Introduction
Thyroid nodules are a common disease of the neck,
with malignant nodules accountings for 5–15% of them (1). The pathological classification of
thyroid cancer (TC) includes papillary, follicular and
undifferentiated types. Except from painless nodules, TC usually
has no other specific clinical symptom, making it harder to detect
at an early stage. In recent years, the incidence of TC has
gradually increased, ranking 9th among all tumors worldwide
(2–5). Early diagnosis of TC and surgical
treatment are particularly important for the prognosis of
patients.
The most accurate examination for preoperative
diagnosis of TC, thyroid fine needle aspiration cytology (FNAC) is
unable to distinguish between thyroid follicular carcinoma and
follicular adenoma. Recent studies found that measuring TgAb could
help differentiate TC from indeterminate nodules subjected to FNAC
(6–8).
TgAb, an IgG glycoprotein secreted by lymphoid B
cells, and thyroid peroxidase antibodies (TPOAb) usually indicates
autoimmune thyroid diseases (AITD) when they are positive. These
two antibodies could also be elevated in patients with TC (9). Recently, more and more studies have
focused on the relationship between thyroid antibodies and TC. An
early study found that the prevalence rate of positive TgAb in
patients with TC was 2.5 times as much as in the general population
(9). Kim et al (10) first reported that TgAb could be used
as an independent predictor for TC diagnosis (OR=1.80, 95%
CI=1.29–2.58), regardless of the presence of AITD, especially in
younger patients (11). Furthermore,
TC patients with positive TgAb levels had a worse prognosis after
surgery (12,13). However, this issue remains
controversial. The present meta-analysis was therefore performed to
systematically evaluate the association between positive thyroid
antibodies (TgAb and TPOAb) and the risk of TC.
Materials and methods
Search strategy
A systematic search was conducted using three
electronic databases (Pubmed, Embase and Cochrane library) to
retrieve potentially relevant articles published before October
2018. The search strategy comprised the terms (all fields) ‘thyroid
cancer’, ‘thyroid carcinoma’, ‘thyroid neoplasm’, or ‘thyroid
nodule’, and ‘thyroglobulin antibody’, ‘thyroglobulin
autoantibody’, ‘thyroid peroxidase antibody’, ‘thyroid peroxidase
autoantibody’, ‘thyroid antibody’, ‘thyroid autoantibody’, ‘TgAb’
or ‘TPOAb’. We also searched for relevant articles from references
of the original paper and review articles.
Selection criteria
The inclusion criteria were as follows: i) Studies
explored the association between preoperative serum thyroid
antibodies (TgAb or TPOAb), as a categorical variable, and the risk
of TC; ii) patients with TC and thyroid benign nodules were
classified into case group and control group, respectively; iii)
the diagnosis of TC was based on preoperative FNAC or postoperative
histological biopsy. Reviews, duplicate literatures, the meeting
abstract and the studies that did not provide odds ratio (OR) and
the corresponding 95% confidence intervals (CI) data adjusted by
multivariate logistic regression analysis were excluded.
Data extraction
Two researchers independently extracted important
information from the selected literature. The extracted data
included: The first author, year of publication, study location,
sample size, cancer types, confounding factors, OR values and 95%CI
adjusted by multivariate logistic regression analysis.
Quality evaluation
The Newcastle-Ottawa Scale (14) was used to evaluate the quality of the
selected literature. This scale is three dimensional: Selection,
comparability and exposure, with a score range of 0–9. Scores of
0–6 were classified as low-quality studies and scores of 7–9 as
high-quality studies.
Statistical analysis
Statistical analysis was carried out using STATA
14.0 software. The heterogeneity across studies was estimated using
the I-squared statistic and Cochran's Q-test. If there was
significant heterogeneity (I2>50% or P<0.10), the
random effects model was used for meta-analysis. Next, a Galbraith
plot was used to investigate the source of heterogeneity from
single studies. At the same time, meta-regression and subgroup
analyses were performed based on the characteristics of studies to
identify factors that contributed to heterogeneity. Otherwise, the
fixed effects model was used. The pooled OR and 95%CI were
calculated to evaluate the association between positive thyroid
antibodies and the risk of TC. Z test was used to determine the
significance of this association. Sensitivity analysis was
performed to explore the influence of a single study on the overall
risk estimate by omitting one study in each turn. Begg rank
correlation test and Egger's regression test were used to estimate
the publication bias, and the result of meta-analysis was corrected
using the trim-and-fill method, if publication bias was identified.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Literature search and study
characteristics
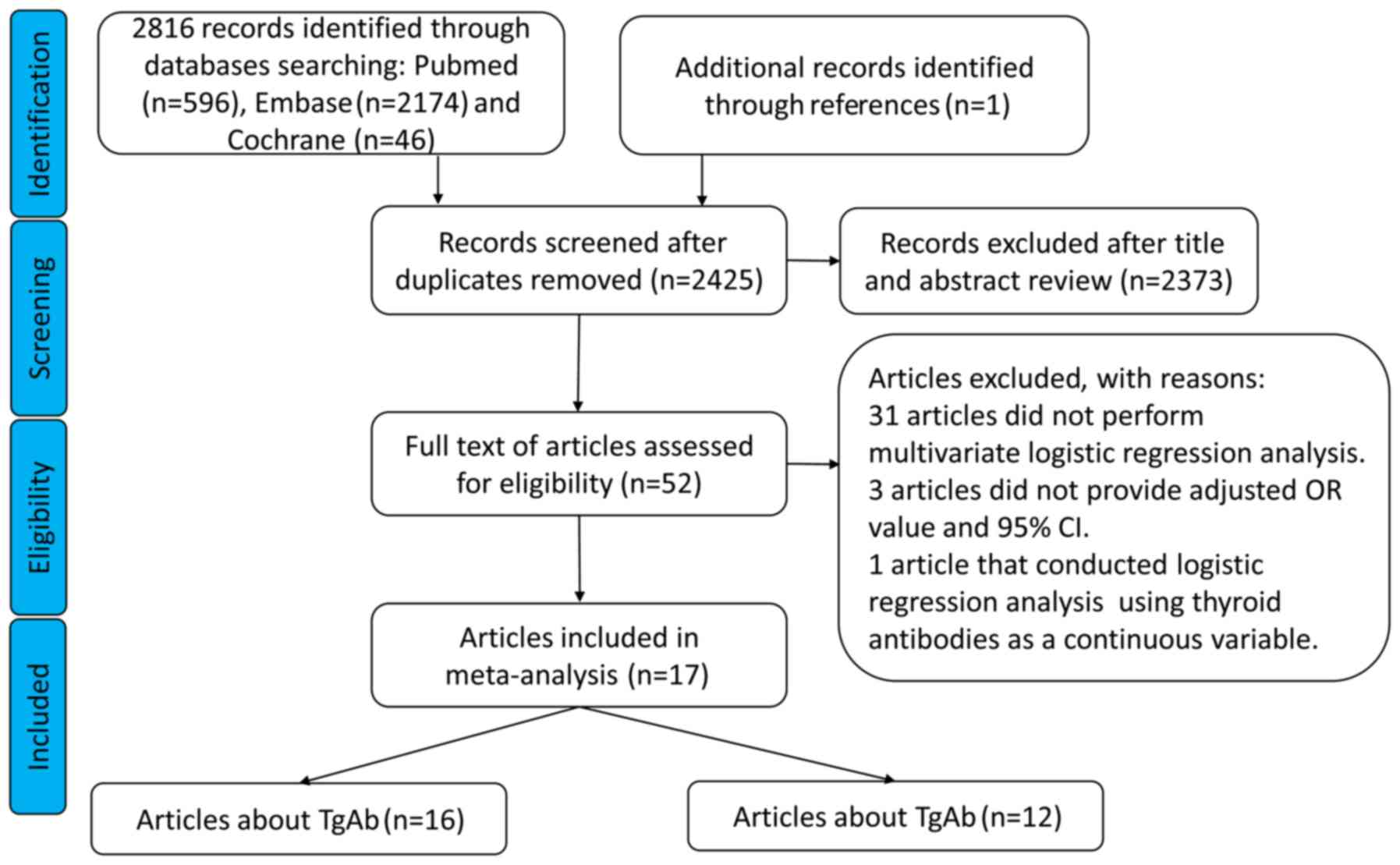
Our search identified 2,817 potentially relevant
articles, out of which 392 were duplicates and were deleted
(Fig. 1). After reading the title
and abstract, 2,373 articles were deleted based on the exclusion
criteria. Next, the full text of the remaining 52 articles was
reviewed and 35 more were excluded. Finally, 17 articles were
included in the meta-analysis (7,8,10,11,15–27). The
characteristics of those articles are listed in Table I.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| Author, year | Country | Sample size
(case/control) | Cancer types | Confounding factors
adjusted for in the original study | OR or RR
(95%CI) | Quality score | (Refs.) |
|---|
| Zhu et al,
2018 | China | 90/285a; Noneb | TC | Age, nodule size,
TSH | 2.59
(1.25–5.37)a;
Noneb | 8 | (15) |
| Liu et al,
2018 | China |
524/2,460a; 524/2,460b | TC | Age, nodule size,
TSH, TPOAb/TgAb | 4.435
(1.902–10.345)a; 0.901
(0.346–2.350)b | 9 | (16) |
| Zhao et al,
2017 (male) | China |
276/193a;
Noneb | PTC | Age, TSH,
TPOAb/TgAb, nodule size, nodule number | 3.21
(1.36–7.57)a;
Noneb | 9 | (17) |
| Zhao et al,
2017 (female) | China |
844/728a;
844/728b | PTC | Age, TSH,
TPOAb/TgAb, nodule size, nodule number | 1.80
(1.38–2.36)a; 1.98
(1.44–2.73)b | 9 | (17) |
| Liu et al,
2017 | China |
927/927a;
927/927b | PTC | Age, gender,
TSH | 2.35
(1.82–3.04)a; 1.58
(1.21–2.05)b | 6 | (11) |
| Zeng et al,
2016 | China |
578/620a;
Noneb | PTC | Age, nodule
size | 4.894
(2.520–9.505)a;
Noneb | 8 | (18) |
| He et al,
2016 | China |
189/748a;
194/764b | TC | Age, gender, TSH,
TPOAb/TgAb | 1.53
(0.91–2.56)a; 0.92
(0.54–1.57)b | 8 | (19) |
| Qin et al,
2015 | China |
237/1,401a; 237/1,401b | DTC | Age, gender, nodule
size, nodule number, TSH, TPOAb/TgAb | 2.10
(1.40–3.15)a; 1.2
(0.71–2.04)b | 9 | (20) |
| Li et al,
2015 | China |
1,967/7,228a; 1,967/7,228b | TC | 18 confounding
factorsc | 1.20
(1.02–1.42)a; 2.83
(2.39–3.36)b | 7 | (21) |
| Vasileiadis et
al, 2014 | Greece |
389/447a;
Noneb | PTC | Age, gender, HT,
TSH, nodule size | 1.86
(1.21–2.53)a;
Noneb | 9 | (8) |
| Grani et al,
2014 | Italy |
78/1,131a; Noneb | MN | Age, nodule size,
nodule number, TPOAb/TgAb | 0.98
(0.41–2.38)a;
Noneb | 6 | (22) |
| Azizi et al,
2014 | America |
233/1,790a; 233/1,790b | MN | Age, gender | 2.24
(1.57–3.19)a; 1.19
(0.88–1.61)b | 7 | (23) |
| Wu et al,
2013 | China |
537/1,595a; 537/1,595b | PTC | Age, gender, TSH,
TPOAb/TgAb | 1.921
(1.431–2.580)a; 1.945
(1.195–3.165)b | 9 | (24) |
| Lun et al,
2013 | China |
636/1,631a; 634/1,627b | PTC | Age, gender, HT,
TSH, TPOAb/TgAb | 1.89
(1.47–2.44)a; 1.19
(0.88–1.61)b | 8 | (25) |
| Boi et al,
2013 | Italy |
189/1,472a; 189/1,472b | MN | Age, gender, TSH,
TPOAb/TgAb | 1.67
(1.05–2.67)a; 2.15
(1.42–3.25)b | 7 | (26) |
| Azizi et al,
2011 | America |
253/2,247a; 253/2,247b | MN | Age, gender, nodule
number, TSH | 1.57
(1.11–2.23)a; 1.12
(0.83–1.51)b | 7 | (7) |
| Kim et al,
2010 | Korea |
296/1,342a; Noneb | MN | Age, gender, nodule
number, nodule size, TSH | 1.61
(1.12–2.33)a;
Noneb | 6 | (10) |
| Boelaert et
al, 2006 | Britain | Nonea; 91/1,138b | MN | TSH | Nonea; 1.19 (0.6–2.35)b | 7 | (27) |
Meta-analysis
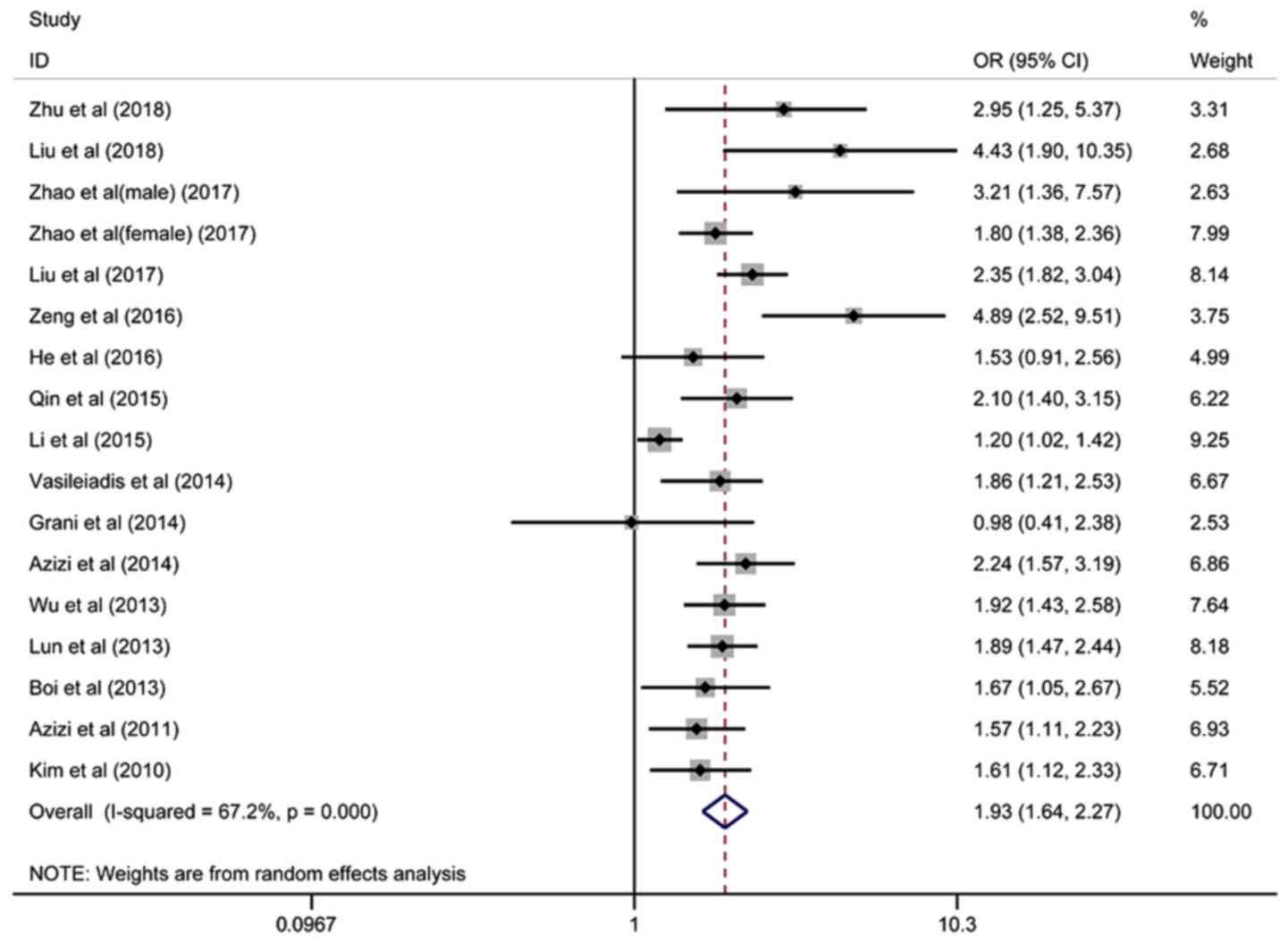
Sixteen articles containing 17 studies on the
association between TgAb and the risk of TC involving 34,488
patients were included, and the pooled OR was 1.93 (95%
CI=1.64–2.27, I2=67.2%), based on the random-effects
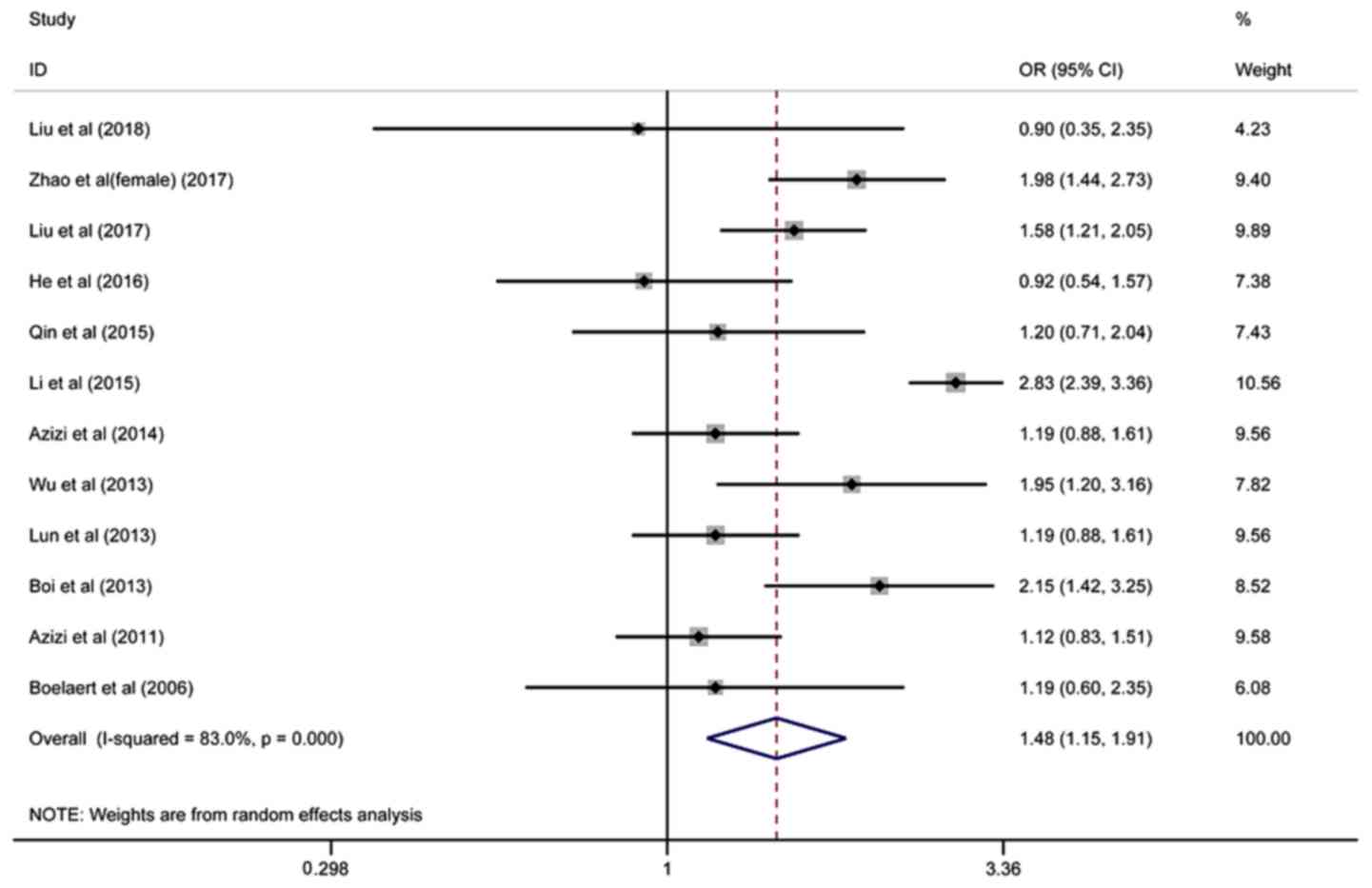
model (Fig. 2). Twelve studies on
the association between TPOAb and the risk of TC involving 30,007
patients were included, and the pooled OR was 1.48 (95%
CI=1.15–1.91, I2=83.0%), based on the random-effects
model (Fig. 3).
Heterogeneity analysis
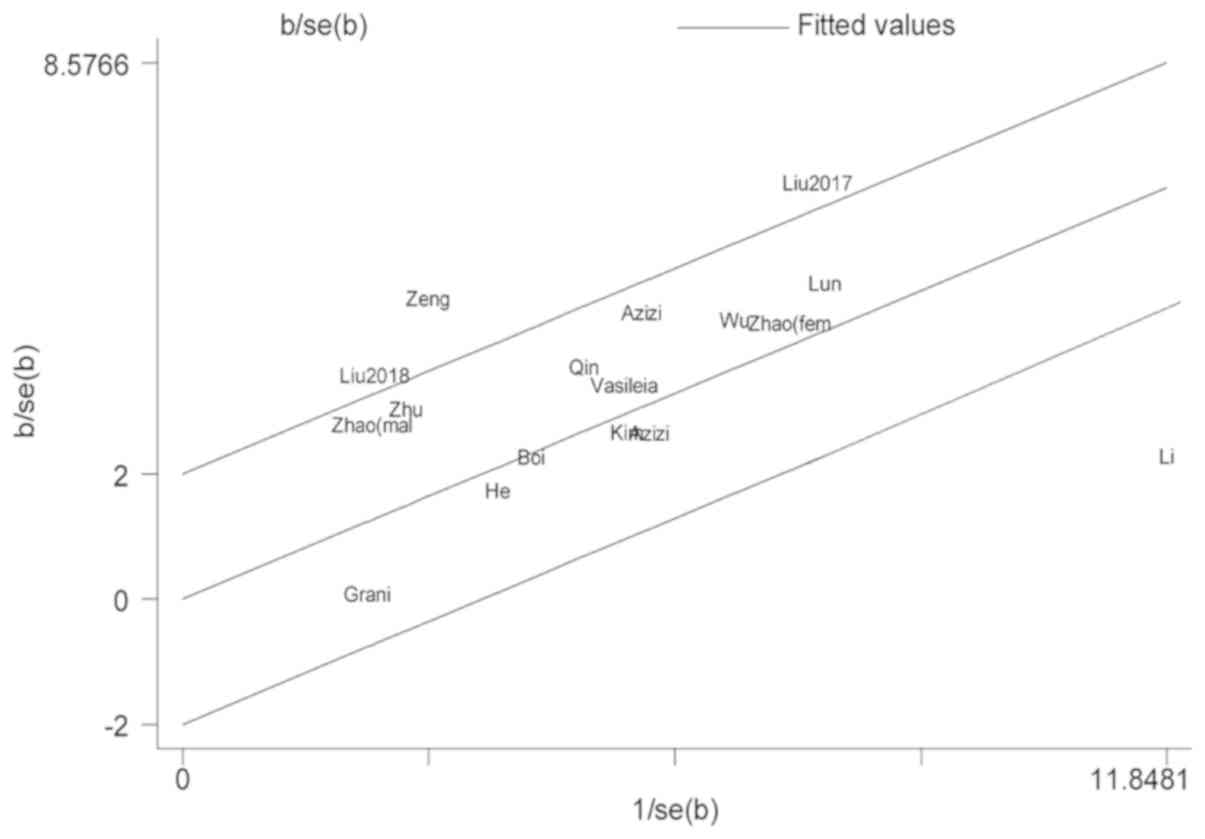
As mentioned above, there was significant
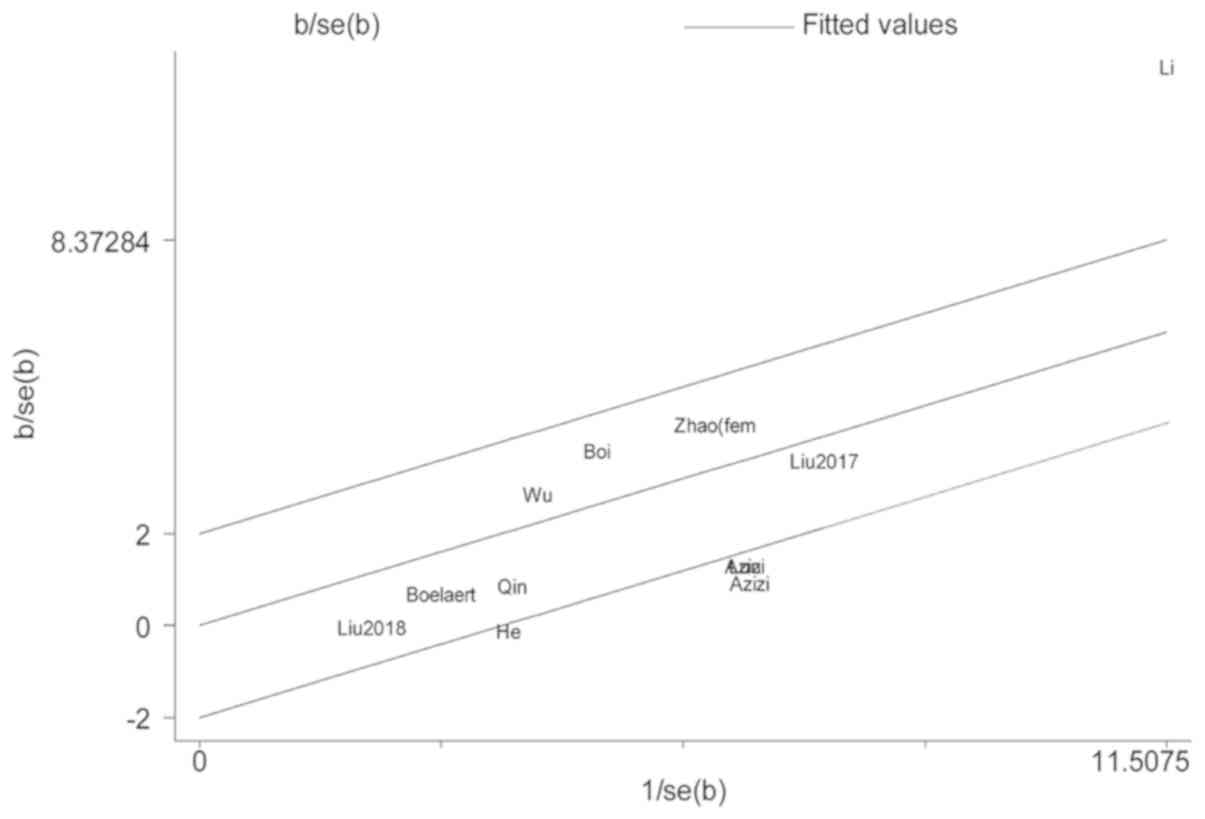
heterogeneity across the included studies. In studies about TgAb,
the Galbraith radial plot showed that two studies caused the
heterogeneity (18,21) (Fig.
4). After removing the two studies, we found that the rest of
the included studies were homogeneous (I2=9.7%, P=0.35)
and the association between positive TgAb and the increased risk TC
did not change (OR=1.93, 95%CI=1.74–2.14). In addition,
meta-regression analysis was conducted based on the characteristics
of studies including study location, cancer types, sample size and
confounding factors. The results indicated that confounding factors
(gender and thyroid nodule number) were responsible for 74.6% of
the heterogeneity (P<0.05).
In studies on TPOAb, Li et al (21) and Zhao et al (17) (female) study data were the causes of
heterogeneity (Fig. 5). The
association between positive TPOAb and increased risk of TC did not
change (OR=1.33, 95%CI=1.13–1.57) and the heterogeneity became not
significant (I2=36.5%, P=0.12) following the removal of
the two studies. Meta-regression analysis did not identify the
characteristics of studies that led to heterogeneity. It only
showed non-significant heterogeneity in the PTC group.
Subgroup analysis
In studies on TgAb, positive TgAb was associated
with an increased risk of TC in all subgroups. A stronger
association was found in studies that did not adjust the thyroid
nodule number (OR=2.14, 95% CI=1.82–2.52), as compared with studies
that did (OR=1.61, 95% CI=1.29–2.00; P=0.04).
In studies on TPOAb, no association between TPOAb
and the risk of TC was observed in the TC group and big sample size
group (Table II).
 | Table II.Subgroup analysis to probe
differences in the pooled OR values between studies included in the
meta-analysis. |
Table II.
Subgroup analysis to probe
differences in the pooled OR values between studies included in the
meta-analysis.
| A, TgAb |
|---|
|
|---|
|
|
| Test of
heterogeneity |
|
|---|
|
|
|
|
|
|---|
| Variables | Pooled OR
(95%CI) | I2
(%) | P-value | P-value |
|---|
| Study location |
|
|
| 0.34 |
|
Occident (n=5) | 1.78
(1.48–2.14) | 0.2 | 0.405 |
|
| Asia
(n=12) | 2.05
(1.66–2.53) | 75.4 | 0 |
|
| Cancer type |
|
|
| 0.29 |
| TC
(n=4) | 1.99
(1.14–3.46) | 78.8 | 0.003 |
|
| DTC
(n=1) | 2.10
(0.89–2.65) | – | – |
|
| PTC
(n=7) | 2.11
(1.77–2.50) | 42.8 | 0.105 |
|
| MN
(=5) | 1.72
(1.42–2.07) | 2.6 | 0.392 |
|
| Sample size |
|
|
| 0.16 |
|
>1,200 (=12) | 1.81
(1.52–2.15) | 68.3 | 0 |
|
|
<1,200 (=5) | 2.48
(1.65–3.75) | 58.4 | 0.048 |
|
| Adjusted for nodule
number |
|
|
| 0.04 |
| Yes
(n=7) | 1.61
(1.29–2.00) | 60.8 | 0.018 |
|
| No
(n=10) | 2.14
(1.82–2.52) | 38.9 | 0.099 |
|
| Adjusted for nodule
size |
|
|
| 0.76 |
| Yes
(n=10) | 2.04
(1.54–2.70) | 76.1 | 0 |
|
| No
(n=7) | 1.95
(1.72–2.20) | 0 | 0.489 |
|
| Adjusted for
gender |
|
|
| 0.18 |
| Yes
(n=13) | 1.80
(1.56–2.09) | 61.00 | 0.002 |
|
| No
(n=4) | 2.90
(1.48–5.69) | 67.10 | 0.028 |
|
|
| B,
TPOAb |
|
|
|
| Test of
heterogeneity |
|
|
|
|
|
|
|
Variables | Pooled OR
(95%CI) | I2
(%) | P-value | P-value |
|
| Study location |
|
|
| 0.53 |
|
Occident (n=4) | 1.35
(1.00–1.82) | 57.3 | 0.071 |
|
| Asia
(n=8) | 1.55
(1.13–2.12) | 84.2 | 0 |
|
| Cancer type |
|
|
| 0.66 |
| TC
(n=3) | 1.41
(0.56–3.51) | 89.9 | 0 |
|
| DTC
(n=1) | 1.20
(0.71–2.03) | – | – |
|
| PTC
(n=4) | 1.60
(1.27–2.02) | 50 | 0.112 |
|
| MN
(=4) | 1.35
(1.00–1.82) | 57.3 | 0.071 |
|
| Sample size |
|
|
| 0.85 |
|
>2,000 (=6) | 1.46
(0.95–2.26) | 90.6 | 0 |
|
|
<2,000 (=6) | 1.54
(1.21–1.95) | 48.3 | 0.085 |
|
| Adjusted for nodule
number |
|
|
| 0.46 |
| Yes
(n=4) | 1.69
(1.03–2.78) | 91 | 0 |
|
| No
(n=8) | 1.39
(1.14–1.69) | 43.2 | 0.091 |
|
| Adjusted for nodule
size |
|
|
| 0.27 |
| Yes
(n=4) | 1.79
(1.14–2.79) | 80 | 0.001 |
|
| No
(n=8) | 1.36
(1.13–1.63) | 47.5 | 0.064 |
|
Sensitivity analysis
Sensitivity analysis found that the removal of any
studies on TgAb or TPOAb did not affect the pooled OR values and
95% CI, suggesting that the results of our meta-analysis were
stable and not influenced by a single study.
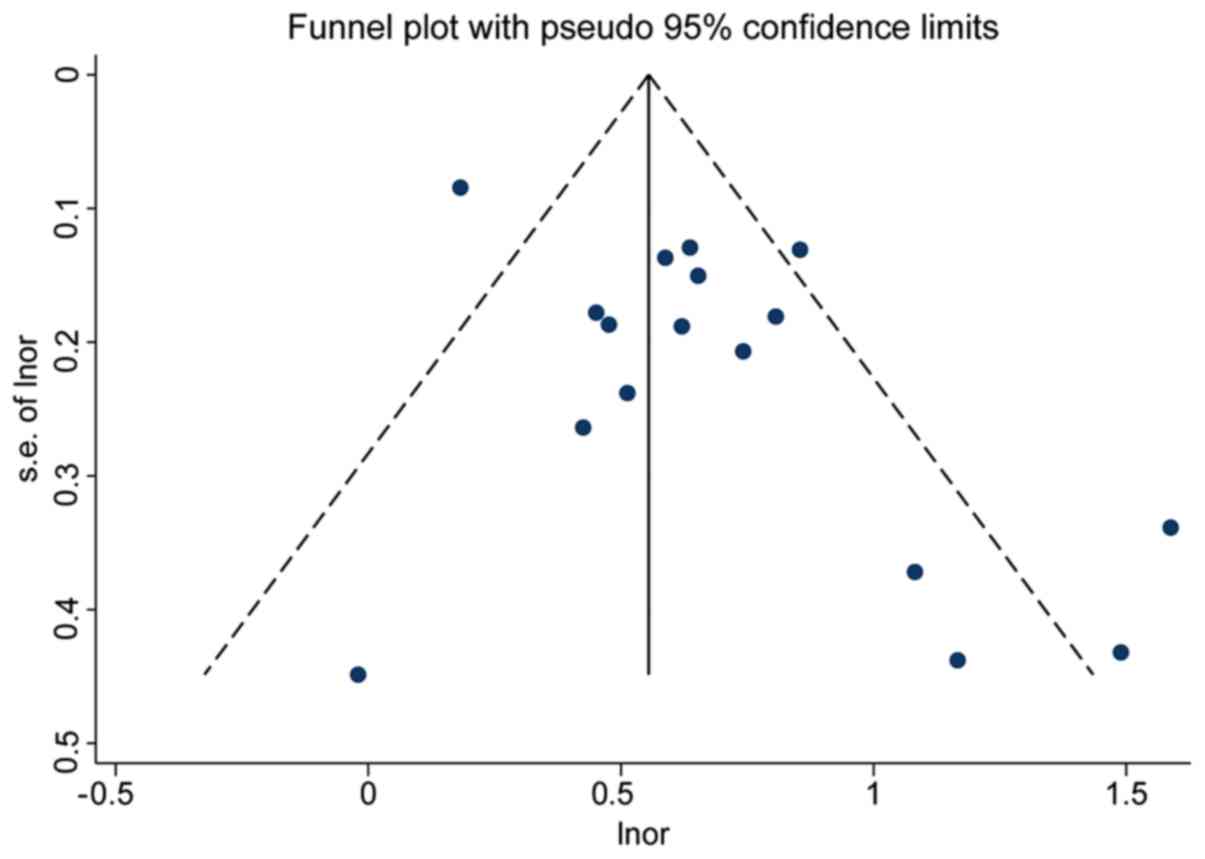
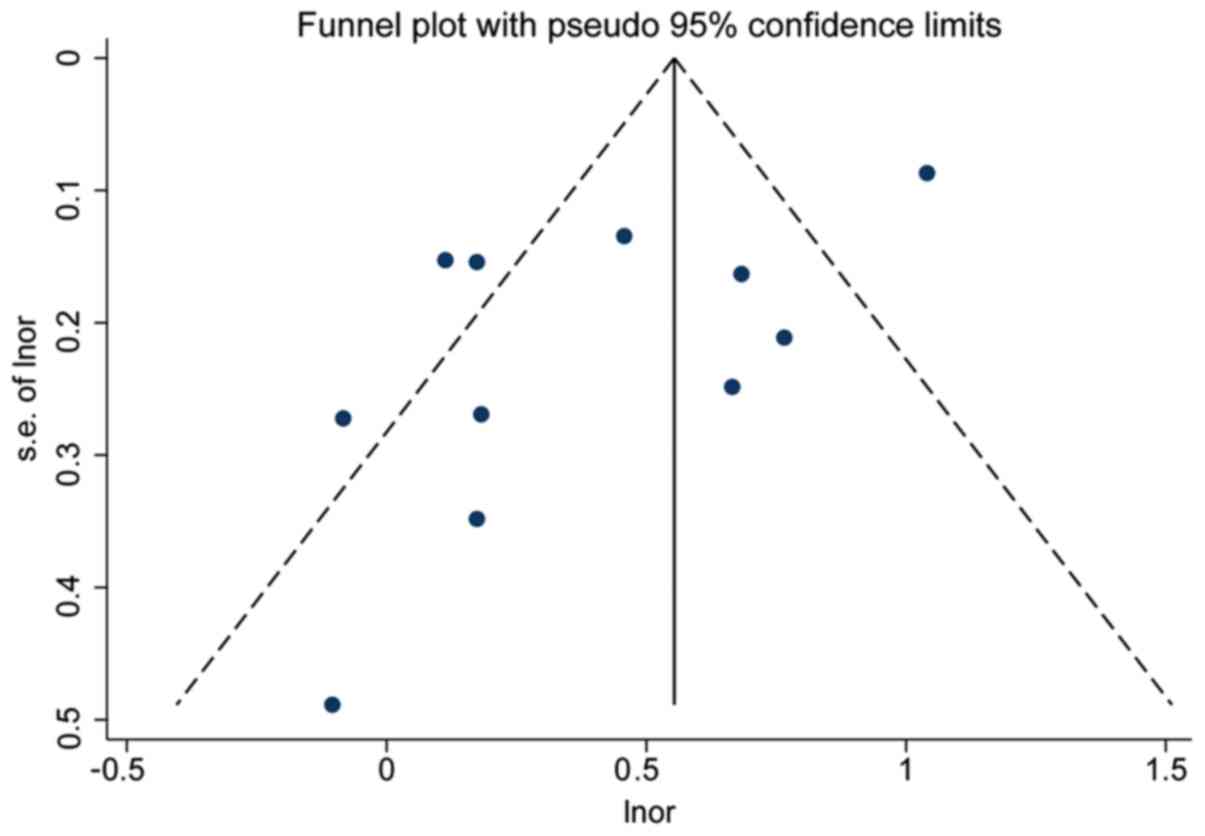
Publication bias
In studies on TgAb, the funnel plot was
asymmetrical, indicating the presence of potential publication bias
(P=0.02; Fig. 6), whereas
publication bias did not exist (P=0.28) following the removal of
the data from the study by Li et al (21), which was the biggest sample size
study and source of heterogeneity. This suggested that the
essential difference between smaller and larger studies that arises
from heterogeneity across studies was the cause of the asymmetry of
the funnel plot (28). Despite that,
the trim-and-fill method was used right away and the corrected OR
value was found to be 1.61 (95% CI=1.36–1.91), which was not
significantly different from the original OR values, proving the
authenticity of the meta-analysis. In studies on TPOAb, both the
funnel plot and the Egger test indicated the presence of
publication bias (P<0.01), but no publication bias was observed
(P=0.11) following the removal of the data from the study by Li
et al (21) (Fig. 7).
Discussion
The identification of benign and malignant thyroid
nodules has always been in the center of clinical attention. It is
controversial whether thyroid antibodies are a risk factor for TC
(22,29–31). In
the present meta-analysis, TgAb-positive patients were found twice
as likely to develop TC as TgAb-negative patients, suggesting that
positive TgAb is a risk factor for TC. Though positive TPOAb is
associated with an increased risk of TC, this association did not
exist in some subgroups, which may have been due to the small
sample size of those subgroups.
Furthermore, the result of the meta-analysis was
consistent with a diagnostic study conducted by Hosseini et
al (29), which found that the
sensitivity and specificity of TgAb for the diagnosis of TC was
16.04% (95% CI=11.37–21.68) and 90.67% (95% CI=85.66–94.38),
respectively. Therefore, positive TgAb is specific for TC, although
negative TgAb has little value in eliminating the diagnosis of TC.
Certain studies reported that the lower sensitivity of TgAb may
arise from the limitations of assay methods, since using different
TgAb assays could discover discrepancies in the TgAb status
(32–34).
It is well-known that TgAb, combined with TPOAb,
used to be a hallmark of AITD. Several studies have discovered an
obvious association between AITD with TC, and reported that the
coexistence with AITD may be one cause of the elevated serum
thyroid antibodies in patients with TC (35–37).
However, positive TgAb remained a risk factor for TC in studies
which excluded AITD patients (24,25). A
study showed that the exposure of thyroglobulin antigen during
tumor formation could cause an increase in serum TgAb through
immune responses (38). Other
studies found that thyroglobulin had ~40 antigenic sites, which
were different between TC and AITD patients (39,40). TC
patients exhibited clearly higher core fucose content and an
increasing trend of TgAb sialylation (41). Further research could improve the
predictive value of TgAb for TC by detecting TC-specific TgAb
fragments.
The present meta-analysis had several following
advantages: First, all included studies had performed a
multivariate logistic regression analysis that controlled the
effects of confounding factors, which fully demonstrated the
independent predictive value of TgAb for TC. Secondly, our analysis
included a large sample data from a total of 34,488 thyroid nodules
patients in 17 studies on TgAb. Although a moderate to high
heterogeneity was observed among those studies
(I2=67.2%), the pooled OR value did not change following
the removal of the two studies that caused the heterogeneity. It
was also found that the differences in confounding factors (thyroid
nodule number and gender) controlled by the study caused 74.6% of
the heterogeneity. This may be due to differences in cancer rates
among people with different genders or thyroid nodule numbers.
Nevertheless, the results of both the sensitivity analysis and the
trim-and-fill method supported the accuracy of our meta-analysis on
the association between positive TgAb and the increased risk of
TC.
However, the present meta-analysis also had certain
limitations. First, most of the included studies were retrospective
case-control studies and cross-sectional studies, which were unable
to articulate the causal relationship between TgAb and TC. As a
result, a multi-center and prospective cohort study is required to
further investigate this issue. Secondly, publications bias was
identified in our meta-analysis on TgAb. Even though the
heterogeneity across studies, particularly that by Li et al
(21) that had too big a sample size
and was the main source of heterogeneity, led to this bias
(28), the result of the
trim-and-fill method supported the authenticity of our
meta-analysis. Finally, in studies on TPOAb, even though we
discovered that the high heterogeneity was derived from single
studies (I2=83.0%), we failed to identify the
characteristics of these studies that led to heterogeneity. We
therefore did not conduct a meta-analysis on the association
between TPOAb and the risk of TC.
In conclusion, positive TgAb is an independent risk
factor for TC. The association between positive TPOAb and the risk
of TC remains to be elucidated.
Acknowledgements
Not applicable.
Funding
The Graduate Science Foundation Project of
University of South China supported the present study (grant no.
2018KYY497).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YaX, QZ, QAL and SLY conceived and designed the
experiments. YaX, QZ, QAL, SLY and YoX performed the experiments.
YaX and QZ analyzed the data. YaX, QAL, SLY, QZ and YoX wrote the
paper.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frates MC, Benson CB, Doubilet PM,
Kunreuther E, Contreras M, Cibas ES, Orcutt J, Moore FD Jr, Larsen
PR, Marqusee E and Alexander EK: Prevalence and distribution of
carcinoma in patients with solitary and multiple thyroid nodules on
sonography. J Clin Endocrinol Metab. 91:3411–3417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morris LG, Tuttle RM and Davies L:
Changing trends in the incidence of thyroid cancer in the united
states. JAMA Otolaryngol Head Neck Surg. 142:709–711. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
La Vecchia C, Malvezzi M, Bosetti C,
Garavello W, Bertuccio P, Levi F and Negri E: Thyroid cancer
mortality and incidence: A global overview. Int J Cancer.
136:2187–2195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du L, Wang Y, Sun X, Li H, Geng X, Ge M
and Zhu Y: Thyroid cancer: Trends in incidence, mortality and
clinical-pathological patterns in Zhejiang Province, Southeast
China. BMC Cancer. 18:2912018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karatzas T, Vasileiadis I, Zapanti E,
Charitoudis G, Karakostas E and Boutzios G: Thyroglobulin
antibodies as a potential predictive marker of papillary thyroid
carcinoma in patients with indeterminate cytology. Am J Surg.
212:946–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Azizi G and Malchoff CD: Autoimmune
thyroid disease: A risk factor for thyroid cancer. Endocr Pract.
17:201–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vasileiadis I, Boutzios G, Charitoudis G,
Koukoulioti E and Karatzas T: Thyroglobulin antibodies could be a
potential predictive marker for papillary thyroid carcinoma. Ann
Surg Oncol. 21:2725–2732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spencer CA, Takeuchi M, Kazarosyan M, Wang
CC, Guttler RB, Singer PA, Fatemi S, LoPresti JS and Nicoloff JT:
Serum thyroglobulin autoantibodies: Prevalence, influence on serum
thyroglobulin measurement, and prognostic significance in patients
with differentiated thyroid carcinoma. J Clin Endocrinol Metab.
83:1121–1127. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim ES, Lim DJ, Baek KH, Lee JM, Kim MK,
Kwon HS, Song KH, Kang MI, Cha BY, Lee KW and Son HY: Thyroglobulin
antibody is associated with increased cancer risk in thyroid
nodules. Thyroid. 20:885–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Li C, Zhao W and Wang Y:
Hashimoto's thyroiditis is an important risk factor of papillary
thyroid microcarcinoma in younger adults. Horm Metab Res.
49:732–738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ernaga-Lorea A, Hernandez-Morhain MC,
Anda-Apinaniz E, Pineda-Arribas JJ, Migueliz-Bermejo I,
Eguílaz-Esparza N and Irigaray-Echarri A: Prognostic value of
change in anti-thyroglobulin antibodies after thyroidectomy in
patients with papillary thyroid carcinoma. Clin Transl Oncol.
20:740–744. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trimboli P, Zilioli V, Imperiali M and
Giovanella L: Thyroglobulin autoantibodies before radioiodine
ablation predict differentiated thyroid cancer outcome. Clin Chem
Lab Med. 55:1995–2001. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. 2009, http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
|
|
15
|
Zhu C, Li S, Gao X, Zhu G, Song M and Gao
F: Retrospective analysis of thyroid nodules: Thyroid cancer risk
factors in suzhou, china. Clin Lab. 64:333–338. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Zheng D, Li Q, Tang X, Luo Z, Yuan
Z, Gao L and Zhao J: A predictive model of thyroid malignancy using
clinical, biochemical and sonographic parameters for patients in a
multi-center setting. BMC Endocr Disord. 18:17–23. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao H, Li H and Huang T: High urinary
iodine, thyroid autoantibodies, and thyroid-stimulating hormone for
papillary thyroid cancer risk. Biol Trace Elem Res. 184:317–324.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng R, Shou T, Yang KX, Shen T, Zhang JP,
Zuo RX, Zheng YQ and Yan XM: Papillary thyroid carcinoma risk
factors in the yunnan plateau of southwestern China. Ther Clin Risk
Manag. 12:1065–1074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He LZ, Zeng TS, Pu L, Pan SX, Xia WF and
Chen LL: Thyroid hormones, autoantibodies, ultrasonography, and
clinical parameters for predicting thyroid cancer. Int J
Endocrinol. 2016:82158342016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin J, Yu Z, Guan H, Shi L, Liu Y, Zhao N,
Shan Z, Han C, Li Y and Teng W: High thyroglobulin antibody levels
increase the risk of differentiated thyroid carcinoma. Dis Markers.
2015:6486702015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li T, Sheng J, Li W, Zhang X, Yu H, Chen
X, Zhang J, Cai Q, Shi Y and Liu Z: A new computational model for
human thyroid cancer enhances the preoperative diagnostic efficacy.
Oncotarget. 6:28463–28477. 2015.PubMed/NCBI
|
|
22
|
Grani G, Calvanese A, Carbotta G,
D'Alessandri M, Nesca A, Bianchini M, Del Sordo M, Vitale M and
Fumarola A: Thyroid autoimmunity and risk of malignancy in thyroid
nodules submitted to fine-needle aspiration cytology. Head Neck.
37:260–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Azizi G, Keller JM, Lewis M, Piper K,
Puett D, Rivenbark KM and Malchoff CD: Association of hashimoto's
thyroiditis with thyroid cancer. Endocr Relat Cancer. 21:845–852.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu X, Lun Y, Jiang H, Gang Q, Xin S, Duan
Z and Zhang J: Coexistence of thyroglobulin antibodies and thyroid
peroxidase antibodies correlates with elevated thyroid-stimulating
hormone level and advanced tumor stage of papillary thyroid cancer.
Endocrine. 46:554–560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z,
Wang F, Duan Z, Xin S and Zhang J: Hashimoto's thyroiditis as a
risk factor of papillary thyroid cancer may improve cancer
prognosis. Otolaryngol Head Neck Surg. 148:396–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boi F, Minerba L, Lai ML, Marziani B,
Figus B, Spanu F, Borghero A and Mariotti S: Both thyroid
autoimmunity and increased serum TSH are independent risk factors
for malignancy in patients with thyroid nodules. J Endocrinol
Invest. 36:313–320. 2013.PubMed/NCBI
|
|
27
|
Boelaert K, Horacek J, Holder RL,
Watkinson JC, Sheppard MC and Franklyn JA: Serum thyrotropin
concentration as a novel predictor of malignancy in thyroid nodules
investigated by fine-needle aspiration. J Clin Endocrinol Metab.
91:4295–4301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lau J, Ioannidis JP, Terrin N, Schmid CH
and Olkin I: The case of the misleading funnel plot. BMJ.
333:597–600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hosseini S, Payne RJ, Zawawi F, Mlynarek
A, Hier MP, Tamilia M and Forest VI: Can preoperative thyroglobulin
antibody levels be used as a marker for well differentiated thyroid
cancer? J Otolaryngol Head Neck Surg. 45:312016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gabalec F, Srbova L, Nova M, Hovorkova E,
Hornychova H, Jakubikova I, Ryska A and Cap J: Impact of
hashimoto's thyroiditis, TSH levels, and anti-thyroid antibody
positivity on differentiated thyroid carcinoma incidence.
Endokrynol Pol. 67:48–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Selek A, Cetinarslan B, Tarkun I, Canturk
Z, Ustuner B and Akyay Z: Thyroid autoimmunity: Is really
associated with papillary thyroid carcinoma? Eur Arch
Otorhinolaryngol. 274:1677–1681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Donegan D, McIver B and
Algeciras-Schimnich A: Clinical consequences of a change in
anti-thyroglobulin antibody assays during the follow-up of patients
with differentiated thyroid cancer. Endocr Pract. 20:1032–1036.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
D'Aurizio F, Metus P, Ferrari A, Caruso B,
Castello R, Villalta D, Steffan A, Gaspardo K, Pesente F, Bizzaro
N, et al: Definition of the upper reference limit for thyroglobulin
antibodies according to the national academy of clinical
biochemistry guidelines: Comparison of eleven different automated
methods. Auto Immun Highlights. 8:82017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pickett AJ, Jones M and Evans C: Causes of
discordance between thyroglobulin antibody assays. Ann Clin
Biochem. 49:463–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee IS, Hsieh AT, Lee TW, Lee TI and Chien
YM: The association of thyrotropin and autoimmune thyroid disease
in developing papillary thyroid cancer. Int J Endocrinol.
2017:59403672017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Li H, Ji QH, Zhu YX, Wang ZY,
Wang Y, Huang CP, Shen Q, Li DS and Wu Y: The clinical features of
papillary thyroid cancer in hashimoto's thyroiditis patients from
an area with a high prevalence of hashimoto's disease. BMC Cancer.
12:6102012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baser H, Topaloglu O, Tam AA, Evranos B,
Alkan A, Sungu N, Dumlu EG, Ersoy R and Cakir B: Higher TSH can be
used as an additional risk factor in prediction of malignancy in
euthyroid thyroid nodules evaluated by cytology based on bethesda
system. Endocrine. 53:520–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fiore E, Rago T, Latrofa F, Provenzale MA,
Piaggi P, Delitala A, Scutari M, Basolo F, Di Coscio G, Grasso L,
et al: Hashimoto's thyroiditis is associated with papillary thyroid
carcinoma: Role of TSH and of treatment with L-thyroxine. Endocr
Relat Cancer. 18:429–437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Latrofa F, Ricci D, Grasso L, Vitti P,
Masserini L, Basolo F, Ugolini C, Mascia G, Lucacchini A and
Pinchera A: Characterization of thyroglobulin epitopes in patients
with autoimmune and non-autoimmune thyroid diseases using
recombinant human monoclonal thyroglobulin autoantibodies. J Clin
Endocrinol Metab. 93:591–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sinclair D: Clinical and laboratory
aspects of thyroid autoantibodies. Ann Clin Biochem. 43:173–183.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao L, Liu M, Gao Y, Huang Y, Lu G, Gao
Y, Guo X and She B: Glycosylation of sera thyroglobulin antibody in
patients with thyroid diseases. Eur J Endocrinol. 168:585–592.
2013. View Article : Google Scholar : PubMed/NCBI
|