. (ix)2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Betka J, Mrzena L, Astl J, Nemec J, Vlcek
P, Taudy M and Skrivan J: Surgical treatment strategy for thyroid
gland carcinoma nodal metastases. Eur Arch Otorhinolaryngol. 254
(Suppl 1):S169–S174. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scheumann GF, Seeliger H, Musholt TJ, Gimm
O, Wegener G, Dralle H, Hundeshagen H and Pichlmayr R: Completion
thyroidectomy in 131 patients with differentiated thyroid
carcinoma. Eur J Surg. 162:677–684. 1996.PubMed/NCBI
|
|
21
|
Travagli JP, Cailleux AF, Ricard M, Baudin
E, Caillou B, Parmentier C and Schlumberger M: Combination of
radioiodine (131I) and probe-guided surgery for persistent or
recurrent thyroid carcinoma. J Clin Endocrinol Metab. 83:2675–2680.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rubello D, Salvatori M, Ardito G, Mariani
G, Al-Nahhas A, Gross MD, Muzzio PC and Pelizzo MR: Iodine-131
radio-guided surgery in differentiated thyroid cancer: Outcome on
31 patients and review of the literature. Biomed Pharmacother.
61:477–481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salvatori M, Ardito G, Pelizzo MR, Mariani
G, Gross M, Al-Nahhas A and Rubello D: Treatment of local and
regional recurrences of differentiated thyroid cancer by
radio-guided surgery with iodine-131. Nucl Med Rev Cent East Eur.
9:119–124. 2006.PubMed/NCBI
|
|
24
|
Lucchini R, Puxeddu E, Calzolari F,
Burzelli F, Monacelli M, D'Ajello F, Macaluso R, Giammartino C,
Ragusa M, De Feo P, et al: Recurrences of thyroid well
differentiated cancer: Ultrasonography-guided surgical treatment.
Minerva Chir. 63:257–260. 2008.PubMed/NCBI
|
|
25
|
Duprez R, Lebas P, Marc OS, Mongeois E,
Emy P and Michenet P: Preoperative US-guided hook-needle insertion
in recurrent lymph nodes of papillary thyroid cancer: A help for
the surgeon. Eur J Radiol. 73:40–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sippel RS, Elaraj DM, Poder L, Duh QY,
Kebebew E and Clark OH: Localization of recurrent thyroid cancer
using intraoperative ultrasound-guided dye injection. World J Surg.
33:434–439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gulec SA, Eckert M and Woltering EA: Gamma
probe-guided lymph node dissection (‘gamma picking’) in
differentiated thyroid carcinoma. Clin Nucl Med. 27:859–861. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grant CS, Thompson GB, Farley DR, Richards
ML, Mullan BP and Hay ID: The value of positron emission tomography
in the surgical management of recurrent papillary thyroid
carcinoma. World J Surg. 32:708–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borsò E, Grosso M, Boni G, Manca G,
Bianchi P, Puccini M, Arganini M, Cabria M, Piccardo A, Arlandini
A, et al: Radioguided occult lesion localization of cervical
recurrences from differentiated thyroid cancer: Technical
feasibility and clinical results. Q J Nucl Med Mol Imaging.
57:401–411. 2013.PubMed/NCBI
|
|
30
|
Molina MA, Goodwin WJ, Moffat FL, Serafini
AN, Sfakianakis GN and Avisar E: Intra-operative use of PET probe
for localization of FDG avid lesions. Cancer Imaging. 9:59–62.
2009.PubMed/NCBI
|