Introduction
Hodgkin's lymphoma (HL) is a clonal B-lymphocyte
neoplasm commonly affecting younger patients. HL has characteristic
pathological stages, highlighting the paramount importance of a
timely and effective clinical management. Epidemiological studies
on HL from Saudi Arabia are scarce (1). Recent cancer researchers have yet to
identify tumor-specific biomarkers with inter-individual variations
(i.e., polymorphisms). It has been hypothesized that genetic
signatures may enable genetic prediction of cancer prognosis.
Obviously, this successful prediction will determine the diagnostic
and prognostic value of the association between the biomarker and
the clinical outcome (2). HL is the
ninth most common malignancy in the Gulf Cooperation Council (GCC)
States, and its incidence has continuously increased over the past
decade among GCC nationals (3). The
2013 Saudi Cancer Registry report revealed that the median age of
HL at diagnosis was 25 years (4). In
most Western countries, HL has two peaks of incidence, at the age
range of 10–35 years old and at a later age >60 years old.
However, in the Middle East and Asia, HL is more common during
childhood (5). Based on a study by
Al-Diab et al (6) on a Saudi
and Middle Eastern population, it is hypothesized that the rapid
improvement in the living standards and healthcare systems over the
past two decades has affected the clinical behavior of HL.
The diagnosis of HL is confirmed by the presence of
Hodgkin and Reed-Sternberg cells (HRS) (7,8). The
Revised European-American Lymphoma (REAL) classification, which was
later adopted by the World Health Organization (WHO) scheme,
concluded that HL comprises two well-defined entities: Nodular
lymphocyte-predominant (NLP) HL and the more common variant,
classical HL (CHL), which was sub-classified into four subtypes:
Nodular sclerosis (NS), mixed-cellularity (MC), lymphocyte-depleted
(LD), and lymphocyte-rich (LR) (7,9). The
diagnosis of HL should be followed by staging, which is achieved
using the Ann Arbor staging scheme or its Cotswold modification;
the latter classifies HL as low-grade (stages I and II) and
high-grade (stages III and IV) (10). To date, the most widely accepted risk
stratification scoring system for high-grade HL is the
International Prognostic Score (IPS), with a higher score
predicting a worse outcome (11–13).
Another prognostic factor for early-stage HL is the erythrocyte
sedimentation rate (ESR); an increased ESR is predictive of early
relapse and poor prognosis (14).
The diagnosis of HL relies on morphological
examination and immunophenotyping of the HRS cells. This may be
achieved by immunohistochemical (IHC) staining of formalin-fixed
paraffin-embedded (FFPE) sections, or the multi-parametric flow
cytometry technique for fresh tissue specimens. These reliable
assays display the same accuracy, and can directly detect HRS
lymphoma cells and evaluate the background cells as an adjunct
diagnostic test (15). Flow
cytometry enables sensitive detection of antigens for which
antibodies may not be available in IHC staining. In addition to the
ease in defining a distinct cell population based on their size,
granularity and presence of specific tumor cell markers, it
excludes dead cells. Similarly, the FFPE tissue specimens are not
suitable for flow cytometric analysis, as it requires viable,
unfixed cells (8,16).
In most HL cases, HRS cells constitute the minority
of the cellular milieu, while the vast majority of the tumor
microenvironment (TME) cells are immune non-neoplastic cellular
components (9,17). There are four TME patterns observed
that form the basis of the different CHL subtypes (17). Furthermore, TME cells are outnumbered
by tumor-associated macrophages (TAMs), which participate in the
recruitment of more macrophages and other immune cells (18). TAMs promote peritumoral inflammation
and avoid immune destruction, as they exhibit TAM-specific
tumor-sustaining properties (19).
Macrophages were found to support tumor growth and suppress the
immune response that targets tumor cells (20). Increased numbers of TAMs have been
reported to confer a poor prognosis, inferior outcomes and
shortened survival in HL (6). Ree
and Kadin reported that the presence of large numbers of TAMs is
correlated with a high incidence of clinical-laboratory relapse
within 2 years of therapy (21).
Increased numbers of TAMs in the TME should be taken into
consideration to improve prognostic prediction standards and to
plan appropriate therapeutic strategies (22). However, although advances in therapy
may achieve a disease-free survival, this is accompanied by
increased risk of secondary cancers, most commonly in HL patients
with a family history of cancer (23). The currently available therapeutic
protocol is unsuccessful in 20–30% of HL cases, as the current
predictive systems cannot accurately identify these high-risk
patients (24,25). Therefore, minimizing the adverse
effect of TME cells on treatment success and reducing tumor
progression may be achieved via therapeutic inhibition of TAMs.
Therapeutic targeting of these chemo-protective TAMs may improve
the efficiency of standard treatments (17,19,26,27).
The gene signature of HL is routinely validated in
FFPE tissues using IHC. CD163 antigen expression by TAMs was
evaluated by computer image analysis or quantified manually by
pathologists. Scoring was performed by estimating the relative
percentage of CD163-positive cells in relation to overall
cellularity (18). The
overexpression of the CD163 antigen is an important pre-treatment
measure and may be used to identify patients with an unfavorable
prognosis or at high risk for relapse. On the other hand, patients
with a favorable prognosis should not be over-treated (28). This gene signature of TAMs was
validated in several cohort studies, which have demonstrated that
increased CD163 expression on IHC was directly associated with
treatment failure and a poor event-free survival. The adverse
prognostic significance of CD163 has been confirmed to be a
predictive biomarker for HL patients (18,20,24,29).
Yoon et al (30) concluded
that a high CD163 immunostaining intensity is particularly
associated with a decreased rate of complete remission (CR) and is
considered as a specific reliable prognostic indicator in HL. By
contrast, Azambuja et al (31), did not observe such an association in
their study population. The CD163 gene is a scavenger receptor
cysteine-rich (SRCR) protein-coding gene; it is a restricted
monocyte and tissue macrophage marker, as demonstrated by IHC
(32,33). CD163 is considered to be a better TAM
marker compared with CD68 in HRS cell-rich areas, with less
non-specific staining of the background (34). A single-nucleotide polymorphism (SNP)
in the CD163 gene may modify the CD163 protein phenotype,
particularly if these SNPs are located within the regulatory region
that can alter the protein expression and, thus, the functional
properties of the CD163 protein (35).
Materials and methods
Sample collection
CHL comprises the majority of HL cases. A review of
our records (between 1990 and 2015) at John Hopkins Aramco
Healthcare Center (JHAH) in Saudi Arabia revealed that CHLs
represented >85% of HL cases. Although HL may be curable, a
marked percentage of HL cases (20–30%) fail to respond to therapy.
Early identification of those cases has become an important
objective for clinical research. The aim of this retrospective
case-control study was to investigate the significance of the CD163
antigen expression and the presence of the related SNPs on the
prognosis of CHL in Saudi patients. A total of 100 formalin-fixed
paraffin-embedded (FFPE) tissue samples of patients previously
diagnosed with CHL were collected. Patients were selected from all
histological CHL subclasses and stages. A total of 20 FFPE normal
lymphoid samples (mainly tonsils) that had been collected within
the same period from subjects with no known personal or family
history of cancer were used as negative controls for the IHC
staining evaluation. In addition, 100 EDTA peripheral blood samples
were collected from healthy blood donors to be used as a study
control group for the molecular genotyping assay. The study
protocol received ethical approval from the Institutional Review
Board at John Hopkins Aramco Healthcare Center.
IHC staining and evaluation
All the FFPE tissue blocks were re-cut into two
representative sections that were mounted on slides and designated
as CD30 and CD163. In addition, a normal lymph node tissue section
was included as a quality control in each slide. All tissues were
processed in the fully automated staining instrument Ventana
Benchmark Ultra (Ventana Medical Systems, Inc., Tucson, AZ, USA)
for antigen retrieval and immune staining. IHC included monoclonal
antibody CD30 (clone BerH2, cat. no. 790–2926, Roche Diagnostics,
Basel, Switzerland) and CD163 (clone 10D6, product code:
NCL-L-CD163, Leica Biosystems Ltd., Newcastle, UK; dilution 1:20),
using a multimeric UltraView Universal DAB detection kit and
Ventana iView™ DAB Detection kit (Ventana Medical Systems,
Inc.).
IHC staining was evaluated by a pathologist to
select the areas that contained CD30-positive HRS cells; these were
analyzed for CD163 expression on the surrounding TAMs. Sections
lacking CD30-positive cells, areas with fibrosis, blood vessels,
reactive lymph nodes, necrosis and artifacts were excluded from the
analysis field to minimize areas of non-specific background
staining. The tissue sections were examined manually using a Nikon
ECLIPSE E600 microscope (Nikon Corporation, Tokyo, Japan) under ×40
lenses, equivalent to 1 high-power field of 0.5 mm in diameter and
an area of 0.196 mm2. The number of CD30-positive cells
in at least 10 fields was counted and a mean was calculated. For
CD163-stained slides, the number of CD163-positive
monocytes/macrophages surrounding a central cluster of
CD30-positive cells was counted in 10 fields, then the mean was
also calculated. We herein intended to introduce a new methodology
for calculating a ‘prognosis index’ of CD163, which was calculated
by dividing the mean number of CD163-positive TAMs by the mean
number of CD30-positive cells. Although CD30 IHC may stain
activated B lymphocytes in addition to HRS cells, it is used as a
baseline to differentiate reactive B lymphocytes from malignant HRS
cells. CD30-positive reactive B lymphocytes were counted in the
normal lymph node tissues (i.e., negative controls), selecting the
inter-follicular rather than the intrafollicular areas or
sinusoidal spaces, and selecting the scattered non-clustered cells
in that area. CD30-positive cells were used at denominator for the
calculated CD163 index to eliminate errors and yield a more
specific absolute number reflecting a more realistic index value.
The equation used was as follows:
CD163 prognosis index=Mean number of CD163-positive
TAMs/mean number of CD30-positive cells.
Genotyping by polymerase chain
reaction (PCR) analysis
All FFPE sections of the CHL and control groups were
assayed for molecular PCR genotyping. Two normal tonsillar fresh
tissue specimens were also obtained to serve as PCR negative
controls. In addition, two fresh lymph node tissue samples from
newly diagnosed CHL patients served as PCR positive controls. Total
genomic DNA was extracted from patients' FFPE and control group
tissues, in addition to EDTA peripheral blood samples of the study
control group, using QIAamp DNA FFPE Tissue (250) kit (cat. no.
56404, Qiagen Inc., USA) and Qiagen Mini Spin column technique,
according to the manufacturer's instructions and
specifications.
Genotyping assay was performed for seven CD163 gene
SNPs, including four randomly selected SNPs at the promoter region
(rs61729, rs11054197, rs11054195 and rs75608120), in addition to
three different randomly selected SNPs at the protein-coding region
(rs200642325, rs61729510 and rs150018775) in both control and CHL
patient samples. PCR was performed using 96-well plates and
TaqMan® GTXpress™, Genotyping Assays, Human, SM Kit
(cat. no. 4351379; Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The PCR reaction was performed with an
Applied Biosystems 7500 Real Time PCR system following the standard
manufacturer's protocols and the following conditions: Enzyme
activation at 95°C for 20 sec, then total of 40 cycles of both
denaturation at 95°C for 15 sec and annealing/extension at 60°C for
60 sec.
Statistical analysis
All statistical analyses were performed using SPSS
software, version 22.0 (IBM Corp., Armonk, NY, USA). The results
were considered statistically significant at P≤0.05. CD163 IHC
optimum threshold was determined using SPSS software based on the
maximal χ2 value of the log-rank test for disease
relapse (DR), classified as low- and high-risk. DR was defined as
the time between treatment initiation and disease progression or
relapse. Overall survival (OS) was defined as the time between
treatment initiation and death from any cause. The t-test was used
for the association of the CD163 indices (high and low) with DR and
OS. Fisher's exact probability (Chi-squared) test was used to
investigate any association of all the studied SNPs with DR and OS
in CHL patients. One-way analysis of variance was applied to
investigate the association of the CD163 indices with different
SNPs, and also for different HL stages and CHL subtypes. The
correlation of the CD163 prognosis index with ESR for low-stage
CHL, and with IPS score for high-stage CHL, were analyzed by
t-tests.
Survival curves were constructed using the
Kaplan-Meier method and assessed for statistical significance using
the log-rank test. Genotype and allele frequencies for all used
SNPs were estimated using the online version of SHEsis Software
(http://analysis.bio-x.cn/myAnalysis.php). Fisher's
exact probability (Chi-squared) test was used to evaluate any
associations of all the studied SNPs with DR and OS in the CHL
patients included in this study.
Results
Demographic characteristics of the
studied Saudi CHL patients
Our data demonstrated that 44% of the patients were
female and 56% were male, with a male: Female ratio of 1.3:1.0. The
CHL patients' age at diagnosis ranged from 3 to 80 years, with a
median age of 42 years. The majority (78%) of the patients were
aged <45 years (Table I). The
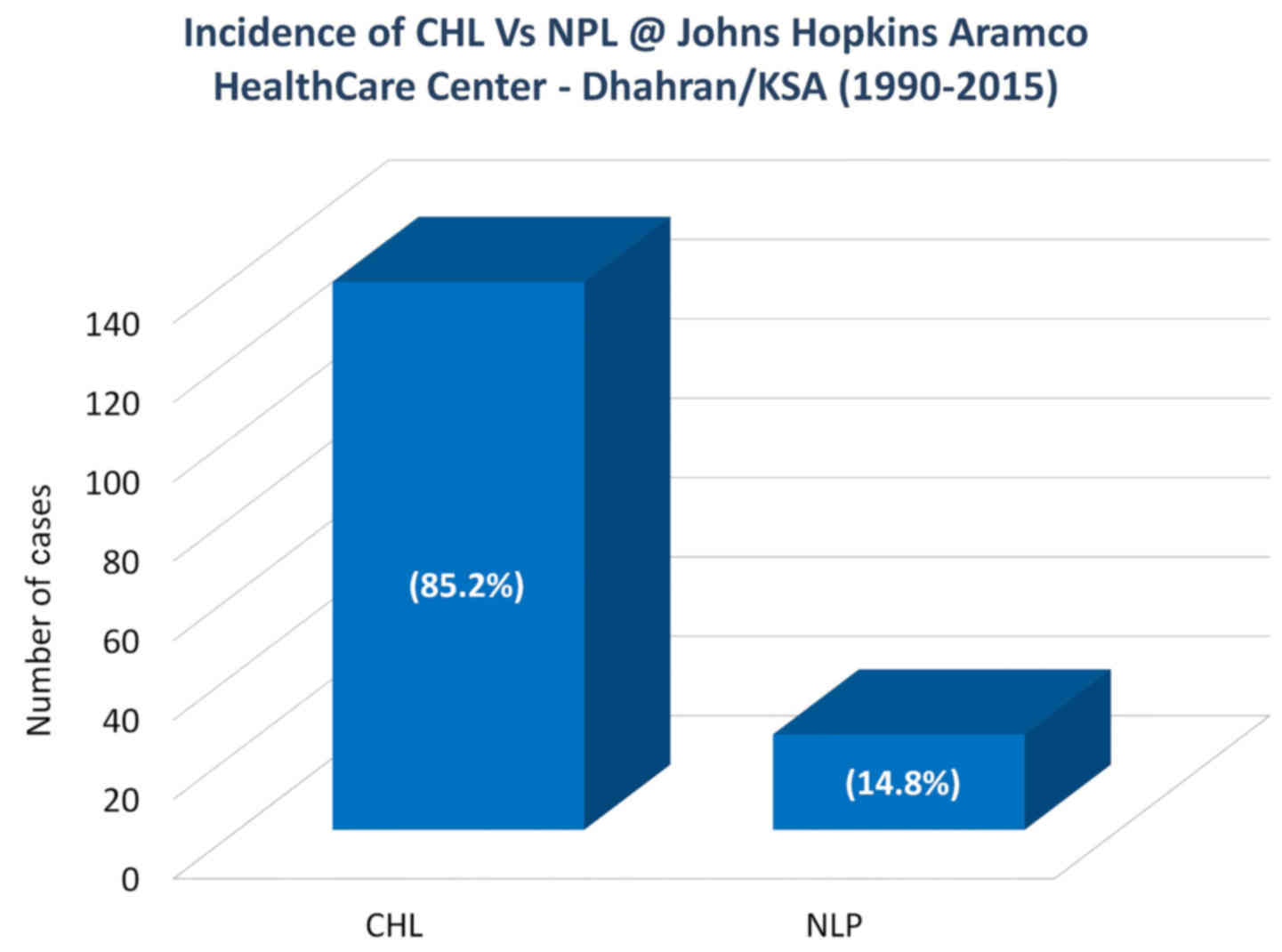
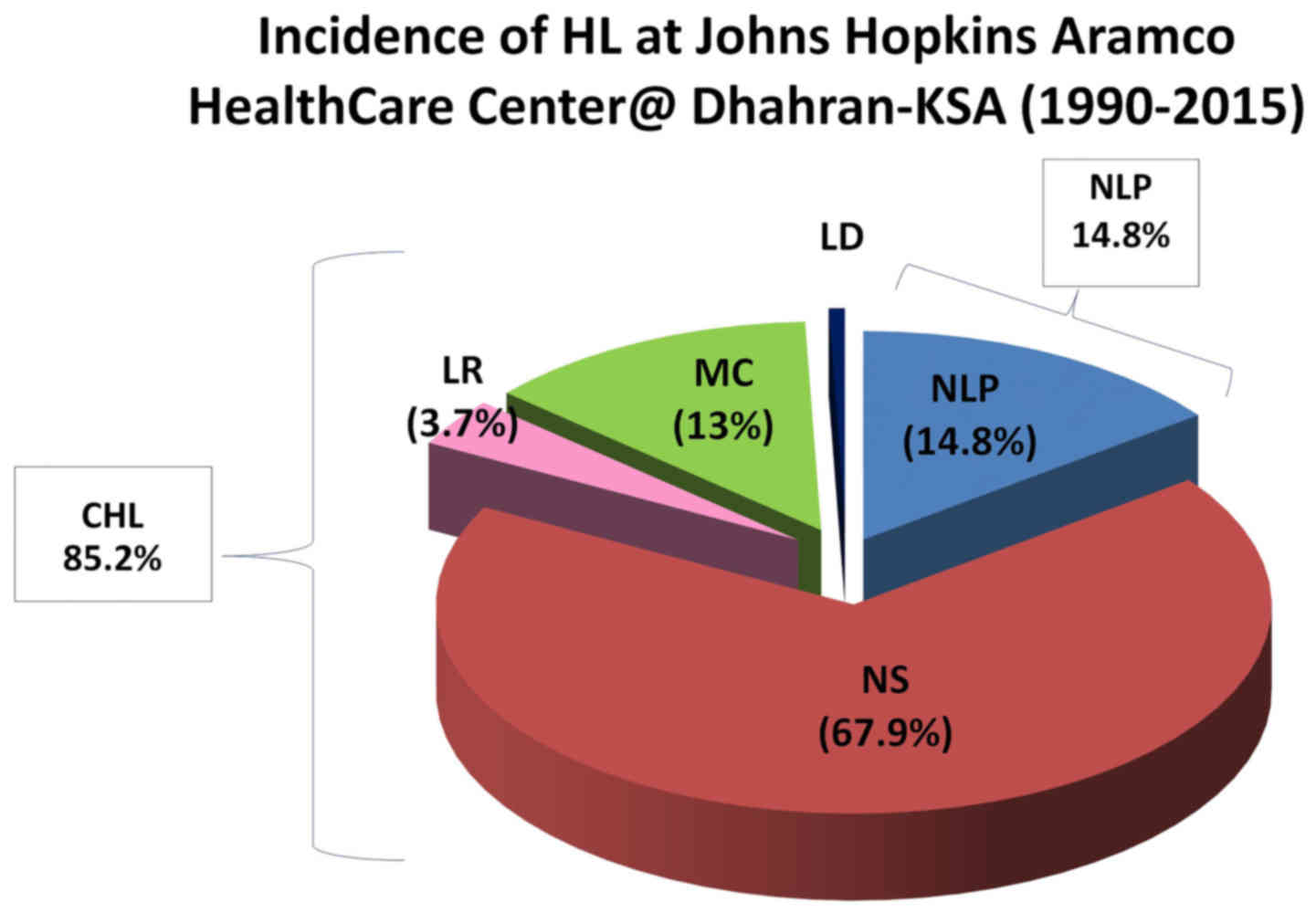
aggregated data also revealed that the CHL entity constituted 85.2%
of all newly diagnosed HL cases at JHAH, whereas NLP accounted for
14.8% of all cases over the past 26 years (Fig. 1). Moreover, ~81% of the cases
diagnosed at JHAH exhibited a higher prevalence of the NS subtype
of CHL compared with the other subtypes (Table I; Fig.
2). In addition, data analysis demonstrated that stage II and
III CHLs were the most common among the studied Saudi patients
(41.3 and 33.7%, respectively; Table
I).
 | Table I.Demographic characteristics of CHL
cases in the Saudi population at the John Hopkins Aramco HealthCare
Center during the period between 1990 and 2015. |
Table I.
Demographic characteristics of CHL
cases in the Saudi population at the John Hopkins Aramco HealthCare
Center during the period between 1990 and 2015.
| Demographic
characteristics | Total no. (n) | % |
|---|
| Age (years) | 100 |
|
|
<45 | 78 | 78.0 |
|
>45 | 27 | 27.0 |
| Sex | 100 |
|
|
Male | 56 | 56.0 |
|
Female | 44 | 44.0 |
| CHL stage | 92 |
|
| I | 8 | 8.7 |
| II | 38 | 41.3 |
|
III | 31 | 33.7 |
| IV | 15 | 16.3 |
| CHL subtype | 100 |
|
| NS | 81 | 81.0 |
| MC | 13 | 13.0 |
| LR | 5 | 5.0 |
| LD | 1 | 1.0 |
| Treatment received
(response) | 100 |
|
|
Curative | 98 | 98.0 |
|
Palliative | 2 | 2.0 |
| Relapse | 100 |
|
| No
relapse | 84 | 84.0 |
|
Relapsed | 16 | 16.0 |
| Overall
survival | 100 |
|
| Did not
survive | 11 | 11.0 |
|
Survived | 89 | 89.0 |
CD163 IHC staining
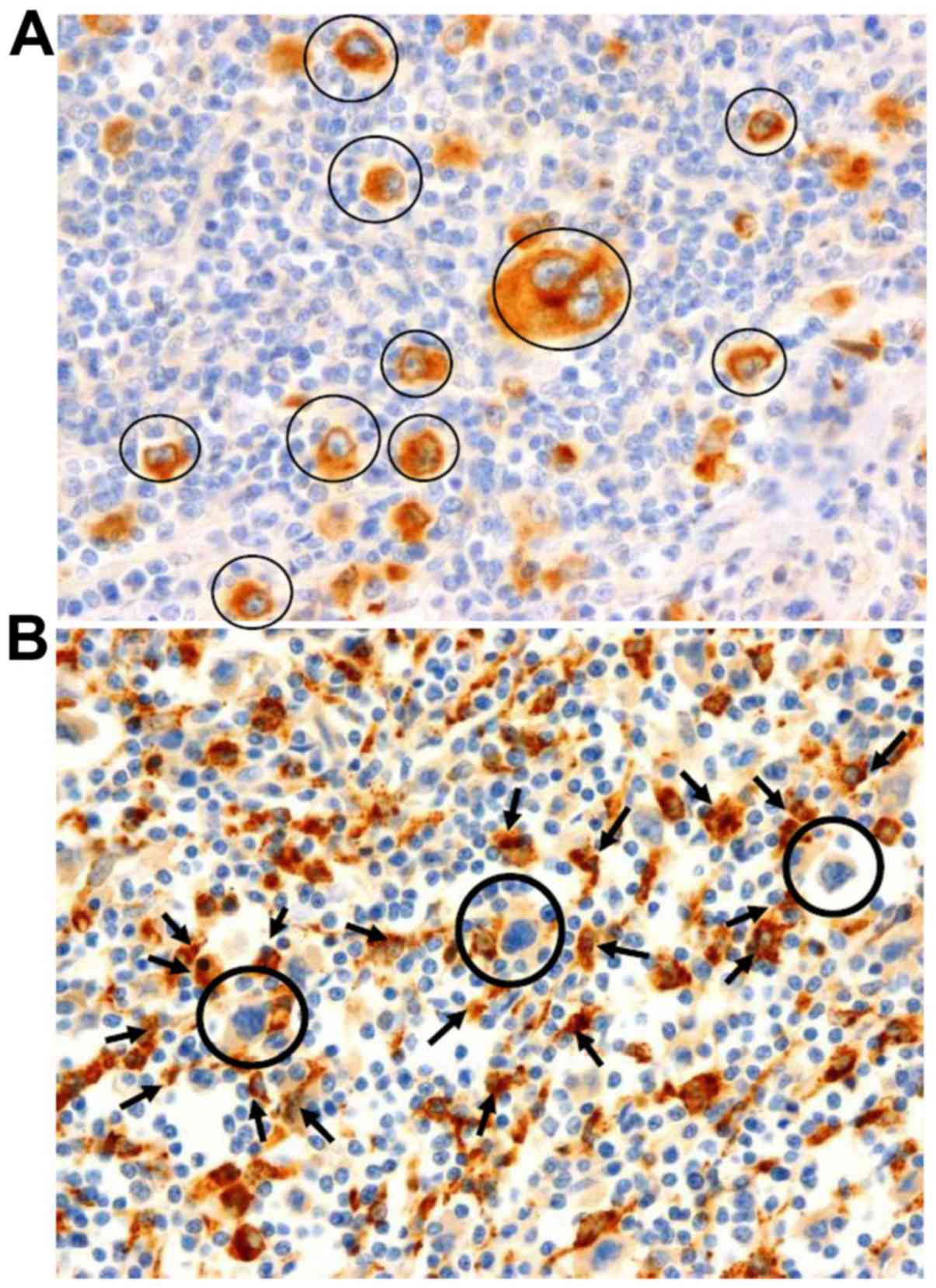
The microscopic examination of IHC staining for
CD163 revealed that this protein is expressed on TAMs as
surface/membranous and cytoplasmic/lysosomal staining in cases with
a higher number of TAMs around HL cells; however, it was mainly
expressed as cytoplasmic/lysosomal staining in cases with a lower
number of TAMs surrounding HL cells. Only CD163-positive TAMs
(Fig. 3B) surrounding a central
cluster of CD30-positive cells (Fig.
3A) were taken into consideration to calculate the CD163 index
in this study. The CD163 prognosis index was calculated by dividing
the number of CD163-positive macrophages by the total number of
CD30-positive HRS cells in CHL patients, and by the number of
CD30-positive reactive B-lymphocytes in the control group. When
comparing the index mean of CD163 expressed on TAMs in CHL patients
against that of the control group using Student's t-test, a
statistically significant difference was observed (P<0.001;
Table II).
 | Table II.Comparison of the CD163 index between
CHL patients and control subjects. |
Table II.
Comparison of the CD163 index between
CHL patients and control subjects.
| Indices | CHL patients
(n=100) | Controls
(n=20) | t-test P-value |
|---|
| CD163 (mean) | 13.5 | 2.8 | <0.001 |
Determining the threshold of the CD163
prognosis index
The CD163 prognosis index threshold was determined
using t-test to compare the CD163 IHC mean of the relapsed cases
vs. that of the non-relapsed patients at several cut-off values (5,
7, 10, 15, 20 and 25) of the CD163 index. A threshold value of 15
reflected significant differences between the relapsed and
non-relapsed patient groups, with a P-value of 0.008. Accordingly,
CHL patients were divided into two stratification groups: Low-risk
(lower CD163 index, ≤15.0) and high-risk (higher CD163 index,
>15.0). In this study, 69/100 (69%) of CHL patients had a low
CD163 index and 31/100 (31%) had a high CD163 index (Table III).
 | Table III.CHL risk stratification groups at the
cut-off value of the CD163 index in comparison with controls. |
Table III.
CHL risk stratification groups at the
cut-off value of the CD163 index in comparison with controls.
|
| CHL patients | Controls |
|
|---|
|
|
|
|
|
|---|
| CD163 IHC index (at
cut-off) | n | % | n | % | P-value |
|---|
| Low-risk CHL
(≤15) | 69 | 69.0 | 20 | 100.0 | <0.001 |
| High-risk CHL
(>15) | 31 | 31.0 | 0 | 0 |
|
| Total | 100 | 100.0 | 20 | 100.0 |
|
Association of CD163 IHC prognostic
index with DR and OS in CHL
The analysis revealed that there was a statistically
significant direct association of the CD163 index with DR and OS,
with a Pearson's Chi-squared P-value of <0.001. Additionally,
the CD163 index at the determined threshold level (15.0) was
directly associated with DR (P=0.022, Fisher's exact test=0.034);
however, it was not significantly associated with OS rate (P=0.173,
Fisher's exact test=0.189).
Genotyping analysis of CD163 gene
SNPs
Upon CD163 gene SNPs analysis, genotype and allele
frequencies for all seven studied SNPs were calculated using the
SHEsis Software. The analysis demonstrated that all CD163 gene SNPs
were at Hardy-Weinberg equilibrium (HWE) among controls (Fisher's
P-value >0.05), which indicated that our population was
homogeneous, excluding rs75608120 SNP, which is located at the
promoter region of the CD163 gene and was not in HWE among controls
(Fisher's P-value <0.001), indicating that our population was
not homogeneous for this SNP (Table
IV). The theory behind HWE states that in a given population,
allele frequency as well as genotype frequency of any gene will
persist among consecutive generations, unless evolutionary
influences interfere.
 | Table IV.Genotype frequencies of seven
different SNPs in CD163 gene protein sequence in 100 CHL patients
and 100 controls. |
Table IV.
Genotype frequencies of seven
different SNPs in CD163 gene protein sequence in 100 CHL patients
and 100 controls.
| A, Genotype
frequency (CHL patients) |
|---|
|
|---|
|
| CD163 promoter
sequence SNPs | CD163 exon
SNPs |
|---|
|
|
|
|
|---|
| Hardy-Weinberg
equilibrium test | Prom SNP 1
rs61729515 | Prom SNP 2
rs11054197 | Prom SNP 3
rs11054195 | Prom SNP 4
rs75608120 | Gene SNP 1
rs200642325 | Gene SNP 2
rs61729510 | Gene SNP 3
rs150018775 |
|---|
|
| CC | 0.00 | AA | 0.01 | AA | 0.00 | CC | 0.01 | AA | 0.00 | CC | 1.00 | GG | 0.00 |
|
| CT | 0.00 | AG | 0.15 | AT | 0.00 | CT | 0.01 | AG | 0.00 | CT | 0.00 | GT | 0.00 |
|
| TT | 1.00 | GG | 0.84 | TT | 1.00 | TT | 0.98 | GG | 1.00 | TT | 0.00 | TT | 1.00 |
| χ2 |
|
| 0.13 |
|
| 42.88 |
|
| 0.00 |
|
|
| df |
|
| 1 |
|
| 1 |
|
| 1 |
|
|
| Fisher's
P-value |
|
| 0.721 |
|
| 0.000 |
|
| 1.000 |
|
|
| Pearson's
P-value |
|
| 0.721 |
|
| 0.000 |
|
| 1.000 |
|
|
|
| B, Genotype
frequency (control group) |
|
|
| CD163 promoter
sequence SNPs | CD163 exon
SNPs |
|
|
|
|
| Hardy-Weinberg
equilibrium test | Prom SNP 1
rs61729515 | Prom SNP 2
rs11054197 | Prom SNP 3
rs11054195 | Prom SNP 4
rs75608120 | Gene SNP 1
rs200642325 | Gene SNP 2
rs61729510 | Gene SNP 3
rs150018775 |
|
|
| CC | 0.00 | AA | 0.00 | AA | 0.00 | CC | 0.01 | AA | 0.00 | CC | 0.99 | GG | 0.00 |
|
| CT | 0.00 | AG | 0.09 | AT | 0.00 | CT | 0.03 | AG | 0.00 | CT | 0.01 | GT | 0.00 |
|
| TT | 1.00 | GG | 0.91 | TT | 1.00 | TT | 0.96 | GG | 1.00 | TT | 0.00 | TT | 1.00 |
| χ2 |
|
| 0.22 |
|
| 14.79 |
|
| 0.00 |
|
|
| df |
|
| 1 |
|
| 1 |
|
| 1 |
|
|
| Fisher's
P-value |
|
| 0.638 |
|
| 0.000 |
|
| 0.959 |
|
|
| Pearson's
P-value |
|
| 0.638 |
|
| 0.000 |
|
| 0.959 |
|
|
Association of CD163 gene SNPs with DR
and OS of CHL
Chi-square analysis of allele genotype of the
studied CD163 gene SNPs revealed that there is no statistically
significant association with DR or OS in CHL, except for one CD163
SNP (rs75608120), which is located in the promoter region, and
exhibited a statistically significant association with DR
(P=0.032), but not with OS (Table
V).
 | Table V.Significance of the correlation
between CD163 SNPs with the DR and OS rates of the CHL cases among
Saudi patients at the John Hopkins Aramco HealthCare Center during
the period between 1990 and 2015. |
Table V.
Significance of the correlation
between CD163 SNPs with the DR and OS rates of the CHL cases among
Saudi patients at the John Hopkins Aramco HealthCare Center during
the period between 1990 and 2015.
|
| CHL patients | Control group |
|
|
|---|
|
|
|
|
|
|
|---|
| CD163 promoter
region SNPs | n=100 | % | n=100 | % | DR P-value
NCa | OS P-value
NCa |
|---|
| SNP1
(rs61729515) |
|
|
|
|
|
|
|
Homozygous for wild-type
allele | 0 | 0 | 0.0 | 0 |
|
|
| Heterozygous | 0 | 0 | 0.0 | 0 |
|
|
|
Homozygous for mutant
allele | 100 | 100 | 100.0 | 100 |
|
|
| SNP2
(rs11054197) | 100 |
| 100 |
| 0.203 | 0.573 |
|
Homozygous for wild-type
allele | 1 | 1 | 0.0 | 0 |
|
|
| Heterozygous | 15 | 15 | 9.0 | 9 |
|
|
|
Homozygous for mutant
allele | 84 | 84 | 91.0 | 91 |
|
|
| SNP3
(rs11054195) | 100 |
| 100 |
| 0.637 | 0.637 |
|
Homozygous for wild
allele | 0 | 0 | 0.0 | 0 |
|
|
| Heterozygous | 0 | 0 | 0.0 | 0 |
|
|
|
Homozygous for mutant
allele | 100 | 100 | 100.0 | 100 |
|
|
| SNP4
(rs75608120) | 100 |
| 100 |
| 0.032 | 0.09 |
|
Homozygous for wild
allele | 1 | 1 | 1.0 | 1 |
|
|
| Heterozygous | 1 | 1 | 3.0 | 3 |
|
|
|
Homozygous for mutant
allele | 98 | 98 | 96.0 | 96 |
|
|
Testing the study hypothesis
In order to determine whether CD163 protein
overexpression is stimulated by the presence of any SNP at the gene
promoter region, or even at the protein-coding region, the
calculated CD163 prognostic index was evaluated for its association
with its SNPs using analysis of variance (ANOVA). The analysis
revealed that none of the studied SNPs were associated with the
CD163 IHC protein expression (P=0.690). Thus, the null hypothesis
was rejected.
Association of CD163 IHC prognostic
index with ESR for low-stage CHL
The CD163 IHC-determined index was assessed via
Student's t-test for association with ESR, as this predictive
biomarker is already used to assess the CHL risk for low-stage HL
(I and II). The analysis demonstrated a direct significant
association between the ESR and the CD163 index (P<0.001): The
CD163 index was higher in CHL cases with an ESR of >20 mm/h
(Table VI).
 | Table VI.Association of ESR and IPS score with
the CD163 index in HL patients. |
Table VI.
Association of ESR and IPS score with
the CD163 index in HL patients.
| Variables | n | % | P-value
(t-test) |
|---|
| ESR (stages I and
II), mm/h | 46 |
| <0.001 |
|
<20 | 20 | 43.3 |
|
|
>20 | 26 | 56.6 |
|
| IPS (stages III and
IV) | 46 |
| <0.001 |
|
<3 | 18 | 39.1 |
|
|
>3 | 7 | 15.2 |
|
Association of the CD163 IHC
prognostic index with IPS score for high-stage CHL
The statistical t-test was used to assess the
association of the IHC CD163 index with IPS, as it is the accepted
tool used for predicting the HL risk for high-stage CHL (III and
IV). The analysis confirmed a significant association between IPS
and the CD163 index (P<0.001): The CD163 index was higher in CHL
cases with an IPS of >3 (Table
VI).
CHL survival curve based on the
established CD163 prognostic index threshold
The total number of relapsed and deceased CHL
patients in our study was 27/100 (27%). A total of 16 CHL patients
(16%) constituted the known relapsed CHL cases within a period
ranging from 1 month to 12 years, with a median of 3.2 years. A
total of 11/100 CHL patients (11%) succumbed to the disease within
the same time period. A total of 11 CHL known cases died from the
disease, the majority (9 out of 11) of them succumbed within the
first 5 years, while the minority (2 out of 11) survived for >5
years. The median OS was ~3.5 years in our patient population
(Table VII).
 | Table VII.Summary of DR and OS in the studied
CHL patients at the John Hopkins Aramco HealthCare Center. |
Table VII.
Summary of DR and OS in the studied
CHL patients at the John Hopkins Aramco HealthCare Center.
|
| DR | OS |
|---|
|
|
|
|
|---|
|
| 0–6 months | 6 months-5
years | 5–10 years | >10 years | <5 years | >5 years |
|---|
| No. of CHL
patients | 3/100 | 10/100 | 2/100 | 1/100 | 9/100 | 2/100 |
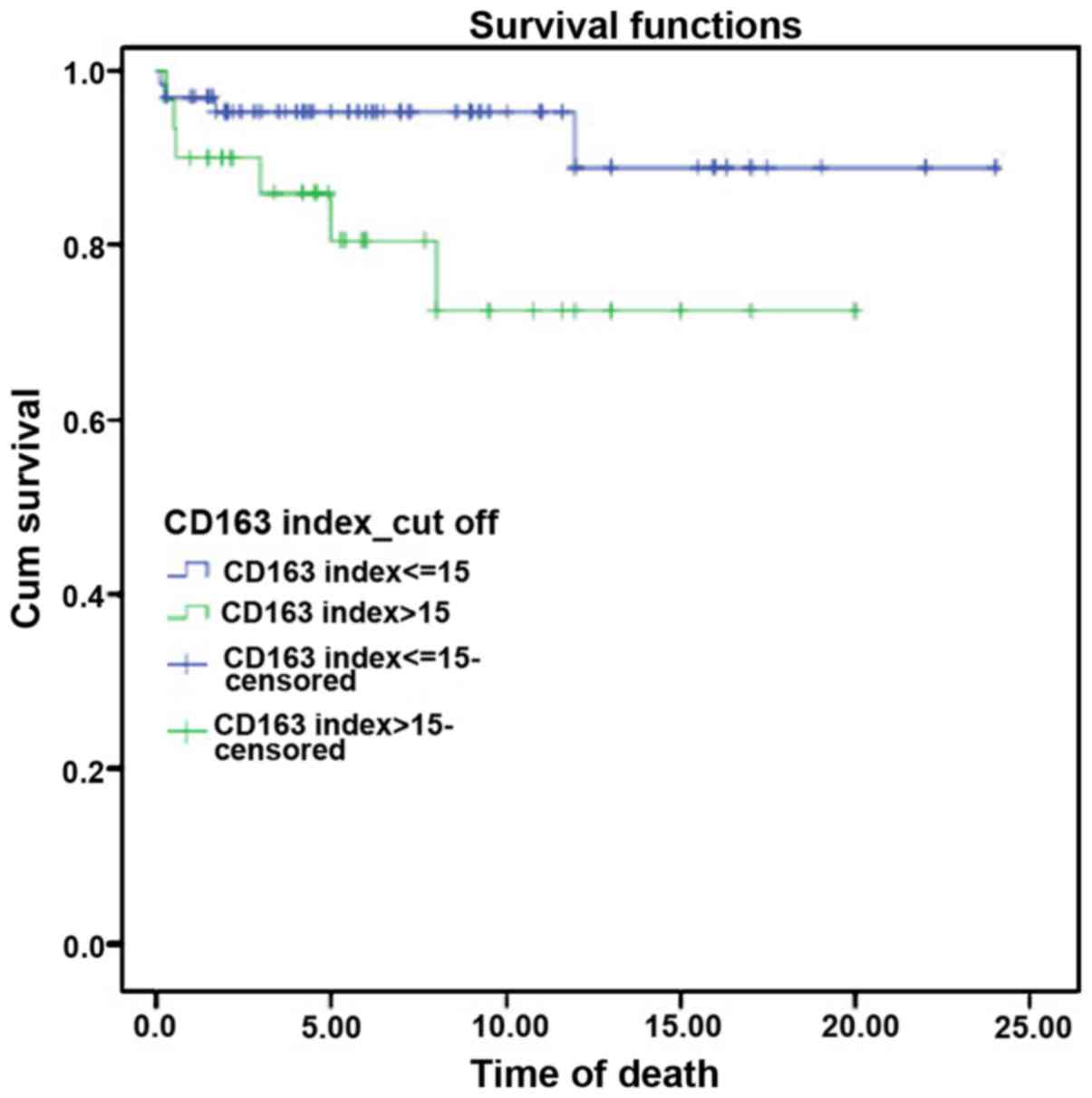
A survival curve was constructed for the CD163 index
using the Kaplan-Meier method, which calculates the cumulative
survival rate. Accordingly, the CD163 index was statistically
significant in high-risk CHL patients (i.e., CD163 index >15.0)
in comparison with the low-risk patients (i.e., CD163 index ≤15.0).
Subsequently, there was an observable decline in the survival of
high-risk CHL patients (Fig. 4).
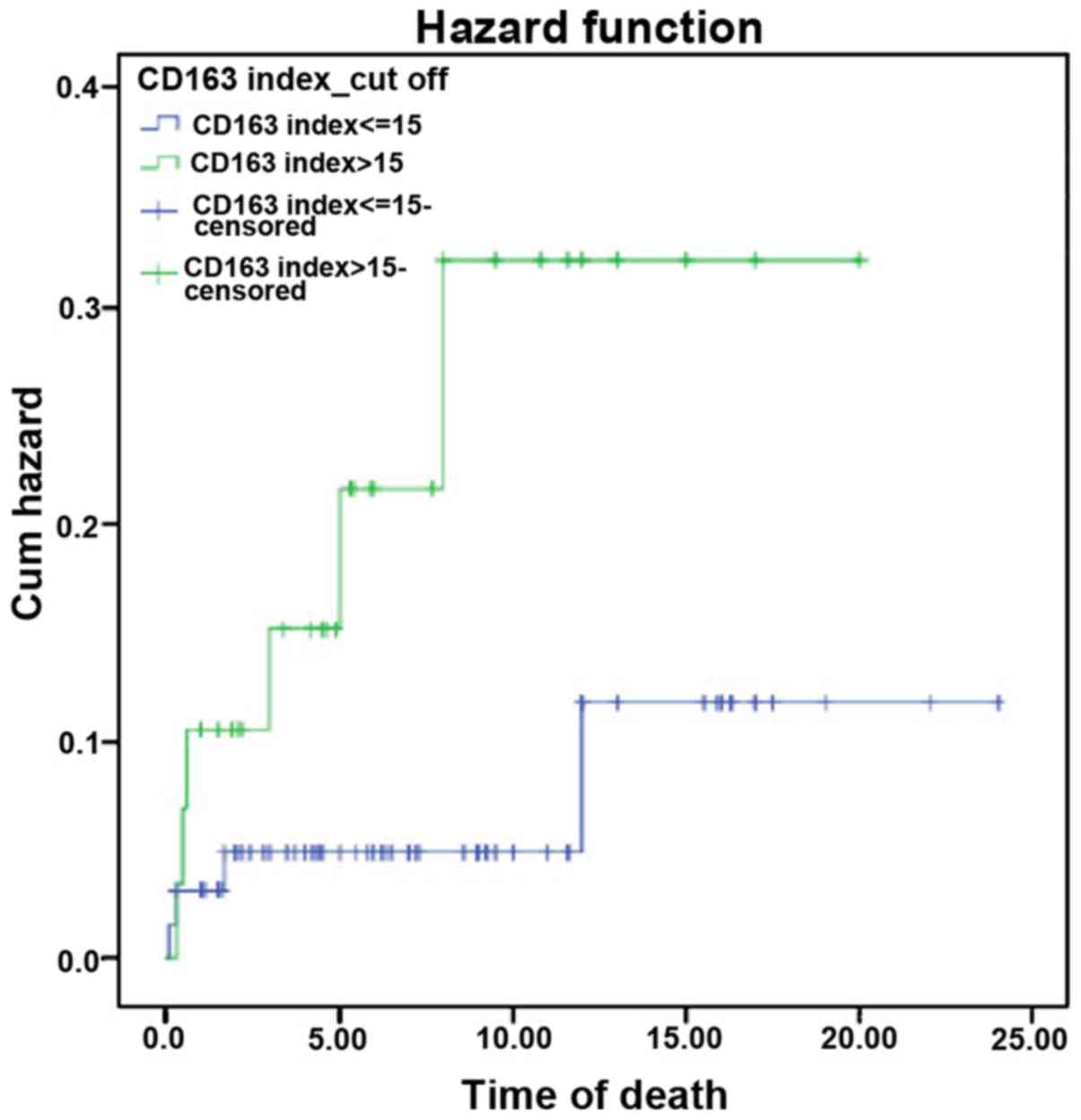
Similarly, the hazard function analysis demonstrated a higher
cumulative hazard in the high-risk CHL group (Fig. 5). The survival and the hazard
function were assessed using the log-rank test, which revealed a
significant association between the CD163 index and survival rate,
with a P-value of 0.039.
Discussion
In the present study, we first attempted to
standardize the evaluation of CD163 expression on TAMs using IHC,
and to determine a threshold level for CHL patients to help
stratify disease prognosis. To investigate the effect of CD163
protein expression on CHL prognosis, the association of CD163 IHC
with DR and OS was evaluated and found to be statistically
significant (P<0.001). This finding confirms that an increased
number of TAMs overexpressing CD163 is significantly associated
with reduced progression-free and overall survival after standard
chemotherapy (with or without radiotherapy). Therefore, this
antigen may be considered as a prognostic biomarker for CHL in
Saudi patients.
We attempted to determine the threshold of IHC
staining level of CD163 in order to stratify the CHL cases into
low- and high-risk groups. Based on the maximal χ2 value
of the log-rank test with regard to relapse and survival rate
(i.e., DR and OS), the CD163 index threshold was determined as
15.0, with a Pearson's Chi-squared P-value of 0.008 and a Fisher's
exact test P-value of 0.014. This threshold value for CD163 was
significantly correlated with the number of relapsed CHL patients
(P=0.022). Moreover, our study demonstrated that CD163 protein
expression was significantly correlated with ESR in low-stage (I
and II) and with IPS in high-stage (III and IV) CHL, with a P-value
of <0.001. This finding supports the use of CD163 expression
levels in addition to ESR and IPS as biomarkers for predicting the
prognosis of CHL.
To complete the study, we performed genetic typing
of 7 SNPs in the 5′ region of the CD163 locus in 100 CHL patients
and an equivalent number (i.e., n=100) of healthy controls. The 7
polymorphisms were selected on the basis that they may guide, drive
or affect the protein expression level of CD163. To the best of our
knowledge, no previous study has investigated CD163 gene
polymorphisms, or their correlation with the level of protein
expression or CHL prognosis. We herein attempted to correlate the
presence of different CD163 gene SNPs with CHL prognosis by
calculating the statistical significance of these SNP genotypes
with the DR and OS of CHL. Using the Chi-squared test, we found
that all the studied CD163 SNPs were insignificantly correlated
with DR and OS in CHL patients, except for rs75608120 (a
polymorphism located in the promoter region). This SNP exhibited a
significant correlation with the DR of CHL (P=0.032), but not with
OS (P=0.090). One of the main goals of the present study was to
determine whether CD163 protein overexpression is guided or
stimulated by the presence of any one SNP in CHL patients compared
with healthy controls. Upon multivariate ANOVA, no significant
correlation was identified between CD163 expression on IHC and the
studied SNPs in CHL patients (P=0.690), although the rs75608120
polymorphism may represent a separate single genetic predictive
biomarker for CHL prognosis. In addition, the lack of an
association between CD163 IHC and its studied SNPs in CHL may be
due to the small number of CHL patients included in the present
study. Further investigation is required to study more or even all
the SNPs at the promoter region; however, this may indicate that
the protein overexpression of CD163 on TAMs in CHL cases is not
directly caused by any genetic polymorphism in its gene, but rather
the result of immunological pathophysiology of TME crosstalk.
In conclusion, the present study provided valuable
insight into the molecular genetics and antigenicity of CD163 and
its effect on the prognosis of CHL in Saudi patients. CD163
overexpression on TAMs was confirmed to be significantly associated
with early relapse and reduced survival post-therapy. To the best
of our knowledge, the present study was also the first to define a
specific genetic pattern that is clearly associated with the
clinical outcome of CHL. Accordingly, CD163 is a useful predictive
antigenic and genetic biomarker for CHL prognosis in Saudi
patients. Our findings may pave the way for improving the clinical
management of CHL in the future through molecular analyses for gene
expression and IHC, and they may help develop better therapeutic
protocols incorporating pharmacogenetics and monoclonal antibodies
specifically designed to block TAM receptors for CD163. Anti-CD163
antibodies may be applied for the treatment of HL along with the
currently available therapeutic options.
Acknowledgements
Warmest thanks to my colleagues at Arabian Gulf
University, Mr. Ali Mehaimeed, Mr. Muhallab, Dr Nouriddin and Dr
Sonia Bourguiba, as well as Mr. AbuAqla, Mr. Khalid Khunaizi and Dr
Ali Rabaan at Johns Hopkins Aramco Healthcare (JHAH), who provided
the necessary technical assistance and efficient support. Special
thanks to Dr Ahmed Alsagheir and Dr Adel Alkhatti from the Oncology
institute at JHAH. I am grateful to have such support from all the
pioneers and philanthropists around me.
Funding
The present study was supported by the Arabian Gulf
University-College of Medicine and Medical Sciences, Bahrain. The
funder had no role in the study design, data collection and
analysis, decision to publish, or preparation of the
manuscript.
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
HSA and WR designed the experiments; HSA performed
the experiments, data validation and formal analysis, and wrote the
manuscript; WR, AD and MF reviewed and revised the manuscript; AD
acquired funding. All authors have read and approved the final
version of this manuscript.
Ethics approval and consent to
participate
Ethics approval for the present study was obtained
from the Institutional Review Board. Written informed consent was
provided by the subjects regarding the use of their tissue samples
for research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rauf MS, Akhtar S and Maghfoor I: Changing
trends of adult lymphoma in the kingdom of Saudi Arabia-comparison
of data sources. Asian Pac J Cancer Prev. 16:2069–2072. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leukemia and Lymphoma Society, . American
Cancer Society Cancer Facts and Figures 2016; GLOBOCAN 2012;
ClinicalTrials.gov. CRI grantee progress reports and other CRI
grantee documents. 2016, http://www.cancerresearch.org/cancer-immunotherapy/impacting-all-cancers/lymphoma#sthash.9EkuimDH.dpuf
91November 14–2014
|
|
3
|
Al-Madouj AN, Eldali A and Al-Zahrani AS:
Ten year cancer incidence among nationals of the GCC states. Gulf
center for cancer control and prevention, king faisal specialist
hospital and research center. 2011, http://www.moh.gov.bh/Content/Files/Publications/GCC%20Cancer%20Incidence%202011.pdfSeptember
1–2011
|
|
4
|
Cancer Incidence Report Saudi Arabia 2013,
. http://www.chs.gov.sa/Ar/HealthCenters/NCC/CancerRegistry/CancerRegistryReports/2013June
1–2016
|
|
5
|
Maggioncalda A, Malik N, Shenoy P, Smith
M, Sinha R and Flowers CR: Clinical, molecular, and environmental
risk factors for Hodgkin lymphoma. Adv Hematol. 2011:7362612011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Diab AI, Siddiqui N, Sogiawalla FF and
Fawzy EM: The changing trends of adult Hodgkin's disease in Saudi
Arabia. Saudi Med J. 24:617–622. 2003.PubMed/NCBI
|
|
7
|
Piccaluga PP, Agostinelli C, Gazzola A,
Tripodo C, Bacci F, Sabattini E, Sista MT, Mannu C, Sapienza MR,
Rossi M, et al: Pathobiology of hodgkin lymphoma. Adv Hematol.
2011:9208982011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Montes-Moreno S: Hodgkin's lymphomas: A
tumor recognized by its microenvironment. Adv Hematol.
2011:1423952011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith LB: Nodular lymphocyte predominant
Hodgkin lymphoma: Diagnostic pearls and pitfalls. Arch Pathol Lab
Med. 134:1434–1439. 2010.PubMed/NCBI
|
|
10
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the committee on Hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
11
|
Hasenclever D and Diehl V: A prognostic
score for advanced Hodgkin's disease. International prognostic
factors project on advanced Hodgkin's disease. N Engl J Med.
339:1506–1514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Canioni D, Deau-Fischer B, Taupin P,
Ribrag V, Delarue R, Bosq J, Rubio MT, Roux D, Vasiliu V, Varet B,
et al: Prognostic significance of new immunohistochemical markers
in refractory classical Hodgkin lymphoma: A study of 59 cases. PLoS
One. 4:e63412009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moccia AA, Donaldson J, Chhanabhai M,
Hoskins PJ, Klasa RJ, Savage KJ, Shenkier TN, Slack GW, Skinnider
B, Gascoyne RD, et al: International prognostic score in
advanced-stage Hodgkin's lymphoma: Altered utility in the modern
era. J Clin Oncol. 30:3383–3388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henry-Amar M, Friedman S, Hayat M, Somers
R, Meerwaldt JH, Carde P, Burgers JM, Thomas J, Monconduit M and
Noordijk EM: Erythrocyte sedimentation rate predicts early relapse
and survival in early-stage Hodgkin disease. The EORTC lymphoma
cooperative group. Ann Intern Med. 114:361–365. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roshal M, Wood BL and Fromm JR: Flow
cytometric detection of the classical hodgkin lymphoma: Clinical
and research applications. Adv Hematol. 2011:3870342011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dunphy CH: Applications of flow cytometry
and immunohistochemistry to diagnostic hematopathology. Arch Pathol
Lab Med. 128:1004–1022. 2004.PubMed/NCBI
|
|
17
|
Scott DW and Steidl C: The classical
Hodgkin lymphoma tumor microenvironment: Macrophages and gene
expression-based modeling. Hematology Am Soc Hematol Educ Program.
2014:144–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan KL, Scott DW, Hong F, Kahl BS, Fisher
RI, Bartlett NL, Advani RH, Buckstein R, Rimsza LM, Connors JM, et
al: Tumor-associated macrophages predict inferior outcomes in
classic Hodgkin lymphoma: A correlative study from the E2496
Intergroup trial. Blood. 120:3280–3287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mallmann MR, Schmidt SV and Schultze JL:
Macrophages in human cancer: Current and future aspects. Atlas
Genet Cytogenet Oncol Haematol. 16:765–774. 2012.
|
|
20
|
Ruhrberg C and De Palma M: A double agent
in cancer: Deciphering macrophage roles in human tumors. Nat Med.
16:861–862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ree HJ and Kadin ME:
Macrophage-histiocytes in Hodgkin's disease. The relation of
peanut-agglutinin-binding macrophage-histiocytes to
clinicopathologic presentation and course of disease. Cancer.
56:333–338. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo B, Cen H, Tan X and Ke Q:
Meta-analysis of the prognostic and clinical value of
tumor-associated macrophages in adult classical Hodgkin lymphoma.
BMC Med. 14:1592016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sud A, Thomsen H, Sundquist K, Houlston RS
and Hemminki K: Risk of second cancer in Hodgkin lymphoma survivors
and influence of family history. J Clin Oncol. 35:1584–1590. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steidl C, Lee T, Shah SP, Farinha P, Han
G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al:
Tumor-associated macrophages and survival in classic Hodgkin's
lymphoma. N Engl J Med. 362:875–885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sánchez-Espiridión B, Montalbán C, López
A, Menárguez J, Sabín P, Ruiz-Marcellán C, Lopez A, Ramos R,
Rodríguez J, Cánovas A, et al: A molecular risk score based on 4
functional pathways for advanced classical Hodgkin lymphoma. Blood.
116:e12–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gregory AD and Houghton AM:
Tumor-associated neutrophils: New targets for cancer therapy.
Cancer Res. 71:2411–2416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wahl LM and Kleinman HK: Tumor-associated
macrophages as target for cancer therapy. J Natl Cncer Inst.
90:1583–1584. 1998. View Article : Google Scholar
|
|
28
|
Derenzini E and Younes A: Predicting
treatment outcome in classical Hodgkin lymphoma: Genomic advances.
Genome Med. 3:262011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barros MH, Segges P, Vera-Lozada G, Hassan
R and Niedobitek G: Macrophage polarization reflects T cell
composition of tumor microenvironment in pediatric classical
Hodgkin lymphoma and has impact on survival. PLoS One.
10:e01245312015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoon DH, Koh YW, Kang HJ, Kim S, Park CS,
Lee SW, Suh C and Huh J: CD68 and CD163 as prognostic factors for
Korean patients with Hodgkin lymphoma. Eur J Haematol. 88:292–305.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azambuja D, Natkunam Y, Biasoli I, Lossos
IS, Anderson MW, Morais JC and Spector N: Lack of association of
tumor-associated macrophages with clinical outcome in patients with
classical Hodgkin's lymphoma. Ann Oncol. 23:736–742. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lau SK, Chu PG and Weiss LM: CD163: A
specific marker of macrophages in paraffin-embedded tissue samples.
Am J Clin Pathol. 122:794–801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ritter M, Buechler C, Langmann T, Orso E,
Klucken J and Schmitz G: The scavenger receptor CD163: Regulation,
promoter structure and genomic organization. Pathobiology.
67:257–261. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harris JA, Jain S, Ren Q, Zarineh A, Liu C
and Ibrahim S: CD163 versus CD68 in tumor associated macrophages of
classical Hodgkin lymphoma. Diagn Pathol. 7:122012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El-Zein R, Monroy CM, Etzel CJ, Cortes AC,
Xing Y, Collier AL and Strom SS: Genetic polymorphisms in DNA
repair genes as modulators of Hodgkin disease risk. Cancer.
115:1651–1659. 2009. View Article : Google Scholar : PubMed/NCBI
|