Introduction
Breast cancer is one of the most common malignant
tumors that endanger the health of women (1), with an estimated 252,710 new cases in
the United States in 2017 (2) and
26,000 new cases in Canada (3) in
2016. A total of 1 in 8 women in the United States will have breast
cancer in their lifetime (4).
Approximately 1.5 million women worldwide develop breast cancer
every year, and ~500,000 women succumb to breast cancer (5). Early detection and advances in
screening have led to a 5-year survival rate approaching 90%, and
in the United States, almost 3 million people are living with a
prior diagnosis of breast cancer (2). The treatment options usually include a
combination of surgery, cytotoxic chemotherapy, radiation therapy
and molecularly targeted endocrine therapy, depending on the type
of breast cancer diagnosed (6).
At present, anthracyclines and taxanes are the two
major classes of drugs for breast cancer treatment. Anthracyclines
(Table I) are among the most
commonly used and effective drugs in breast cancer treatment. In
the past 30 years, they have become an important component of
adjunctive and palliative therapy for breast cancer. Anthracyclines
belong to a class of antineoplastic antibiotics, which interfere
with cell replication by acting on the DNA at several levels,
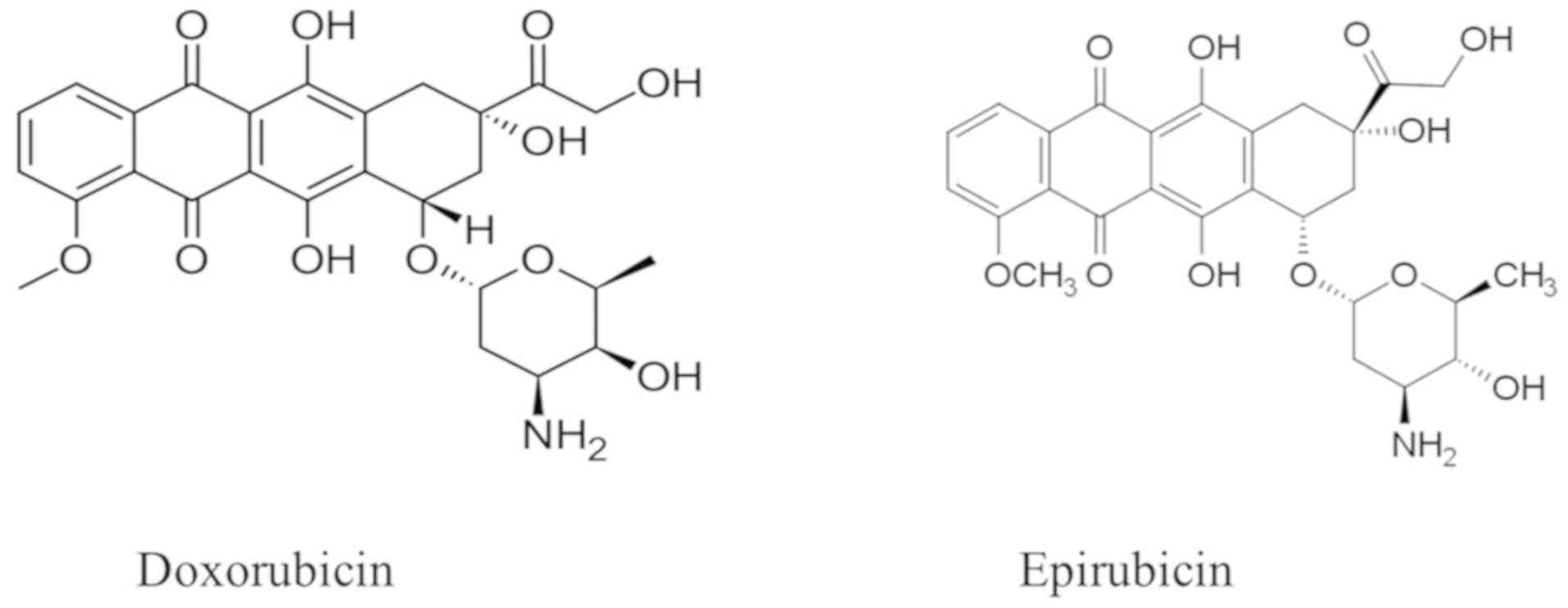
showing an effect in every phase of the cell cycle. Doxorubicin and
epirubicin (Fig. 1) are commonly
used in clinical practice. Administration is only via an
intravenous infusion; metabolism is hepatic and excretion via the
bile route, while urinary elimination accounts for approximately
1/6 of the total amount. Although anthracyclines exhibit a range of
toxic effects, including transient myelosuppression, mucositis and
hair loss, cardiotoxicity still remains a prominent risk since it
may be permanent and progressive, leading to multimorbidity and
severely impacting quality of life in patients with breast cancer.
Acute cardiotoxicities, as well as the potential effect of
cumulative doses, increasing the risk of congestive heart failure,
are crucial and should be considered when deciding on a treatment
strategy. The present study presents a concise review of the
literature, focusing on anthracycline-induced cardiotoxicity, its
pathophysiology, prevention, monitoring and outcomes. A
comprehensive literature review has been conducted. A bibliographic
search was performed in the Cochrane, Medline, PubMed, Scopus, Web
of Science and Scielo databases. Databases were searched
systematically using the following key words: Anthracyclines,
breast cancer, risk factors, prevention and treatment, combined
with cardiotoxicity, cardiomyopathy or heart failure.
 | Table I.Commonly used chemotherapeutics in
the treatment of breast cancer. |
Table I.
Commonly used chemotherapeutics in
the treatment of breast cancer.
| Cancer
treatment | Cardiovascular
adverse effects |
|---|
| Anthracyclines (eg,
epirubicin, doxorubicin, daunorubicin, idarubicin,) | Ventricular
fibrillation, myocarditis, heart failure, ventricular tachycardia,
left ventricular dysfunction, pericarditis, atrial
fibrillation |
| Taxanes (e.g.,
paclitaxel) | Ventricular ectopy,
bradycardia, heart block |
| Alkylating agents
(e.g., cyclophosphamide, cisplatin, mitomycin, ciplastin,
ifosfamide) | Heart failure,
heart block, supraventricular tachycardia, congestive heart
failure, left ventricular dysfunction, bradycardia, atrial
fibrillation, arterial thrombosis, myocarditis, pericarditis |
| Endocrine therapy
(eg, anastrozole, letrozole, tamoxifen) | Thromboembolism,
valvular dysfunction, venous thrombosis, heart failure,
pericarditis, peripheral atherosclerosis, dysrhythmia |
| Cyclin-dependent
kinase 4/6 inhibitors (e.g., ribociclib, palbociclib, lapatinib,
sorafenib, lapatinib) | QTc
prolongation |
| Antimetabolites
(e.g., capecitabine, 5-fluorouracil, cytarabine, methotrexate) | Congestive heart
failure, ventricular tachycardia, coronary artery spasm,
ventricular fibrillation, coronary thrombosis, atrial
fibrillation |
| HER-2–directed
therapies (e.g., pertuzumab, trastuzumab) Radiation therapy | Heart failure, left
ventricular dysfunction, Cardiomyopathy, coronary artery disease,
valvular disease pericar dial disease, arrhythmias |
Mechanism of cardiotoxicity
The pathophysiology of cardiotoxicity induced by
anthracyclines has not been fully elucidated. Until recently, the
most widely accepted hypothesis was that cardiotoxicity by
anthracycline is due to reactive oxygen species (ROS) generated by
the quinone moiety common to all anthracyclines. However, a recent
study by Zhang et al (7)
supports an alternative model, reporting that the toxicity is
caused as anthracyclines block the function of topoisomerase II β
(TopIIB) causing double-stranded DNA breaks, which in turn leads to
activation of p53 tumor-suppressor protein, mitochondrial
dysfunction, and the generation of ROS that result in cardiac cell
death.
Role of reactive oxygen species
The pathological changes include cardiomyocyte
apoptosis or necrosis, loss of myofibrils, expansion of the
sarcoplasmic reticulum and mitochondrial swelling (8). Those can be explained by the mechanism
of action of anthracyclines. In the process of metabolism, the
semiquinone radical of the C-ring is reduced by several single
electron oxidoreductases to form a semiquinone free radical.
Semiquinone free radicals react with oxygen to produce superoxide
anions, and the latter can disproportionate into hydrogen peroxide.
Doxorubicin can also bind to nitrous oxide synthase, which leads to
reactive nitrogen, particularly the production of peroxy-nitrite.
In addition, anthracycline antitumor drugs can also generate free
radicals through non-enzymatic pathways. These reactive oxygen
species and reactive nitrogen can cause mitochondrial functional
damage, energy imbalances, even cardiomyocyte apoptosis. This is
currently the most studied and most widely accepted mechanism of
myocardial injury, but the clinical application of antioxidants to
prevent anthracycline cardiotoxicity has not achieved the desired
results, putting the ‘reactive oxygen and oxidative stress theory’
into question (9).
Some studies also suggest the doxorubicin-induced
cardiotoxicity is mediated by the doxorubicinol (DOXol) metabolite.
A study found that the anthracycline side chain C-13 can be reduced
to doxorubicin doxorubicinol (DOXol) by NADPH-dependent reductase
in vivo (10). The metabolite
still retains cytotoxicity, but can affect energy metabolism in the
heart muscle, changes in ion concentrations and Ca2+
transport, ultimately leading to a decrease in myocardial
contractile function, and therefore DOXol is thought to be involved
in an anthracycline-type cardiotoxicity mechanism. Animal
experiments found that DOXol selectively accumulates in the heart.
At autopsy, in patients treated with doxorubicin, high
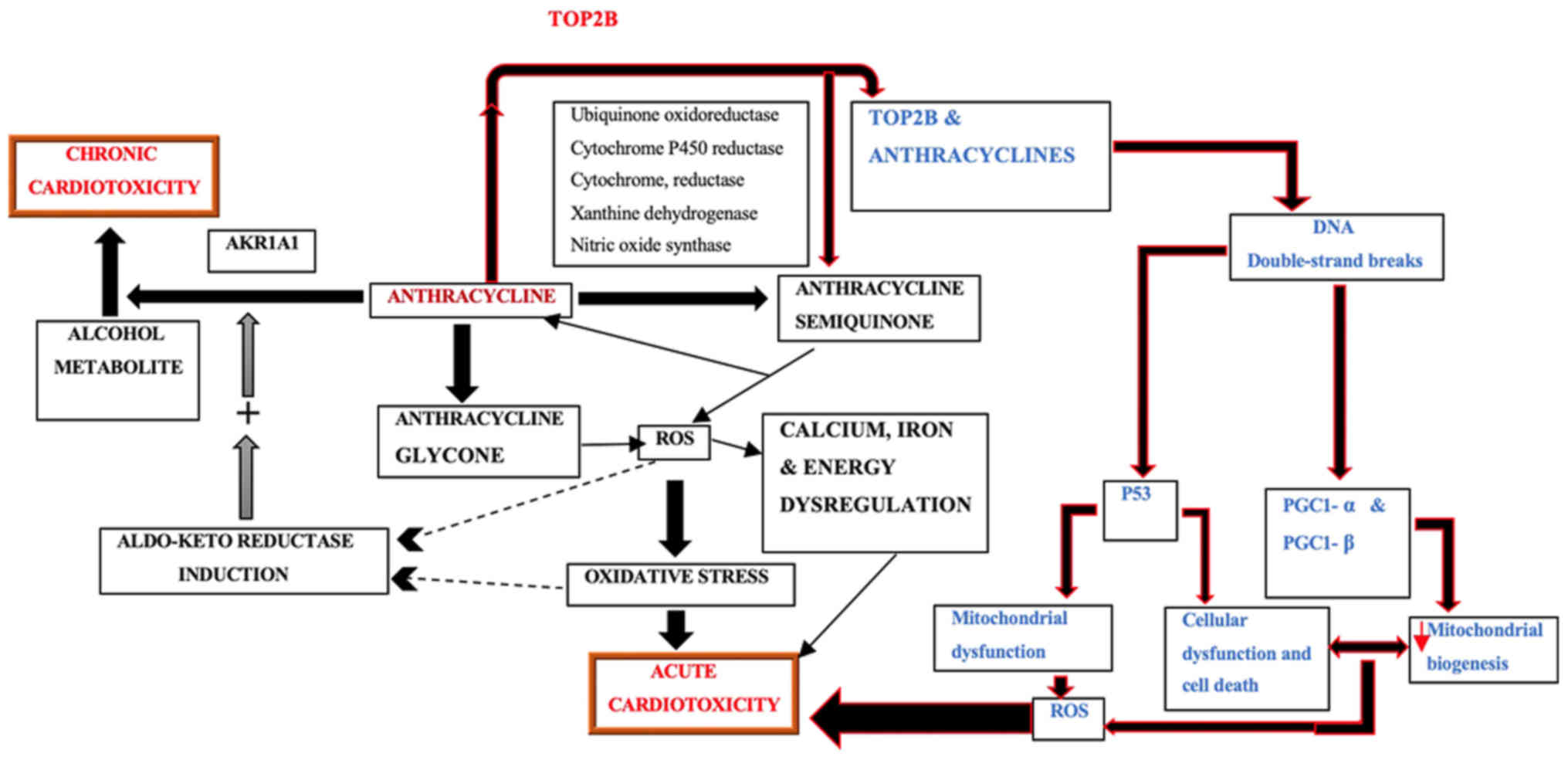
concentrations of DOXol may be found in cardiomyocytes (11). Mordente et al (12) also considered that secondary alcohol
metabolites may play an important role in the development of
anthracycline-induced congestive heart failure and end-stage
cardiomyopathy, and is one of the pathogenic factors of
anthracycline-type cardiotoxicity (Fig.
2). Secondary metabolites are slightly less active at redox
cycling, but markedly more potent at dysregulating calcium and iron
homeostasis. They also produce oxidative stress, ion dysregulation,
and concomitant alterations of cardiac-specific gene expression,
eventually inducing cardiomyopathy.
Some studies also suggest that anticancer drugs,
such as anthracycline, induce cardiotoxicity mediated by the hERG
channel, which is one of several potassium-selective voltage-gated
channels that participate in the control of the electrical activity
of the human heart (13,14). Systemic treatment of cancers with
hERG antagonists may affect cardiac myocytes, resulting in
apoptosis and heart failure (15).
Due to its crucial role in the cardiac action potential, impairment
of hERG channel function can lead to severe cardiac disorders,
manifested by altered QT intervals (13). For instance, inherited
loss-of-function mutations in hERG can cause long QT syndrome,
which predisposes individuals to life-threatening torsades de
pointes arrhythmia (16).
Classification of cardiotoxicity induced by
anthracyclines
The cardiotoxicity induced by these drugs can be
classified as acute, subacute and chronic, which can be further
categorized into type I (early onset) and type II (late onset)
(17,18).
Acute toxicity is rare and reversible, occurring in
the course of administration or within 1 week after administration
as transient arrhythmia, e.g. supraventricular tachycardia,
nonspecific ST segment or T wave abnormality, pericardial
myocarditis syndrome or acute left ventricular failure. The
subacute cardiotoxicity can occur several days to several weeks
after administration, manifesting as acute left heart failure,
myocarditis, and pericarditis. Type I chronic cardiotoxicity
manifests at least one year after the completion of chemotherapy,
mostly years decades after the chemotherapy, mainly as occult
ventricular dysfunction, congestive heart failure, and arrhythmia.
Type II chronic cardiotoxicity is typically caused by novel
biological-targeted antibodies (9,19).
Risk factors for cardiotoxicity induced by
anthracyclines
Cumulative dose
The total cumulative dose of anthracyclines is the
most significant risk factor for cardiac dysfunction (20). There is a clear relationship between
the occurrence of anthracycline cardiotoxicity and the cumulative
dose of the drug. Von Hoff et al (21), in a retrospective analysis, found
that when a patient receives a cumulative dose of doxorubicin at
400, 550 and 700 mg/m2, the incidence of cardiotoxicity
is 3, 7 and 18%, respectively, with dose-limiting toxicity.
Therefore, it is recommended that the cumulative dose of
doxorubicin should not exceed 550 mg/m2. If treated with
epirubicin, the cumulative dose recommendation is to not exceed 900
mg/m2. Ryberg et al (22) performed a risk analysis of 1,097
patients with metastatic breast cancer who had previously been
treated with epirubicin; the maximum cumulative dose for epirubicin
treatment was determined according to the patient's risk level, to
keep the incidence of congestive heart failure below 5%. The
results showed that for patients at an average age of 40, without
other risk factors for congestive heart failure, the recommended
cumulative dose is 806 mg/m2, and an average age of 70,
the maximum cumulative dose is 609 mg/m2.
Age
Von Hoff et al (21) showed that the occurrence of
anthracycline cardiac toxicity was significantly associated with
age. The study included 4,018 patients with a median age of 49, and
the incidence of anthracycline cardiotoxicity increased steadily
with age (P=0.0002). A retrospective analysis by Swain et al
(23) showed that when the
cumulative dosage of doxorubicin reached 400 mg/m2, the
risk of congestive heart failure in patients over 65 years old was
2.25 times higher than that in patients under 65 years old.
For patients of age >65 years, the recommended
dosage ratio of dexrazoxane: Doxorubicin is 10:1; doxorubicin
should be given within 30 min of giving dexrazoxane.
Existing cardiac risk factors
Patients with preexisting cardiac risk factors have
an increased incidence of congestive heart failure when treated
with anthracyclines (24). Studies
by Ryberg et al (22) showed
that patients with cardiac risk factors (such as hypertension,
diabetes, obesity, hyperthyroidism and obstructive lung disease)
had a 3-times greater incidence of cardiac toxicity and were not
affected by the cumulative dose.
Dose reduction reduces the incidence of early
myocardial dysfunction, but does not remove the risk of long-term
problems. A study reported reported that continuous infusions of
free doxorubicin between 48 and 96 h reduce cardiotoxicity
(25). Retrospective analyses have
found that weekly doses of doxorubicin are considerably less
cardiotoxic than administering the drug every 3 weeks.
Radiotherapy
Cardiac radiation exposure markedly increases
myocardial sensitivity to anthracyclines. Concomitant exposure to
cyclophosphamide, bleomycin, vincristine, amsacrine or mitoxantrone
may predispose to cardiotoxicity (26). Black ethnic origin (26) and trisomy 21 (26) increase the risk of early
cardiotoxicity.
An increased incidence of cardiac failure and severe
cardiac abnormalities more frequently occur at longer follow-up
(27).
Cardiotoxicity monitoring
Electrocardiogram (ECG)
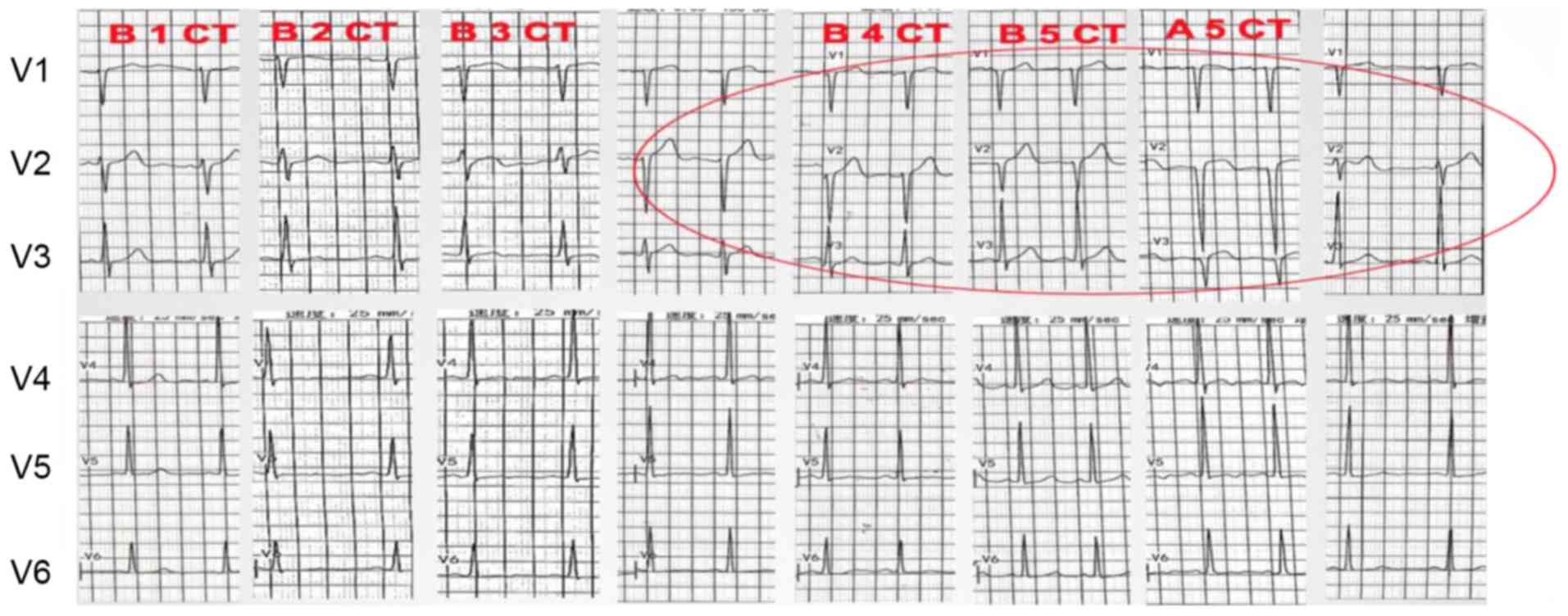
Electrocardiogram changes occur earlier, and are
non-specific, transient and reversible. The most common ECG
abnormalities are ST- and T-wave changes (Fig. 3), sinus tachycardia, QT prolongation,
ventricular premature contractions and transient atrial
contractions. A study showed that prolongation of the QTc interval
predicts a high risk of developing malignant ventricular
arrhythmias (28). ECG is a simple,
convenient and non-invasive method, commonly used for the detection
of cardiotoxicity in clinical practice. However, because it is
affected by many factors, the detection value is not large.
Echocardiography
Echocardiography is a common method for clinical
diagnosis of heart failure, mainly used to detect the left
ventricular ejection fraction (LVEF), and is widely used in
clinical practice because of its non-invasiveness. However,
abnormal changes in left ventricular systolic function or changes
in the morphological structure can only occur when the myocardium
is severely damaged or the overall function of the heart is
impaired (29), and the examination
results are greatly affected by the experience of the sonographer,
which is not conducive to the early detection of lesions. In the
present context, anthracyclines are cardiotoxic and it is necessary
to evaluate the LVEF of a patient every 3 months. If a patient
develops clinical symptoms of cardiotoxicity during anthracycline
treatment, or are asymptomatic with LVEF <45% or a decrease to
the baseline of 15%, then the treatment should be discontinued to
adequately assess the patient's cardiac function, and further
treatment should be administered with caution.
Tissue doppler imaging (TDI)
Tissue doppler imaging (TDI) is an application of
the Doppler effect, based on the traditional color Doppler
technique, for the detection of ventricular muscle, mitral systole
and diastolic frequency, providing ultrasound imaging information
about myocardial motion. Compared with traditional Doppler, the
influence of the cardiac load state is reduced and the result is
more reliable. In a study by Tassan-Mangina et al (30), TDI was able to detect left cardiac
early diastolic and late systolic functional impairment through
follow-up of patients treated with medium-dose anthracycline. If
the isovolumic contraction time at the mitral valve is less than 80
msec, it may indicate late damage to ventricular function. Jurcut
et al (31) studied 16
elderly patients with breast cancer who were treated with liposomal
doxorubicin, and found that the longitudinal and radial
deformations of ventricular systolic phase identified by Doppler
myocardial imaging were comparable to those found using traditional
echocardiography. The strain rate decreased significantly and, in
particular, the radial functional change was more pronounced and
minor lesions to the myocardium were effectively detected.
Radionuclide angiocardiography
Also known as gated multiple gated acquisition
(MUGA), this is a widely used method for detecting the ejection
fraction (21). Mitani et al
(32) found in a retrospective
analysis that, during doxorubicin therapy, using radionuclide
continuous equilibrium monitoring for early detection of the
decrease in LVEF is an effective and economical way of preventing
the development of congestive heart failure. This method has high
reproducibility and can reliably monitor the decrease in LVEF.
However, the accumulation of radiation exposure limits its regular
use. This method is a recommended option when patient treatment or
a clinical trial requires repeated precise testing (33).
Magnetic resonance imaging (MRI)
Cardiac magnetic resonance (CMR) can evaluate heart
morphology, tissue function, metabolism and blood flow changes, can
show the location and extent of myocardial infarction-related edema
and necrosis, and is not affected by cardiac function. Although CMR
is an ideal method for detecting myocardial function and its
damage, repeated examinations are expensive and it is not the
preferred or a routine measure (34).
Endomyocardial biopsy
Cardiac biopsy is currently the most sensitive
indicator of chronic cardiotoxicity induced by anthracycline
treatment (35), but
anthracycline-induced myocardial injury generally presents as
scattered or left ventricular lesions. The majority of biopsy
samples are taken from the right ventricle, which may underestimate
the severity of the lesion. However, cardiac biopsy is not
routinely performed in current practice because of its
invasiveness, so it should not be used as a routine choice in early
monitoring.
Serum biochemical indicators
Cardiac troponin
Cardiac troponin (cTn) is a polypeptide subunit of
the troponin complex. Troponin is an important regulatory protein
involved in muscle contraction and consists of three subunits:
Cardiac troponin C (cTnC), cardiac troponin T (cTnT), and cardiac
troponin (cTnI). When degeneration and necrosis occur in the
myocardium, cTnI and cTnT enter the peripheral blood. Several
studies (36,37) have reported increased levels of
cTnT/TnI in patients treated with anthracycline chemotherapy,
associated with cardiac diastolic dysfunction. cTnT/TnI can detect
early cardiotoxicity caused by anthracyclines such as ADM before
significant LVEF changes occur (38). However, cardiac troponin lacks
specificity in the assessment of cardiotoxicity caused by
anthracycline chemotherapy, and it is necessary to combine other
auxiliary examinations for comprehensive analysis and
diagnosis.
B-type brain natriuretic peptide (BNP)
and N-terminal pro-brain natriuretic peptide (NT-proBNP)
In recent years, BNP and NT-proBNP have garnered
attention in the diagnosis of early cardiac toxicity and have been
gradually applied in clinical practice. A rapid and accurate
indicator of heart failure caused by anthracyclines, BNP contains
32 amino acids, with a signal peptide of 26 amino acids, and has a
half-life of 15 to 2 min. NT-proBNP contains 76 amino acids with a
half-life of 60–120 min (39).
Because NT-proBNP has a long half-life and is stable, it can
accumulate to higher concentrations, so it can diagnose mild
systolic or diastolic heart failure and asymptomatic left
ventricular dysfunction. Zhang et al (40) monitored NT-proBNP in 50 patients with
breast cancer using anthracyclines and found that the value of
NT-proBNP gradually increased with the progress of chemotherapy,
before chemotherapy and after the first course of chemotherapy.
Prevention of cardiotoxicity
Limiting cumulative dose
Maximum doses of anthracyclines have been
implemented. Maximum cumulative doses of 400–550 mg/m2
doxorubicin and 900 mg/m2 epirubicin are currently
recommended (41,42).
Prolonging administration time
Cardiac toxicity can be reduced by weekly low doses
and a prolonged continuous infusion time (24–96 h). An analysis
showed that slow intravenous infusion of doxorubicin (>6 h) over
a prolonged period could reduce the risk of clinical heart failure
and subclinical myocardial injury (43). The cardiotoxicity of the weekly
treatment regimen was less than that of the usual 3-week treatment
regimen (0.8 vs. 2.9%) (44).
Strengthening the monitoring of
cardiotoxicity
During the use of anthracycline drugs, the
monitoring of cardiotoxicity should be strengthened to achieve
early detection and early treatment, to prevent the occurrence of
severe cardiotoxicity. There are currently various reported methods
of monitoring cardiac toxicity: Cardiac endocardium biopsy,
electrocardiogram, echocardiography, myocardial backscatter
integration parameters, tissue Doppler imaging, ischemic modified
albumin, troponin, brain natriuretic peptide and the Tei index.
These monitoring methods have certain limitations, such as
invasiveness and low specificity (45). Therefore, continuous efforts are
needed to find sensitive, specific and non-invasive monitoring
techniques and tools.
Development of liposome
anthracyclines
Liposome anthracycline is used to encapsulate the
drug in a lipid body to protect the drug from degrading and
inactivating in the plasma, and makes use of structural defects,
such as vascular endothelial discontinuity and lymphatic vessel
breakage in the tumor tissue, so that the anthracycline drugs
selectively penetrate the vascular system and enter the tumor
tissue. This reduces the concentration of the drug in the normal
endothelial tissue and can significantly reduce the toxicity to the
heart, while increasing the anti-tumor index and expanding the
antitumor spectrum. Single-use liposome in the treatment of
metastatic breast cancer has the same curative effect as
doxorubicin and the incidence of cardiac toxicity is significantly
lower (13 vs. 29%), the cardiac toxicity occurs later and the
cumulative dose is larger (46).
Oncological strategies to mitigate
cardiotoxicity
Iron chelating agents
Dexrazoxane (DEX), a most promising cardioprotective
agent, has been shown to be effective in reducing both acute and
chronic cardiotoxicity induced by anthracycline therapy (47). It has been widely used in the United
States and Europe for various clinical applications. Dexrazoxane is
mainly used to reduce the incidence and severity of cardiomyopathy
caused by doxorubicin in patients with advanced breast cancer. It
is applicable to patients who receive a dose of 300
mg/m2 of doxorubicin and need to continue to use
doxorubicin. It is only drug approved by the US FDA to protect and
reduce the cardiotoxicity of anthracyclines. Multicenter randomized
controlled clinical trials have shown that dexrazoxane has a
significant cardioprotective effect in breast cancer patients
receiving anthracycline chemotherapy, and does not affect the
antitumor efficacy of anthracyclines (48). Its protection mechanism is mainly
through reducing the production of oxygen free radicals, lipid
peroxidation products and myocardial apoptosis (49).
A multicenter phase II clinical trial in China also
confirmed that dexrazoxane combined with doxorubicin in the
treatment of breast cancer and lymphoma has a significant
protective effect on cardiotoxicity caused by doxorubicin. At the
same time, dexrazoxane not only has a certain protective effect on
the occurrence of cardiotoxicity, but also has a certain reparative
effect on damage already done (50).
The 2011 edition of the Chinese Expert Consensus on the Prevention
of Cardiotoxicity of Anthracycline Anticancer Drugs (51) has also indicated that dexrazoxane has
a cardioprotective effect on patients receiving anthracycline
chemotherapy.
Some authors have suggested that repeated imaging
can be used to detect and assess treatment with iron chelating
agents, especially echocardiography and CMR.
Angiotensin-converting enzyme
inhibitors (ACEI)
These drugs also reduce the cardiotoxicity of
anthracyclines (11). In an early
study, it was concluded that zofenopril has a complete protective
effect on cardiomyocytes from chronic cardiomyopathy caused by
doxorubicin, without affecting the antitumor activity of
doxorubicin (52). A recent rat
experiment also demonstrated that low-dose and high-dose zofenopril
had a complete protective effect against electrophysiological
changes (QT interval prolongation) caused by long-term use of
doxorubicin (1.5 mg/kg qwx5) (53).
β-blockers
Carvedilol is used to treat symptomatic congestive
heart failure, reducing the mortality and hospitalization rates of
cardiovascular disease. Kalay et al (54) demonstrated in a prospective study
that prophylactic use of carvedilol in the treatment of breast
cancer patients treated with anthracyclines protects left
ventricular ejection function.
Antioxidants
Many studies (55–57) have
shown that the most commonly used cardioprotective antioxidants
(such as vitamin E, coenzyme Q10, glutathione and N-acetylcysteine)
are not ideal. Flavonoids (especially monoHER), while having
antioxidant effects, also have iron chelation and carbonyl
reductase inhibition, showing advantages in preventing the
cardiotoxicity of anthracyclines while not affecting their
anti-tumor activity (10). The
monoHER derivative frederine has a complete protective effect in
the rat heart, and its effective dose is only 1/5 of that of
monoHER, although monoHER is more likely to be applied in the
clinic (58).
Treatment of cardiotoxicity
In the early detection of anthracycline-induced
cardiotoxicity symptoms, such as mild arrhythmia, atrial
fibrillation, pericarditis, etc., anthracyclines should be
discontinued by switching to a chemotherapy regimen without
anthracyclines, and the necessary symptomatic treatment should be
given.
When patients have symptoms of congestive heart
failure, anti-heart failure treatments, such as converting enzyme
inhibitors (ACEI), diuretics and β-blockers, can be given. A recent
study showed that administration of ACEI or a combination of
beta-blockers at the early detection of an anthracycline-induced
cardiac insufficiency restored LVEF and reduced cardiac events
(59).
Future directions to avoid cardiotoxicity
induced by anthracyclines
There is no definitive and effective treatment for
dose-dependent cardiotoxicity caused by anthracyclines. The
cardiotoxicity of anthracyclines, in addition to factors related to
the patient and the drug itself, is also associated with
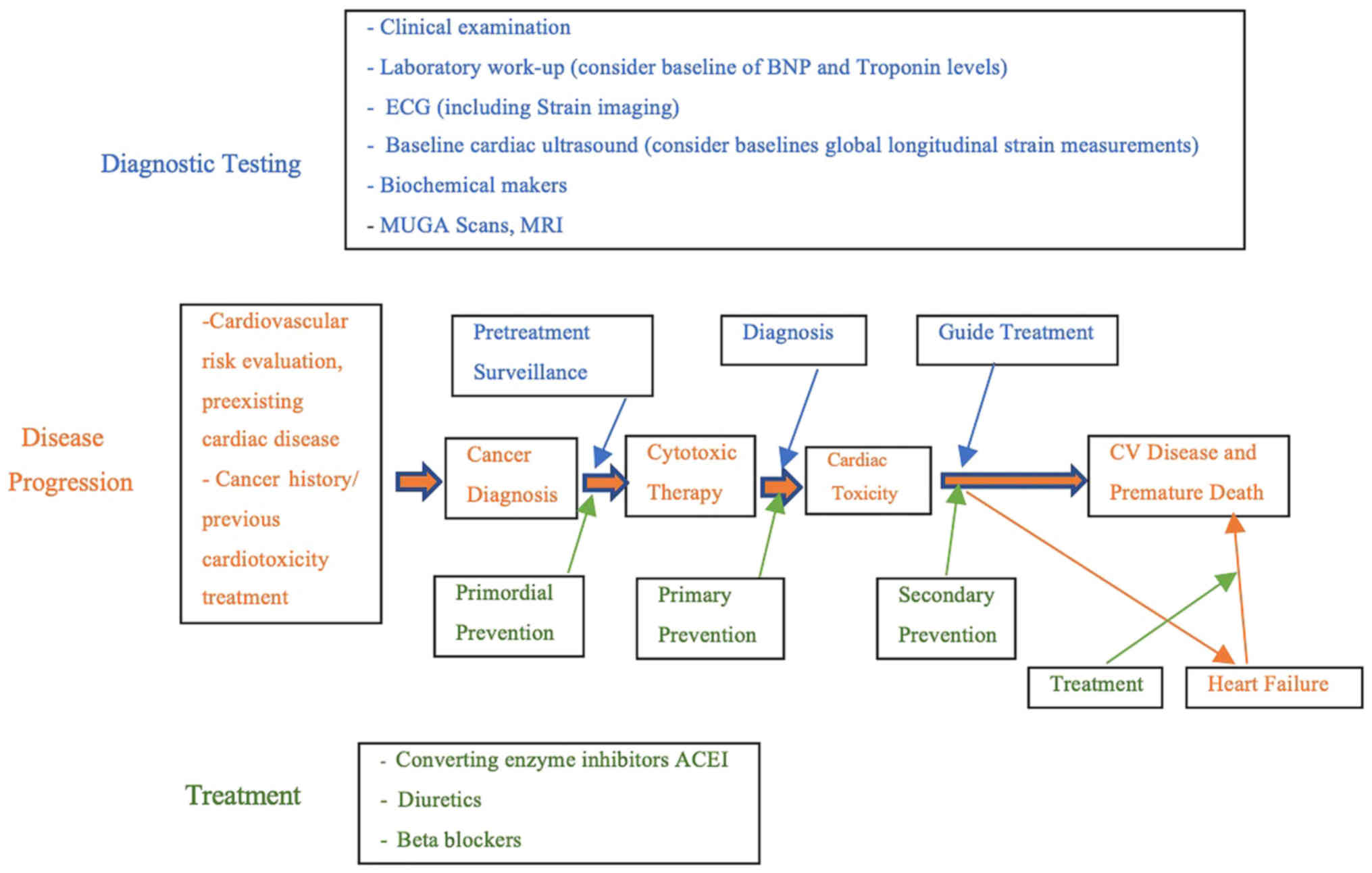
insufficient clinical attention. Monitoring and controlling heart
toxicity caused by anthracyclines requires close cooperation
between oncologists and cardiovascular physicians (18). It is necessary to establish a
treatment monitoring specification for cardiotoxicity in breast
cancer patients, and to fully assess the potential risks of
treatment before it is started, in order to comprehensively
understand patient heart function and tumor status, and to fully
communicate with patients to minimize the risk of heart failure
(Fig. 4).
In conclusion, further understanding of the
mechanisms, monitoring methods, and preventive measures against
cardiotoxicity caused by anthracyclines is of great significance
for the early prevention of the cardiotoxicity of
anthracyclines.
Conclusion
The current research on anthracycline-induced
cardiotoxicity is still unclear and there is no effective
prevention method. Anthracyclines are one of the most important
drugs in the treatment of breast cancer. Therefore, while using
these drugs, the risk factors associated with cardiotoxicity should
be considered, cardiac function should be accurately measured and
the cumulative dosage should be limited; otherwise, a new
anthracycline alternative or liposome may be used to reduce
anthracycline-induced cardiotoxicity. The clinician should develop
a rational chemotherapy regimen based on individual differences and
general conditions, and closely monitor the cardiotoxicity response
induced by chemotherapeutic agents, in combination with various
other examinations. Prevention of chronic cardiotoxicity is
difficult, but regimens for the administration of the
anthracyclines using prolonged infusion carry a lower risk and new
liposomal preparations of doxorubicin offer less cardiotoxicity
than traditional doxorubicin. The cardiac protectant dexrazoxane is
effective in some clinical settings.
The treatment of anthracycline-induced heart failure
is not well studied; it is likely that treatment should be similar
to that for other types of heart failure.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Scientific
Research Foundation for the Returned Overseas Chinese Scholars,
State Education Ministry (grant no. 2015-311), The Shanghai Health
and Family Planning Commission Project (grant nos. 20134298,
201640253 and 201640226), The Shanghai Health and Family Planning
Commission Fund for Qing Nian Yi Shi Training Project (grant no.
2014118), The Shanghai Yangpu District Science and Technology
Commission Project (grant nos. 2016-2017, YP17ZM02 and 2017106),
the Shanghai Yangpu District Health and Family Planning Commission
Project (grant nos. 2011-2013 and 2016-2017, YP17ZM02, 2017106),
the Shanghai Yangpu District Health and Family Planning Commission
Fund for Hao Yi Shi Training Project (grant no. 201742), The
Science Program from Yangpu Center Hospital (grant no. SE1201746),
The Natural Science Foundation of Shanghai (grant no. 18ZR1436000)
and The Fundamental Research Funds for the Central Universities
(grant no. 22120180286).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FC reviewed the published literature, wrote the
manuscript, reviewed and edited the final manuscript. MAFL reviewed
the published literature, wrote sections of the manuscript and the
reference section, and edited the final manuscript. XL reviewed the
published literature, wrote and edited the reference section, and
edited the final manuscript. MW reviewed the published literature,
reviewed and edited the final manuscript. LC, CC and EB reviewed
the published literature, wrote the manuscript, reviewed and edited
the final manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Albini A, Pennesi G, Donatelli F,
Cammarota R, De Flora S and Noonan DM: Cardiotoxicity of anticancer
drugs: The need for cardio-oncology and cardio-oncological
prevention. J Natl Cancer Inst. 102:14–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
SEER Cancer Stat Facts: Female Breast
Cancer. National Cancer Institute; Bethesda, MD: 2016, http://seer.cancer.gov/statfacts/html/breast.htmlApril.
2019
|
|
3
|
Canadian Cancer Society's Advisory
Committee on Cancer Statistics: Canadian cancer statistics 2016.
Canadian Cancer Society. (Toronto, ON). 2016.
|
|
4
|
Cai FF, Kohler C, Zhang B, Wang MH, Chen
WJ and Zhong XY: Epigenetic therapy for breast cancer. Int J Mol
Sci. 12:4465–4487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alvarez RH: Present and future evolution
of advanced breast cancer therapy. Breast Cancer Res. 12 (Suppl
2):S12010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu
YL, Liu LF and Yeh ET: Identification of the molecular basis of
doxorubicin-induced cardiotoxicity. Nat Med. 18:1639–1642. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitry MA and Edwards JG: Doxorubicin
induced heart failure: Phenotype and molecular mechanisms. Int J
Cardiol Heart Vasc. 10:17–24. 2016.PubMed/NCBI
|
|
9
|
Tocchetti CG, Cadeddu C, Di Lisi D,
Femminò S, Madonna R, Mele D, Monte I, Novo G, Penna C, Pepe A, et
al: From molecular mechanisms to clinical management of
antineoplastic drug-induced cardiovascular toxicity: A
translational overview. Antioxid Redox Signal. May 15–2017.(Epub
ahead of print). PubMed/NCBI
|
|
10
|
Kaiserová H, Simůnek T, van der Vijgh WJ,
Bast A and Kvasnicková E: Flavonoids as protectors against
doxorubicin cardiotoxicity: Role of iron chelation, antioxidant
activity and inhibition of carbonyl reductase. Biochim Biophys
Acta. 1772:1065–1074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stewart DJ, Grewaal D, Green RM, Mikhael
N, Goel R, Montpetit VA and Redmond MD: Concentrations of
doxorubicin and its metabolites in human autopsy heart and other
tissues. Anticancer Res. 13:1945–1952. 1993.PubMed/NCBI
|
|
12
|
Mordente A, Meucci E, Silvestrini A,
Martorana GE and Giardina B: New developments in
anthracycline-induced cardiotoxicity. Curr Med Chem. 16:1656–1672.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanguinetti MC and Tristani-Firouzi M:
hERG potassium channels and cardiac arrhythmia. Nature.
440:463–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vandenberg JI, Perry MD, Perrin MJ, Mann
SA, Ke Y and Hill AP: hERG K(+) channels: Structure, function, and
clinical significance. Physiol Rev. 92:1393–1478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jehle J, Schweizer PA, Katus HA and Thomas
D: Novel roles for hERG K(+) channels in cell proliferation and
apoptosis. Cell Death Dis. 2:e1932011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Curran ME, Splawski I, Timothy KW, Vincent
GM, Green ED and Keating MT: A molecular basis for cardiac
arrhythmia: HERG mutations cause long QT syndrome. Cell.
80:795–803. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jaworski C, Mariani JA, Wheeler G and Kaye
DM: Cardiac complications of thoracic irradiation. J Am Coll
Cardiol. 61:2319–2328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jain D, Russell RR, Schwartz RG, Panjrath
GS and Aronow W: Cardiac complications of cancer therapy:
Pathophysiology, identification, prevention, treatment, and future
directions. Curr Cardiol Rep. 19:362017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang G and Liu S: Research progress in
cardiotoxicity of antitumor chemotherapy drugs. J Oncol.
21:1010–1014. 2015.
|
|
20
|
Manrique CR, Park M, Tiwari N, Plana JC
and Garcia MJ: Diagnostic strategies for early recognition of
cancer therapeutics-related cardiac dysfunction. Clin Med Insights
Cardiol. 11:11795468176979832017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Von Hoff DD, Layard MW, Basa P, Davis HL
Jr, Von Hoff AL, Rozencweig M and Muggia FM: Risk factors for
doxorubicin-induced congestive heart failure. Ann Intern Med.
91:710–717. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryberg M, Nielsen D, Cortese G, Nielsen G
and Andersen PK: New insight into epirubicin cardiac toxicity:
Competing risks analysis of 1097 breast cancer patients. J Natl
Cancer Inst. 100:1058–1067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Swain SM, Whaley FS and Ewer MS:
Congestive heart failure in patients treated with doxorubicin: A
retrospective analysis of three trials. Cancer. 97:2869–2879. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tromp J, Steggink LC, Van Veldhuisen DJ,
Gietema JA and van der Meer P: Cardio-oncology: Progress in
diagnosis and treatment of cardiac dysfunction. Clin Pharmacol
Ther. 101:481–490. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hortobagyi GN, Frye D, Buzdar AU, Ewer MS,
Fraschini G, Hug V, Ames F, Montague E, Carrasco CH, Mackay B, et
al: Decreased cardiac toxicity of doxorubicin administered by
continuous intravenous infusion in combination chemotherapy for
metastatic breast carcinoma. Cancer. 63:37–45. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
EBCTCG (Early Breast Cancer Trialists'
Collaborative Group), . Effect of radiotherapy after mastectomy and
axillary surgery on 10-year recurrence and 20-year breast cancer
mortality: Meta-analysis of individual patient data for 8135 women
in 22 randomised trials. Lancet. 383:2127–2135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pein F, Sakiroglu O, Dahan M, Lebidois J,
Merlet P, Shamsaldin A, Villain E, de Vathaire F, Sidi D and
Hartmann O: Cardiac abnormalities 15 years and more after
Adriamycin therapy in 229 childhood survivors of a solid tumors at
the Institute Gustave Roussy. Brit J Cancer. 91:37–44. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmitz KH, Prosnitz RG, Schwartz AL and
Carver JR: Prospective surveillance and management of cardiac
toxicity and health in breast cancer survivors. Cancer. 118 (Suppl
8):S2270–S2276. 2012. View Article : Google Scholar
|
|
29
|
Lang RM, Badano LP, Mor-Avi V, Afilalo J,
Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA,
Kuznetsova T, et al: Recommendations for cardiac chamber
quantification by echocardiography in adults: An update from the
American society of echocardiography and the European association
of cardiovascular imaging. J Am Soc Echocardiogr. 28:1–39.e14.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tassan-Mangina S, Codorean D, Metivier M,
Costa B, Himberlin C, Jouannaud C, Blaise AM, Elaerts J and
Nazeyrollas P: Tissue Doppler imaging and conventional
echocardiography after anthracycline treatment in adults: Early and
late alterations of left ventricular function during a prospective
study. Eur J Echocardiogr. 7:141–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jurcut R, Wildiers H, Ganame J, D'hooge J,
De Backer J, Denys H, Paridaens R, Rademakers F and Voigt JU:
Strain rate imaging detects early cardiac effects of pegylated
liposomal doxorubicin as adjuvant therapy in elderly patients with
breast cancer. J Am Soc Echocardiogr. 21:1283–1289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mitani I, Jain D, Joska TM, Burtness B and
Zaret BL: Doxorubicin cardiotoxicity: Prevention of congestive
heart failure with serial cardiac function monitoring with
equilibrium radionuclide angio-cardiography in the current era. J
Nucl Cardiol. 10:132–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Royen N, Jaffe CC, Krumholz HM,
Johnson KM, Lynch PJ, Natale D, Atkinson P, Deman P and Wackers FJ:
Comparison and reproducibility of visual echocardiographic and
quantitative radionuclide left ventricular ejection fractions. Am J
Cardiol. 77:843–850. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Armstrong AC, Gidding S, Gjesdal O, Wu C,
Bluemke DA and Lima JA: LV mass assessed by echocardiography and
CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc
Imaging. 5:837–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ng R, Better N and Green MD: Anticancer
agents and cardiotoxicity. Semin Oncol. 33:2–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McGowan JV, Chung R, Maulik A, Piotrowska
I, Walker JM and Yellon DM: Anthracycline chemotherapy and
cardiotoxicity. Cardiovasc Drugs Ther. 31:63–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Herman EH, Zhang J, Lipshultz SE, Rifai N,
Chadwick D, Takeda K, Yu ZX and Ferrans VJ: Correlation between
serum levels of cardiac troponin-T and the severity of the chronic
cardiomyopathy induced by doxorubicin. J Clin Oncol. 17:2237–2243.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lipshultz SE, Rifai N, Sallan SE, Lipsitz
SR, Dalton V, Sacks DB and Ottlinger ME: Predictive value of
cardiac troponin T in pedriatic patirnts at risk for myocardial
injury. Circulation. 96:2641–2648. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Porcel JM: Utilization of B-type
natriuretic peptide and NT-proBNP in the diagnosis of pleural
effusions due to heart failure. Curr Opin Pulm Med. 17:215–219.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang N, Zheng L, Zheng J, et al:
Significance of N-terminal pro-brain natriuretic peptide in early
monitoring of anthracycline chemotherapy in elderly patients with
breast cancer. Chin J Gerontol. 6:2931–2933. 2014.
|
|
41
|
Angelis AD, Cappetta D, Berrino L and
Urbanek K: Doxorubicin cardiotoxicity: Multiple targets and
translational perspectives. IntechOpen. https://www.intechopen.com/books/cardiotoxicity/doxorubicin-cardiotoxicity-multiple-targets-and-translational-perspectivesNovember
14–2008
|
|
42
|
De Angelis A, Urbanek K, Cappetta D,
Piegari E, Pia Ciuffreda L, Rivellino A, Russo R, Esposito G, Rossi
F and Berrino L: Doxorubicin cardiotoxicity and target cells: A
broader perspective. Cardiooncology. 2:22016.
|
|
43
|
van Dalen EC, van der Pal HJ, Caron HN and
Kremer LC: Different dosage schedules for reducing cardiotoxicity
in cancer patients receiving anthracycline chemotherapy. Cochrane
Database Syst Rev. CD0050082009.PubMed/NCBI
|
|
44
|
Torti FM, Bristow MR, Howes AE, Aston D,
Stockdale FE, Carter SK, Kohler M, Brown BW Jr and Billingham ME:
Reduced cardiotoxicity of doxorubicin delivered on a weekly
schedule: Assessment by endomyocardial biopsy. Ann Intern Med.
99:745–749. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Koene RJ, Prizment AE, Blaes A and Konety
SH: Shared risk factors in cardiovascular disease and cancer.
Circulation. 133:1104–1114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Harris L, Batist G, Belt R, Rovira D,
Navari R, Azarnia N, Welles L and Winer E; TLC D-99 Study Group, :
Liposome-encapsulated doxorubicin compared with conventional
doxorubicin in a randomized multicenter trial as first-line therapy
of metastastic breast carcinoma. Cancer. 94:25–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: Molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Van Dalen EC, Caron HN, Dickinson HO and
Kremer LC: Cardioprotective interventions for cancer patients
receiving anthracyclines. Cochrane Database Syst Rev.
CD0039172008.PubMed/NCBI
|
|
49
|
Che FF, Liu Y and Xu CG: Study on the
effect and mechanism of dextromethionine on cardiotoxicity induced
by doxorubicin. J Sichuan Univ. 41:24–28. 2010.
|
|
50
|
Gao X, Han Z and Du X: Observation of the
effects of dextromethine on cardiotoxicity induced by epirubicin.
Chin J Cancer Prev Treat. 17:296–298. 2010.
|
|
51
|
Professional Committee of Breast Cancer of
China Anti-Cancer Association, . Basic principles of chemotherapy
for recurrent and metastatic breast cancer. Chin J Med. 9:73–75.
2011.
|
|
52
|
Sacco G, Bigioni M, Evangelista S, Goso C,
Manzini S and Maggi CA: Cardioprotection effects of zofenopril, a
new angiotension-converting enzyme inhibitor, on
doxorubicin-induced cardiotoxicity in the rat. Eur J Pharmacol.
414:71–78. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sacco G, Mario B, Lopez G, Evangelista S,
Manzini S and Maggi CA: ACE inhibition and protection from
doxorubicin-induced cardiotoxicity in the rat. Vascul Pharmacol.
50:166–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kalay N, Basar E, Ozdogru I, Er O,
Cetinkaya Y, Dogan A, Inanc T, Oguzhan A, Eryol NK, Topsakal R and
Ergin A: Protective effects of carvedilol against
anthracycline-induced cardiomyopathy. J Am Coll Cardiol.
48:2258–2262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Elbaky NAA, El-Orabi NF, Fadda LM,
Abd-Elkader OH and Ali HM: Role of N-acetylcysteine and coenzyme
Q10 in the amelioration of myocardial energy expenditure and
oxidative stress, induced by carbon tetrachloride intoxication in
rats. Dose-Response. 16:15593258187901582018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Baker WL, Anglade MW, Baker EL, White CM,
Kluger J and Coleman CI: Use of N-acetylcysteine to reduce
post-cardiothoracic surgery complications: A meta-analysis. Eur J
Cardiothorac Surg. 35:521–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kumar A, Kaur H, Devi P and Mohan V: Role
of coenzyme Q10 (CoQ10) in cardiac disease, hypertension, and
meniere-like syndrome. Pharmacol Ther. 124:259–268. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
van Acker FA, Boven E, Kramer K, Haenen
GR, Bast A and van der Vijgh WJ: Frederine, a new and promising
protector against doxorubicin-induced cardiotoxicity. Clin Cancer
Res. 7:1378–1384. 2001.PubMed/NCBI
|
|
59
|
Mehta LS, Watson KE, Barac A, Beckie TM,
Bittner V, Cruz-Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T, et
al: Cardiovascular disease and breast cancer: Where these entities
intersect: A scientific statement from the American heart
association. Circulation. 137:e30–e66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Khouri MG, Douglas PS, Mackey JR, Martin
M, Scott JM, Scherrer-Crosbie M and Jones LW: Cancer
therapy-induced cardiac toxicity in early breast cancer: Addressing
the unresolved issues. Circulation. 126:2749–2763. 2012. View Article : Google Scholar : PubMed/NCBI
|