Introduction
Lung cancer is the most common malignant tumor
worldwide, with increasing annual incidence and mortality trends
(1). Non-small-cell lung cancer
(NSCLC) accounts for 80–85% of all lung cancers (2). People diagnosed with NSCLC have a low
5-year survival rate (<15%) (3)
and 30–40% patients with locally advanced or metastatic cancer are
not eligible for radical operation (4).
Epidermal growth factor receptor (EGFR) gene
mutations frequently occur in exons 18–21, while deletions in exon
19 and a mutation in exon 21 (specifically the L858R point
mutation) occur during EGFR-tyrosine kinase inhibitor (TKI)
treatment (5). Among the
non-selective Chinese patients with NSCLC, the total rate of EGFR
mutations is ~30% (6). In the
patients with adenocarcinoma, the total rate of EGFR mutations is
50% and, in non-smoking patients with adenocarcinoma, it was 60–70%
(6). EGFR-TKI, a small molecule drug
that is in competition with ATP, binds to the tyrosine kinase of
EGFRs on the cell membrane. Consequently, EGFR-TKI can prevent
tyrosine phosphorylation and inhibit a series of signaling pathways
associated with the formation, proliferation and apoptosis of
malignant cells, so as to inhibit the proliferation of tumor cells
(7).
The existing literature revealed that the EGFR
mutation status (deletions in exon 19 and an exon 21 mutation) was
an important index for predicting the effectiveness of the EGFR-TKI
in the treatment of NSCLC, which is the current standard first-line
treatment (8,9). However, there are only a small number
of reports on the association between the two different mutation
statuses and the response and survival rates following treatment
(10).
The aim of the present study was to demonstrate
that, NSCLC patients with EGFR exon deletions survive longer
following EGFR-TKI treatment than those with exon 21 mutitions.
Materials and methods
Eligibility criteria
The eligibility criteria were the following: i)
Outpatients and inpatients diagnosed with advanced NSCLC (stage
IIIB or IV) who had EGFR mutations (confirmed using second
generation sequencing or the amplification refractory mutation
system method) were enrolled in the Xuzhou Cancer Hospital (Xuzhou,
China) between 1 January 2008 and 31 December 2013 [cancer
classification was done according to the American Joint Committee
on Cancer lung cancer staging criteria (Seventh Edition)] and were
treated with the first-line EGFR-TKI treatment (11); ii) Aged between 18 and 75 years old;
iii) A Eastern Cooperative Oncology Group performance status (PS)
score of ≤2 (12). All the patients
were screened for EGFR gene expression using second generation
sequencing or the amplification refractory mutation system method.
The samples were analyzed using histological approach and iv) The
presence of ≤1 measurable lesion, according to the Response
Evaluation Criteria in Solid Tumors (RECIST) 1.1
guidelines, which was defined as the target lesions measurement
(13).
The detection of EGFR mutation by ARMS-PCR was
performed as follows: Tissues were paraffin embedded and sliced
into 10 µm thick sections. Biopsy samples were stored for a maximum
of 3 years. A microscope was utilized to observe the sections and
to determine the tumor tissue content, the location of which was
then marked. Tumor cells were then enriched to remove the effect of
normal cells on the test results. Subsequently, commercial kits
were utilized to extract human genomic DNA. Extracted DNA was then
determined using a UV spectrophotometer. The optical density (OD)
and concentration of DNA OD260/OD280 was required to be in the
range of 1.8–2.0 and 3–300 ng/µl, respectively. Samples with a
quality out of these ranges were discarded. Extracted DNA was then
immediately tested or stored below −20°C for <6 months. The
negative and positive controls were then set. The positive control
was required to exhibit a typical amplification curve. According to
the Ct value of different fluorescent signal channels, the sample
test results were analyzed to determine whether or not the EGFR
mutation was present.
Medical records and efficacy
evaluation
Treatment plan
A total of 72 patients with advanced NSCLC (IIIB/IV)
who had EGFR mutations (exon 19 deletions or a mutation in exon 21)
were subjected to first-line EGFR-TKI treatment. This treatment
consisted 250 mg/day gefitinib or 150 mg/day erlotinib administered
orally.
Efficacy evaluation
To evaluate the curative effect, the type of
response [either complete response (CR), partial response (PR),
stable disease (SD) or progression disease (PD)], the objective
response rate (ORR) of CR and PR patients, and the disease control
rate (DCR) of CR, PR and SD patients were determined 3 months after
treatment. These criteria were examined according to the
RECIST 1.1 guidelines. Progression-free survival (PFS) was
defined as the duration between treatment initiation and the start
of disease progression or mortality (all-cause). Overall survival
(OS) was defined as the interval between the initiation of
medication and mortality (all-cause) or the end of the follow-up
period. Adverse drug reactions were evaluated according to the
National Cancer Institute Common Terminology Criteria for Adverse
Events (14). Other characteristics
assessed were as follows: Sex, age, histological type, clinical
stage, PS score, smoking history, name of EGFR-TKI administered,
whether the patients received subsequent surgical treatment or
chemotherapy. Non-smokers were defined as patient who smoked ≤100
cigarettes during their lifetime. During the first two months of
EGFR-TKI therapy, all patients underwent imaging examinations as
detailed below, every 8±1 weeks. Patients underwent chest, abdomen
and pelvic computer tomography scans, and brain magnetic resonance
imaging every 3 months until disease progression.
Follow-up
All the patients were regularly followed up by the
telephone every 3 months. The last follow-up was performed 18
months after the last recruitment date.
Statistical analysis
SPSS 17.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analyses. Categorical variables
were reported as numbers and percentages, and continuous variables
were reported as the mean ± standard deviation. Categorical
variables were compared using a chi-square test. The association
between EGFR mutation status, clinical characteristics and the
effect of EGFR-TKI was tested by χ2; PFS and OS were
analyzed with the log-rank test and Cox regression analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patients with deletions in exon 19
have similar clinical characteristics as patients with a mutation
in exon 21
From a total of 72 patients, 37 cases were subjected
to first-line oral gefitinib treatment and 35 to erlotinib; 20
cases were males and 52 cases were females (Table I). Among these cases, patients ≥70
years old accounted for ~51.4%. In addition, 61 cases were
diagnosed with adenocarcinoma and 11 with squamous cell carcinoma.
A total of 33 cases were in the IIIB stage and 39 in the IV stage;
31 cases had PS scores of 0 or 1 and 41 had PS scores of 2. There
were a total of 15 smokers and 57 non-smokers. No statistical
differences in these characteristics were identified between the
exon 19 deletions and exon 21 mutation groups.
 | Table I.Clinical characteristics of 72
patients with advanced non-small cell lung cancer carrying EGFR
mutation. |
Table I.
Clinical characteristics of 72
patients with advanced non-small cell lung cancer carrying EGFR
mutation.
| Clinical
characteristics | Exon 19 deletions
(n=37) | An exon 21 mutation
(n=35) | P-value |
|---|
| Sex |
|
| 0.884 |
| Male | 10 (27.0) | 10 (28.6) |
|
|
Female | 27 (73.0) | 25 (71.4) |
|
| Age (years) |
|
| 0.995 |
|
<70 | 18 (48.6) | 17 (48.6) |
|
| ≥70 | 19 (51.4) | 18 (51.4) |
|
| Histological
type |
|
| 0.820 |
|
Adenocacinoma | 31 (83.8) | 30 (85.7) |
|
|
Squamous | 6 (16.2) | 5 (14.3) |
|
| Clinical stage |
|
| 0.984 |
| IIIB | 17 (45.9) | 16 (45.7) |
|
| IV | 20 (54.1) | 19 (54.3) |
|
| PS score |
|
| 0.974 |
| 0 or
1 | 16 (43.2) | 15 (42.9) |
|
| 2 | 21 (56.8) | 20 (57.1) |
|
| Smoking history |
|
| 0.866 |
|
Smoker | 8 (21.6) | 7 (20.0) |
|
|
Non-smoker | 29 (78.4) | 28 (80.0) |
|
| EGFR-TKI |
|
| 0.642 |
|
Gefitinib | 20 (54.1) | 17 (48.6) |
|
|
Erlotinib | 17 (45.9) | 18 (51.4) |
|
| Sequential
surgery |
|
| 0.968 |
|
Surgery | 1 | 1 |
|
|
Non-surgery | 36 | 34 |
|
| Sequential
chemotherapy |
|
| 0.925 |
|
Chemotherapy | 5 | 32 |
|
|
Non-chemotherapy | 5 | 30 |
|
Patients with deletions in exon 19
exhibit an improved response and survival rate compared with
patients that possess a mutation in exon 21
The curative effect among 72 patients was evaluated;
there were 2 cases of CR, 44 cases of PR, 11 cases of SD and 15
cases of PD, with an ORR of 44% and DCR of 72% (Table II). Furthermore, the patients with
deletions in exon 19 of EGFR included 1 case of CR, 27 cases of PR,
5 cases of SD, 4 cases of PD and an ORR of 75.7%, while patients
with a mutation in exon 21 of EGFR included 1 case of CR, 17 cases
of PR, 6 cases of SD, 11 cases of PD and an ORR of 51.4%. The ORR
of patients with deletions in exon 19 was significantly higher
compared with the patients with a mutation in exon 21
(χ2=4.583; P=0.032). The patients with deletions in exon
19 had a DCR of 89.2%, while those with a mutation in exon 21 had a
DCR of 68.6%. The DCR of patients with deletions in exon 19 was
significantly higher compared with the patients with a mutation in
exon 21 (χ2=4.686; P=0.031).
 | Table II.Efficacy of EGFR-TKI treatment. |
Table II.
Efficacy of EGFR-TKI treatment.
| Group | CR | PR | SD | PD | ORR (%) | P-value | DCR (%) | P-value |
|---|
| Exon 19
deletions | 1 | 27 | 5 | 4 | 75.7 | 0.032 | 89.2 | 0.031 |
| An exon 21
mutation | 1 | 17 | 6 | 11 | 51.4 | 68.6 |
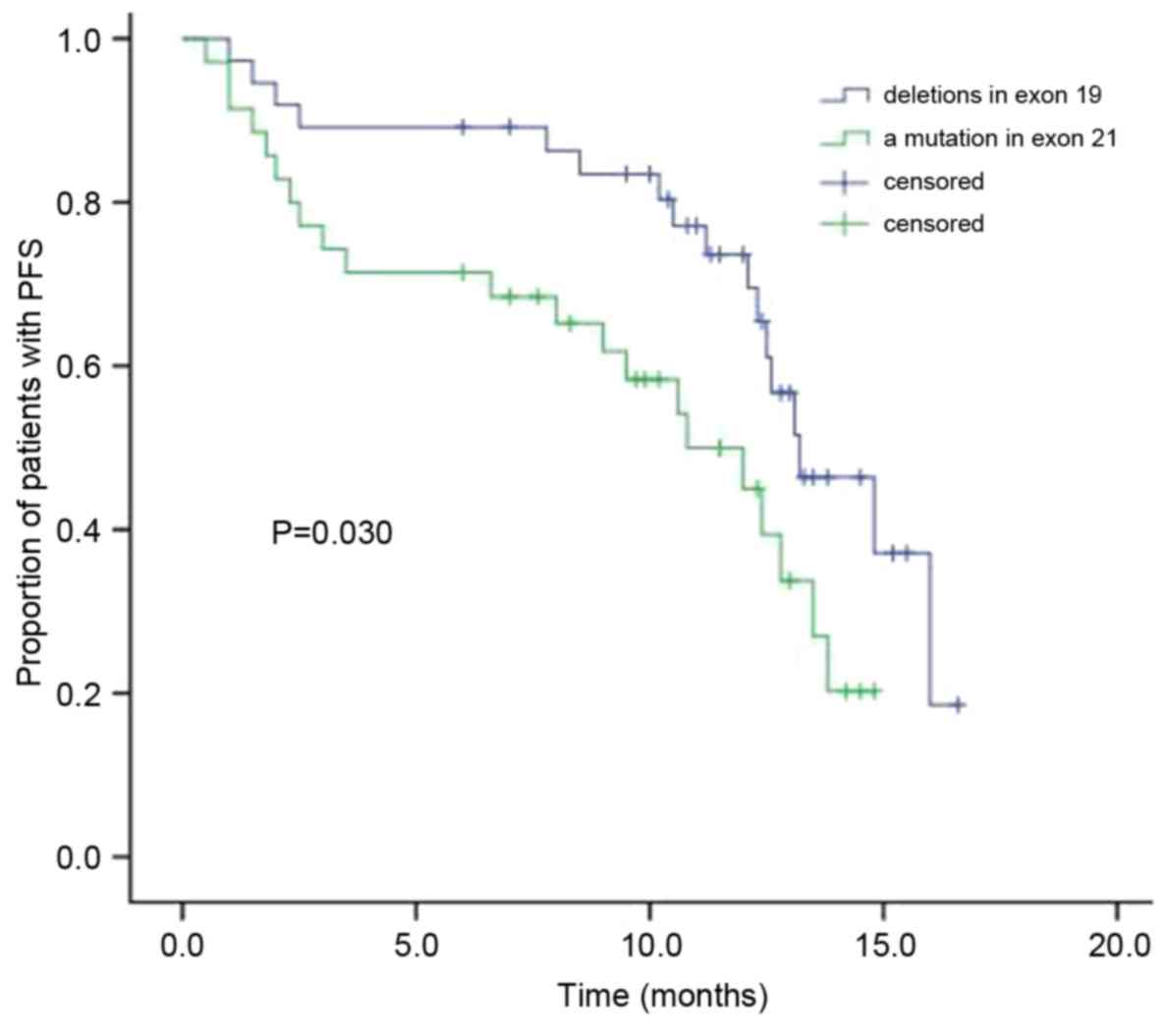
Following EGFR-TKI treatment, the modified median
PFS in the patients with NSCLC who have deletions in exon 19 was
13.2 months, while it was 10.8 months in patients with a mutation
in exon 21 (Fig. 1). The difference
between the two groups' PFS was statistically significant
(χ2=4.700; P=0.030). Furthermore, significant
differences in the PFS were observed between the sexes, patients
with adenocacinoma or squamous, smoking and non-smoking patients,
and patients with mutations in exons 19 and 21 of EGFR (Table III). The results revealed that the
median PFS in females (P=0.009), patients with deletions in exon 19
(P<0.001), patients with adenocarcinoma (P=0.004) and
non-smoking patients (P=0.046) were significantly higher compared
with the median PFS in males, patients with a mutation in exon 21,
patients with squamous cell carcinoma and smoking patients,
respectively. No statistically significant differences in PFS were
observed in relation to the patients' age, tumor stage, PS score
and whether the patient had received gefitinib or erlotinib.
 | Table III.Cox multivariate analysis of PFS and
clinical characteristics among 72 patients with advanced non-small
cell lung cancer carrying EGFR mutation. |
Table III.
Cox multivariate analysis of PFS and
clinical characteristics among 72 patients with advanced non-small
cell lung cancer carrying EGFR mutation.
| Clinical
characteristics | Exp (B) | 95%CI | P-value |
|---|
| Sex |
|
| 0.009 |
| Male
vs. female | 4.600 | 1.455–14.546 |
|
| Age (years) |
|
| 0.468 |
| <70
vs. ≥70 | 1.378 | 0.579–3.280 |
|
| Histological
type |
|
| <0.001 |
|
Adenocacinoma vs.
squamous | 0.011 | 0.001–0.119 |
|
| Clinical stage |
|
| 0.340 |
| IIIB
vs. IV | 0.653 | 0.272–1.567 |
|
| PS score |
|
| 0.748 |
| 0 or 1
vs. 2 | 0.849 | 0.314–2.297 |
|
| Smoking
history |
|
| 0.004 |
| Smoker
vs. non-smoker | 9.742 | 2.026–46.672 |
|
| EGFR-TKI |
|
| 0.789 |
|
Gefitinib vs. erlotinib | 0.887 | 0.368–2.136 |
|
| Group |
|
| 0.046 |
| Exon 19
deletions vs. an exon 21 mutation | 2.256 | 1.013–5.025 |
|
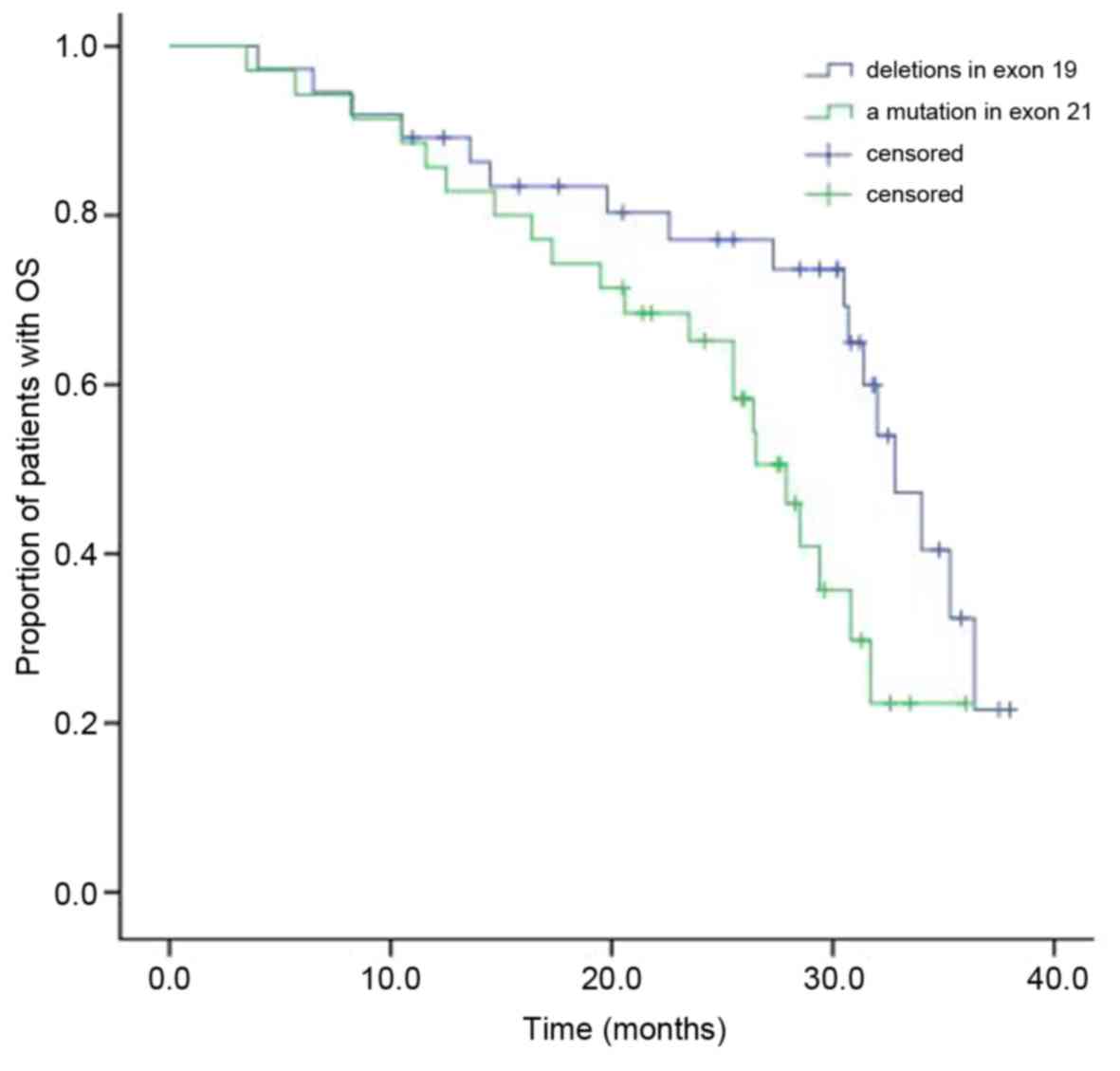
Following EGFR-TKI treatment, the median OS in the
patients with NSCLC who had deletions in exon 19 was 30.2 months,
while it was 25.6 months in patients with a mutation in exon 21
(Fig. 2). The difference between the
two groups' OS was statistically significant (χ2=4.700;
P=0.030). Cox multivariate analysis demonstrated that the median OS
in females (P=0.018), patients with adenocarcinoma (P=0.009) and
non-smoking patients (P=0.003) were significantly higher compared
with the median OS of males, patients with squamous cell carcinoma
and smoking patients, respectively (Table IV). No statistically significant
differences in OS were observed in relation to the patients' age,
tumor stage, PS score, whether the patient had received gefitinib
or erlotinib and whether the patient had mutations in exon 19 or 21
of EGFR.
 | Table IV.Cox multivariate analysis of OS and
clinical characteristics among 72 patients with advanced non-small
cell lung cancer carrying EGFR mutation. |
Table IV.
Cox multivariate analysis of OS and
clinical characteristics among 72 patients with advanced non-small
cell lung cancer carrying EGFR mutation.
| Clinical
characteristics | Exp (B) | 95%CI | P-value |
|---|
| Sex |
|
| 0.018 |
| Male
vs. female | 3.152 | 1.220–8.141 |
|
| Age (year) |
|
| 0.411 |
| <70
vs. ≥70 | 1.455 | 0.595–3.559 |
|
| Histological
type |
|
| 0.009 |
|
Adenocacinoma vs.
squamous | 0.059 | 0.007–0.494 |
|
| Clinical stage |
|
| 0.764 |
| IIIB
vs. IV | 0.875 | 0.366–2.094 |
|
| PS score |
|
| 0.368 |
| 0 or 1
vs. 2 | 0.646 | 0.250–1.671 |
|
| Smoking
history |
|
| 0.003 |
| Smoker
vs. non-smoker | 8.322 | 2.061–33.614 |
|
| EGFR-TKI |
|
| 0.787 |
|
Gefitinib vs. erlotinib | 1.072 | 0.649–1.771 |
|
| Group |
|
| 0.426 |
| Exon 19
deletions vs. an exon 21 mutation | 1.388 | 0.618–3.117 |
|
Patients with deletions in exon 19 are
similarly affected by adverse reactions as patients with a mutation
in exon 21
The most common adverse reactions, which included
rashes (48.6%), diarrhea (26.4%), coughs (2.8%), stomatitis
(4.17%), anorexia (26.4%), nausea (11.1%) and vomiting (4.17%),
occurred in a similar frequency in patients with deletions in exon
19 compared with those with a mutation in exon 21 (Table V). The majority of these reactions
were mild and moderate, while a small number were classified as
third degree adverse reactions according to the National Cancer
Institute Common Terminology Criteria for Adverse Events.
 | Table V.Adverse reactions among 72 patients
with advanced non-small cell lung cancer and epidermal growth
factor receptor mutations. |
Table V.
Adverse reactions among 72 patients
with advanced non-small cell lung cancer and epidermal growth
factor receptor mutations.
| Adverse
reaction | Exon 19 deletions
(n=37) | An exon 21 mutation
(n=35) | χ2 | P-value |
|---|
| Rash | 18 (48.6) | 17 (48.6) | <0.001 | 0.995 |
| Degree
III | 1 | 1 |
|
|
| Degree
IV | 0 | 0 |
|
|
| Diarrhea | 10 (27.0) | 9 (25.7) | 0.016 | 0.899 |
| Degree
III | 0 | 1 |
|
|
| Degree
IV | 0 | 0 |
|
|
| Nausea | 4 (10.8) | 4 (11.4) | 0.007 | 0.934 |
| Degree
III | 0 | 0 |
|
|
| Degree
IV | 0 | 0 |
|
|
| Vomiting | 2 (5.4) | 1 (2.9) | 0.292 | 0.589 |
| Degree
III | 0 | 0 |
|
|
| Degree
IV | 0 | 0 |
|
|
| Fatigue | 2 (5.4) | 2 (5.7) | 0.003 | 0.954 |
| Degree
III | 0 | 0 |
|
|
| Degree
IV | 0 | 0 |
|
|
| Cough | 1 (2.7) | 2 (5.7) | 0.049 | 0.523 |
|
Stomatitis | 2 (5.4) | 1 (2.9) | 0.292 | 0.589 |
|
Anorexia | 9 (24.3) | 10 (28.6) | 0.167 | 0.683 |
|
Interstitial pneumonia | 0 | 0 |
|
|
Discussion
The present study demonstrated that, following
EGFR-TKI treatment for patients with NSCLC, the curative effect in
patients with deletions in exon 19 was significantly higher
compared with patients with a mutation in exon 21 (ORR, 75.7 vs.
51.4%; DCR, 89.2 vs. 68.6%). Furthermore, significantly higher
modified median PFS (13.2 months) and median OS (30.2 months) times
were observed in patients with deletions in exon 19 compared with
patients with a mutation in exon 21 (median PFS, 10.8 months;
median OS, 25.6 months). Additionally, Cox multivariate analysis
revealed that the PFS and OS were higher in female patients,
patients with adenocarcinoma cell carcinoma and non-smoking
patients, and PFS was higher in patients with deletions in exon 19.
No significant differences in PFS and OS were observed in relation
to patients' age, tumor stage, PS score and whether the patient had
received gefitinib or erlotinib, and no significant difference in
OS was identified in patients with exon mutations.
Eight randomized phase III trials (IPASS,
Fist-SIGNAL, W3405, NEJ002, OPTIMAL, EURTAC, LUX-Lung 3, LUX-Lung
6) demonstrated the excellent efficacy of EGFR-TKI against NSCLC.
Compared with platinum-based chemotherapy, which had a 30%
remission rate and median PFS of 5–6 months, EGFR-TKI was
determined to be a better first-line treatment for NSCLC patients
with EGFR mutations, with a 70% remission rate and median PFS of
10–11 months. Consequently, the consistency of EGFR-TKI
demonstrated significant benefit in PFS and ORR as the first-line
treatment in patients with EGFR mutations, thus it was established
as a first-line treatment in these patients. According to some
previous studies, patients with advanced NSCLC, an unknown EGFR
mutation status or without any mutations, the first-line
chemotherapy was revealed to be superior to first-line EGFR-TKI
treatment (15–20). The LUX-Lung 3 clinical trail
demonstrated that the median survival time for the patients with
EGFR exon 19 deletions in the afatinib treatment group (33.3
months) was significantly longer compared with that of the
chemotherapy treatment group (pemetrexed plus cisplatin; 21.1
months). For the patients with EGFR exon 21 mutations in the
aforementioned trial, the survival time in the afatinib treatment
group (27.6 months) was shorter compared with the chemotherapy
treatment group (40.3 months). Furthermore, the LUX-Lung 6 clinical
trail demonstrated that the median survival time for the patients
with EGFR exon 19 deletions in the afatinib treatment group (31.4
months) was significantly longer compared with that of the
chemotherapy treatment group (18.4 months). As for the patients
with EGFR exon 21 mutations, the survival time in the afatinib
treatment group (19.6 months) was shorter compared with that of the
chemotherapy treatment group (24.3 months). The two clinical trials
demonstrated that afatinib could significantly prolong the survival
time for patients with NSCLC and EGFR deletions of exon 19, but it
could not prolong the survival time for the patients with exon 21
mutations (21,22).
Banno et al (23) confirmed that patients with NSCLC and
EGFR exon 19 deletions had a distinct advantage following the
treatment of afatinib compared with the patients with EGFR exon 21
mutations. The report from the NEJ002 study revealed that the
patients with NSCLC and 21 exon mutations exhibited a relatively
poor response to gefitinib, while the patients with NSCLC and exon
19 deletions had a greater response following gefitinib treatment
(24). A meta-analysis revealed
that, following EGFR-TKI as the first-line treatment for patients
with NSCLC, the PFS for the patients with exon 19 deletions were
markedly prolonged compared with patients with EGFR exon 21
mutations, however the study did not analyze OS (25). Additionally, a systematic review and
meta-analysis revealed that in the patients with NSCLC and exon 19
deletions, OS was significantly improved following the EGFR-TKI
treatment compared with those who had chemotherapy, while the
patients with EGFR exon 21 mutations had no OS benefits with
different treatments (5). Another
study demonstrated that among the patients with advanced NSCLC that
were treated with first-line TKI therapy, those with exon 19
deletions had longer PFS compared with those with exon 21 mutations
(25). However, a retrospective
clinical study conducted by Igawa et al (26) determined that no significant
difference in ORR, PFS and OS was identified between patients with
exon 19 deletions and patients with exon 21 mutations treated with
gefitinib. In conclusion, the efficacy and survival rate of the
afatinib treatment in patients with NSCLC and exon 19 deletions was
better compared with those with exon 21 mutations. Only a small
number of studies contradict the aforementioned studies regarding
the comparison of the efficacy and survival rate between the two
different mutations following EGFR-TKI treatment (10).
The studies described in the literature review had
similar results to those obtained in the current study, indicating
that following the administration of EGFR-TKI as the first-line
treatment, patients with advanced NSCLC and deletions in exon 19
have greater ORRs, DCRs, PFS and OS compared with patients with
mutations in exon 21. These rates and survival times were markedly
improved in female patients, non-smoking and adenocarcinoma
patients. Additionally, the two groups manifested predominantly
mild side effects.
Therefore, the EGFR mutation status can be used as a
predictive factor for the efficacy of EGFR-TKI as the first-line
treatment among patients with advanced NSCLC. Furthermore, the
patients with deletions in exon 19 of EGFR have significantly
better outcomes in terms of response and survival rates compared
with patients with a mutation in exon 21 of EGFR. However, due to
the fact that the number of cases in the current study was quite
small, further prospective and multicenter studies are required.
The EGFR mutation status of patients with non-small cell lung
cancer may predict the efficacy and prognosis of EGFR-TKI.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJ performed the studies, participated in collecting
data and drafted the manuscript. MZ and YF performed the
statistical analysis and participated in its design. QL helped to
draft the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of Xuzhou Cancer Hospital (Xuzhou, China) and all
patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EGFR-TKI
|
epidermal growth factor receptor
tyrosine kinase inhibitor
|
|
NSCLC
|
non-small cell lung cancer
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
EGFR
|
epidermal growth factor receptor
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progression disease
|
|
ORR
|
objective response rate
|
|
DCR
|
disease control rate
|
References
|
1
|
Chang JS, Chen LT, Shan YS, Lin SF, Hsiao
SY, Tsai CR, Yu SJ and Tsai HJ: Comprehensive analysis of the
incidence and survival patterns of lung cancer by histologies,
including rare subtypes, in the era of molecular medicine and
targeted therapy: A nation-wide cancer registry-based study from
Taiwan. Medicine (Baltimore). 94:e9692015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cho JH: Immunotherapy for non-small-cell
lung cancer: Current status and future obstacles. Immune Netw.
17:378–391. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kiura K, Ueoka H, Segawa Y, Tabata M,
Kamei H, Takigawa N, Hiraki S, Watanabe Y, Bessho A, Eguchi K, et
al: Phase I/II study of docetaxel and cisplatin with concurrent
thoracic radiation therapy for locally advanced non-small-cell lung
cancer. Br J Cancer. 89:795–802. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuan FC, Kuo LT, Chen MC, Yang CT, Shi CS,
Teng D and Lee KD: Overall survival benefits of first-line EGFR
tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung
cancers: A systematic review and meta-analysis. Br J Cancer.
113:1519–1528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stinchcombe TE: Targeted therapies for
lung cancer. Cancer Treat Res. 170:165–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thatcher N, Chang A, Parikh P, Rodrigues
Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH,
Pemberton K, Archer V and Carroll K: Gefitinib plus best supportive
care in previously treated patients with refractory advanced
non-small-cell Lung Cancer: Results from a randomised,
placebo-controlled, multicentre study (Iressa Survival Evaluation
in Lung Cancer). Lancet. 366:1527–1537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tiseo M, Rossi G, Capelletti M, Sartori G,
Spiritelli E, Marchioni A, Bozzetti C, De Palma G, Lagrasta C,
Campanini N, et al: Predictors of gefitinib outcomes in advanced
non-small lung cancer (NSCLC): Study of a comprehensive panel of
molecular markers. Lung Canser. 67:355–360. 2010. View Article : Google Scholar
|
|
11
|
Rusch VW, Rice TW, Crowley J, Blackstone
EH, Rami-Porta R and Goldstraw P: The seventh edition of the
american joint committee on cancer/international union against
cancer staging manuals: The new era of data-driven revisions. J
Thorac Cardiovasc Surg. 139:819–821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumors:
Recist guideline (version 1.1). Eur J Cancer. 47:228–247. 2009.
View Article : Google Scholar
|
|
14
|
Lakshman A, Modi M, Prakash G, Malhotra P,
Khadwal A, Jain S, Kumari S, Varma N and Varma S: Evaluation of
bortezomib-induced neuropathy using total neuropathy score (Reduced
and Clinical Versions) and NCI CTCAE v4.0 in newly diagnosed
patients with multiple myeloma receiving bortezomib-based
induction. Clin Lymphoma Myeloma Leuk. 17:513–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inoue A, Kobayashi K, Maemondo M, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: North-East Japan Study Group: Updated overall survival
results from a randomized phase III trial comparing gefitinib with
carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer
with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 24:54–59.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen G, Feng J, Zhou C, Wu YL, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Quality of life
(QoL) analyses from OPTIMAL (CTONG-0802), a phase III, randomised,
open-label study of first-line erlotinib versus chemotherapy in
patients with advanced EGFR mutation-positive non-small-cell lung
cancer (NSCLC). Ann Oncol. 24:1615–1622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: West Japan Oncology Group: Gefitinib versus cisplatin plus
docetaxel in patients with non-small-cell lung cancer harbouring
mutations of the epidermal growth factor receptor (WJTOG3405): An
open label, randomised phase 3 trial. Lancet Oncol. 11:121–128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Spanish lung cancer group in
collaboration with groupe français de pneumo-cancérologie and
associazione italiana oncologia toracica: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in Asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han JY, Park K, Kim SW, Lee DH, Kim HY,
Kim HT, Ahn MJ, Yun T, Ahn JS, Suh C, et al: First-SIGNAL:
First-line single-agent iressa versus gemcitabine and cisplatin
trial in never-smokers with adenocarcinoma of the lung. J Clin
Oncol. 30:1122–1128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang
Y, Li W, Hou M, Shi JH, Lee KY, et al: Afatinib versus cisplatin
plus gemcitabine for first-line treatment of Asian patients with
advanced non-small-cell lung cancer harbouring EGFR mutations
(LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet
Oncol. 15:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang JC, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banno E, Togashi Y, Kobayashi Y, Hayashi
H, Mitsudomi T and Nishio K: Afatinib is especially effective
against non-small cell lung cancer carrying an EGFR exon 19
deletion. Anticancer Res. 35:2005–2008. 2015.PubMed/NCBI
|
|
24
|
Fukuhara T, Maemondo M, Inoue A, Kobayashi
K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H,
et al: Factors associated with a poor response to gefitinib in the
NEJ002 study: Smoking and the L858R mutation. Lung Cancer.
88:181–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Sheng J, Kang S, Fang W, Yan Y,
Hu Z, Hong S, Wu X, Qin T, Liang W and Zhang L: Patients with exon
19 deletion were associated with longer progression-free survival
compared to those with L858R mutation after first-line EGFR-TKIs
for advanced non-small cell lung cancer: A meta-analysis. PLoS One.
9:e1071612014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Igawa S, Kasajima M, Ishihara M, Kimura M,
Hiyoshi Y, Asakuma M, Otani S, Katono K, Sasaki J and Masuda N:
Comparison of the efficacy of gefitinib in patients with non-small
cell lung cancer according to the type ofepidermal growth factor
receptor mutation. Oncology. 87:215–223. 2014. View Article : Google Scholar : PubMed/NCBI
|