Introduction
Malignant mesothelioma is an aggressive neoplasm
that arises from the mesothelial cells lining serosal surfaces. The
majority of mesotheliomas arise in the pleura (85.5%), and
malignant peritoneal mesothelioma (MPM) is a rare tumor accounting
for 13.2% of all malignant mesotheliomas (1). The incidence of MPM varies across
countries, but there are reports that it ranges from 0.5 to 3 cases
per 1 million population (2).
According to the World Health Organization, the age-specific
mortality rate of peritoneal malignant mesothelioma is 0.3 per 1
million people, and the mean age at death of MPM is 66.0 years
(3). However, as the number of
patients with MPM is on the increase (2–4),
clinicians and pathologists may acquire more experience with MPM
patients in the future.
Asbestos exposure is the most important risk factor
for malignant mesothelioma, including MPM (4). In addition, irradiation is also a known
risk factor for malignant mesothelioma, and many develop ~10 to 30
years after radiotherapy (5–12). Other risk factors for malignant
mesothelioma have been reported to include other minerals, such as
erionite, thorium and mica (2,4).
However, as these other mineral-related risks are only reported in
case reports, the relative risk of developing MPM has not yet been
quantified (4).
We herein report a case of MPM diagnosed on autopsy
in a patient who succumbed to intestinal obstruction. The patient
had no history of asbestos exposure, but had a history of
radiotherapy for ovarian cancer ~50 years earlier. To the best of
our knowledge, this is the first report of a patient with malignant
mesothelioma that developed this long after radiotherapy.
Case report
An 85-year-old Japanese woman presented at the
Matsumoto Medical Center with abdominal pain and diarrhea in June,
2017. The patient had no history of asbestos exposure; however, she
had a history of several tumor surgeries: Bilateral adnexectomy and
postoperative radiotherapy for ovarian tumors in her 30s (details
unknown), right upper lobectomy for lung adenocarcinoma at the age
of 69 years, thymoma resection at the age of 73 years, and rectal
amputation with artificial anostomy for rectal adenocarcinoma at
the age of 79 years. The patient had not received postoperative
treatment, including radiation therapy, following surgery for
rectal cancer. A physical examination revealed bilateral pleural
effusion, ascites, and lower leg edema. Laboratory tests revealed
an increased white blood cell count (12,270/µl: Reference value
3,300–8,600/µl), anemia (hemoglobin, 7.4 g/dl: Reference value
11.6–14.8 g/dl), hypoalbuminemia (albumin, 1.4 g/dl: Reference
value 4.1–5.1 g/dl), and elevated C-reactive protein level (13.3
mg/dl: Reference value 0.00–0.14 mg/dl). The serum levels of tumor
markers, including carcinoembryonic antigen (CEA) and carbohydrate
antigen 125 (CA125), were not elevated. Therefore a postoperative
adhesive ileus was suspected and the patient was treated with
antibiotics and albumin supplementation, in addition to intravenous
fluid administration, as neither the patient nor her family wished
to have aggressive examinations or treatment. In addition to the
presenting symptoms, vomiting, hematemesis and bleeding appeared in
September, 2017. The patient's general condition gradually
worsened, and she succumbed to cardiopulmonary arrest triggered by
vomiting at ~3 months after the onset of the abdominal
symptoms.
An autopsy was performed with the consent of the
patient's family. Macroscopically, surgical scars were identified
in the right chest and the lower abdominal midline. In the pleural
cavity, clear yellowish pleural effusion (350 ml on the left side
and 250 ml on the right side) and pleural adhesions on the upper
right side were observed. There was no evident pleural plaque
formation. The abdominal cavity contained 3,000 ml of slightly
cloudy, yellowish ascitic fluid, and moderate
intestine-to-intestine and intestine-to-pelvic peritoneum adhesions
were observed. The ileum exhibited adhesions to the pelvic wall
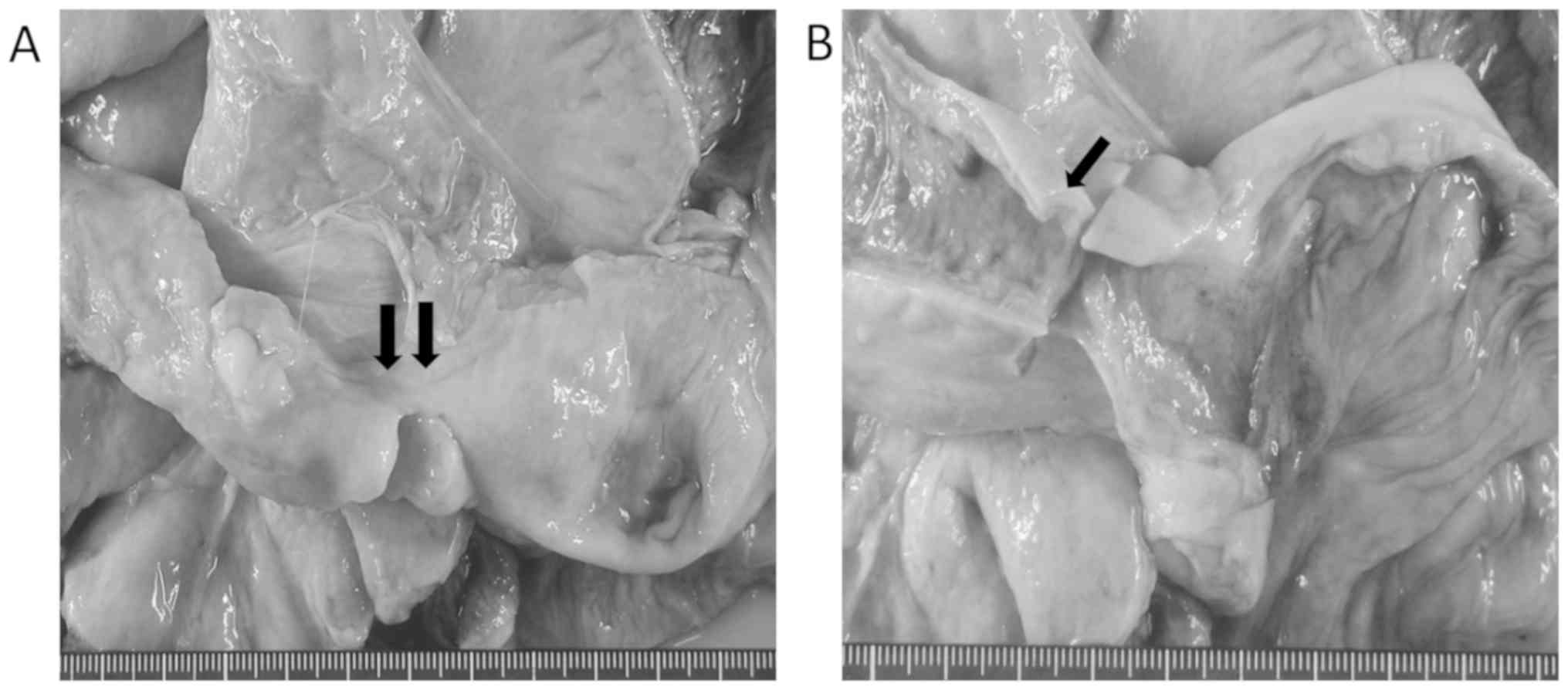
with focal narrowing for a length of ~5 cm (Fig. 1A). On cross section, the intestinal
wall in the area of the ileal narrowing appeared thickened
(Fig. 1B). On histopathological
examination of the thickened ileal wall, proliferating atypical
cells on the peritoneal surface were identified (Fig. 2A and B). These atypical cells had
enlarged nuclei and were arranged in small papillary or tubular
formations (Fig. 2C). No mucin
production was observed. Immunohistochemically, the atypical cells
were positive for cytokeratin (CK) 7 (Fig. 2D), calretinin (Fig. 2F) and podoplanin/D2-40 (Fig. 2G), but negative for CK20 (Fig. 2E), thyroid transcription factor-1
(Fig. 2H), CEA (Fig. 2I), CA125 (Fig. 2J), estrogen receptor (ER) (Fig. 2K), and paired box 8 (PAX8) (Fig. 2L). Therefore, the patient was
diagnosed with MPM of the epithelioid type. MPM with fibrous
thickening of the intestinal wall was limited to the ileum, but
focally involved the serosa of the stomach, jejunum, and the
fibrous capsule of the spleen. No recurrence or metastasis of the
ovarian tumor, thymoma, lung adenocarcinoma, or colorectal
adenocarcinoma were identified locally or systemically.
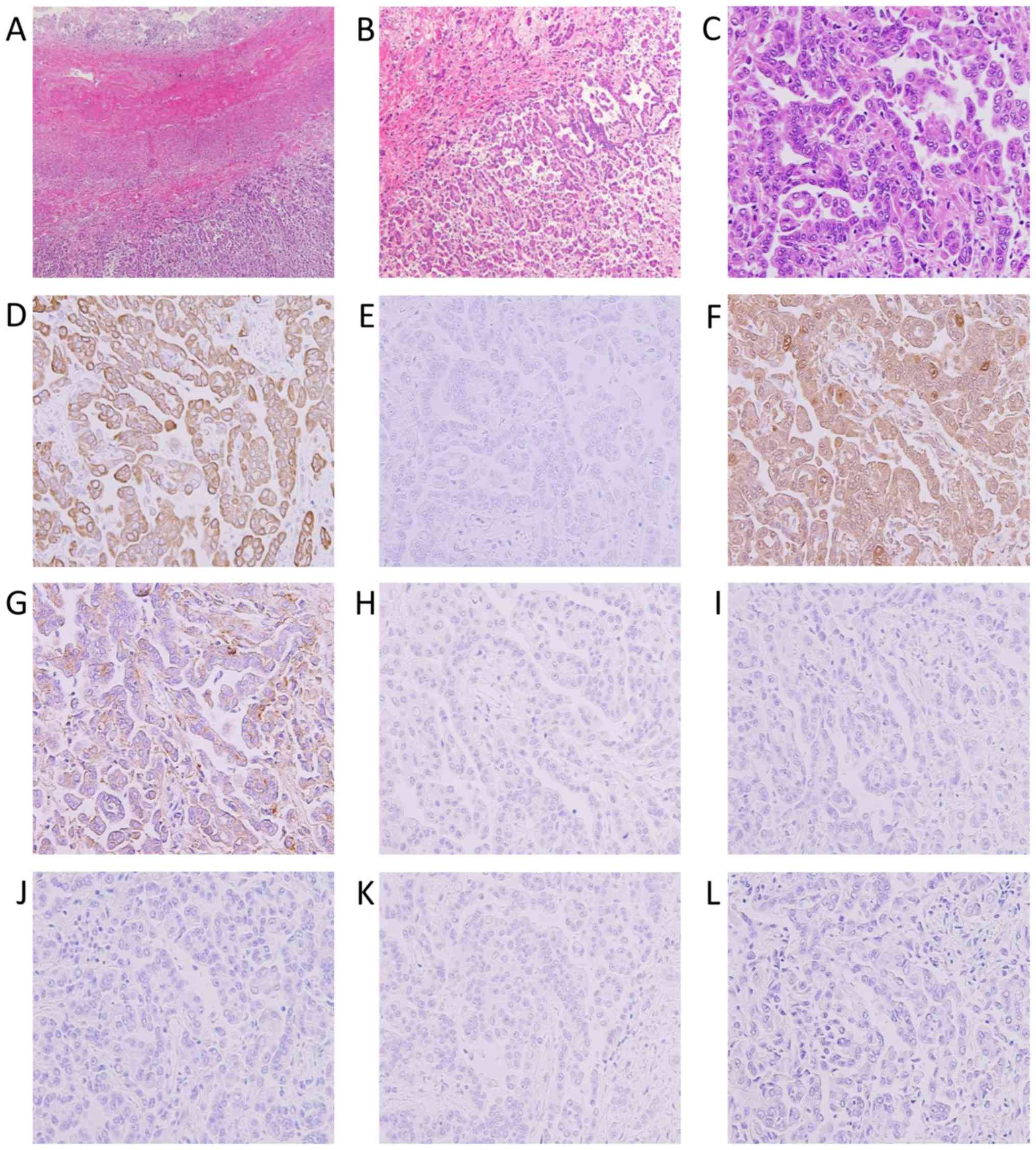 | Figure 2.Histological findings of MPM. (A-C)
Hematoxylin and eosin staining and (D-L) immunohistochemistry for
(D) CK7, (E) CK20, (F) calretinin, (G) D2-40, (H) TTF-1, (I) CEA,
(J) CA125, (K) ER and (L) PAX8. (A) The tumor invaded the
subserosal tissue of the ileum and (B and C) was composed of
epithelial cells arranged in a papilotubular pattern. These cells
were positive for CK7, calretinin and D2-40, and negative for CK20,
TTF-1, CEA, CA125, ER and PAX8. Magnification (A) ×40, (B) ×200 and
(C-L) ×400. MPM, malignant peritoneal mesothelioma; CK,
cytokeratin; TTF, throid transcriprion factor; CEA,
carcinoembryonic antigen; CA125, carbohydrate antigen 125; ER,
estrogen receptor; PAX8, paired box 8. |
Discussion
Clinically, postoperative adhesive ileus and
recurrence or metastasis of the prior cancers was suspected due to
the patient's medical history. In general, the abdominal symptoms
of MPM are non-specific, and may include ascites, retention,
abdominal distension, abdominal pain, weight loss, nausea and
vomiting (4). The imaging and
macroscopic findings of MPM are also non-specific, such as
peritoneal thickening and mass formation (4). Thus, it is difficult to clinically
distinguish MPM from cancer recurrence or metastasis.
18F-fluorodeoxyglucose-positron emission tomography
(PET) was recently reported to be a valuable imaging tool in the
preoperative diagnosis and management of MPM (13). In the future, detailed whole-body
search, including PET examination, may be useful for the early
diagnosis of MPM.
Histologically, MPM is classified into three
subtypes: Epithelioid, sarcomatoid and biphasic. The majority of
MPMs are of the epithelioid type, and display a papillotubular or
solid pattern (4,14). Thus, morphological differentiation
between epithelioid-type MPM and adenocarcinoma is usually
difficult. In particular, morphological differentiation between
epithelioid MPM and serous adenocarcinoma, which arises in the
uterus, ovary, oviduct and the peritoneum, is crucial in female
patients (15). Immunohistochemical
examination is necessary for definitive diagnosis. MPM is generally
positive for mesothelial markers (calretinin, D2-40, Wilms' tumor-1
and CK 5/6) and negative for epithelial markers (several
cytokeratins, such as CK AE1/AE3, CK7 and CAM 5.2), adenocarcinoma
markers (CEA, CA 19-9 and epithelial-specific antigen/Ber-EP 4),
and hormone receptors (ER and progesterone receptor) (4,14,15). In
addition, the Müllerian marker PAX8 is highly positive in serous
adenocarcinoma, but generally negative in MPM (14,16).
Immunohistochemical analysis using a panel of multiple markers is
recommended for accurate diagnosis. In the present case, surgery
for the ovarian tumor had been performed >50 years earlier and
details, including the histological subtype, were unavailable;
however, immunohistochemical examination (positive for calretinin
and D2-40, and negative for PAX8, CA125 and ER) confirmed the
diagnosis of MPM and excluded serous adenocarcinoma.
Asbestos exposure is the most common cause of
malignant mesothelioma, including MPM, particularly in men
(1,4,14).
However, there are several cases in women that are not associated
with asbestos exposure (4). Our
patient had a negative history for occupational asbestos exposure,
as she was a housewife. Furthermore, none of her family members
have developed asbestos-related diseases, such as mesothelioma and
lung cancer, to date. In addition, findings indicating asbestos
exposure, such as pleural plaque formation, were not observed on
autopsy. Therefore, exposure to asbestos was an unlikely cause of
MPM in this case.
However, the patient had a history of multiple
maligancies (ovarian cancer, lung cancer, thymoma and rectal
cancer) and radiotherapy to the pelvis. In this case, although the
patient's history suggested the possibility of a genetic mutation
that increases susceptibility to developing multiple malignancies,
the main lesion of MPM was located in the ileum and adhered to the
pelvis, with superficial spread to a limited serosal area of the
stomach, jejunum and spleen; the location of the lesion
corresponded to the pelvic irradiation site following ovarian
cancer surgery. Malignant mesothelioma can occur after radiotherapy
for tumors, which suggests that direct irradiation may be a risk
factor for its development. Malignant mesotheliomas following
radiation therapy usually develop ~10–30 years after radiotherapy
(5–12). In addition, the US epidemiological
study on radiotherapy (external radiation) for solid tumors
suggested that direct irradiation was associated with the onset of
malignant mesothelioma (17). In
particular, there is an increased risk for >10 years after
irradiation (17). However, the
cumulative incidence of malignant mesothelioma was lower over a
time period of >40 years after irradiation (17). This may be due to the fact that
malignant mesothelioma is a rare disease, and other clinical
factors, such as the onset of other diseases, are involved in
long-term epidemiological surveys. As described above, there have
been several case reports of MPM developing after radiation
therapy. However, there are differences in the clinical data
described in each of those cases. Furthermore, in the present case,
the tissue type of the primary lesion, the irradiation dose and the
duration of the radiation therapy were unknown, as this was an
event from 50 years ago and the medical records had been discarded.
To the best of our knowledge, there have been no reports to date of
MPM developing ~50 years after radiotherapy. However, the
population in Japan is aging, and the number of patients with
malignant mesothelioma is increasing annually (18). Although a number of malignant
mesotheliomas were associated with asbestos exposure in the 1970s,
some may have been related to radiotherapy. As the long-term
prognosis after solid tumor surgery with radiotherapy is expected
to increase due to the recent advances in medical technology, the
incidence of post-irradiation malignant mesothelioma may also
increase. In addition, long-term cancer survivors often develop
other cancers, which makes diagnosis more difficult, as in the
current case; however, malignant mesothelioma should be considered
in the differential diagnosis if a patient has a history of
radiotherapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets during and/or analyzed during the
present study available from the corresponding author on reasonable
request.
Authors' contributions
MO and MK designed the study. MO wrote the
manuscript and assessed the figures and tables. MK, TS, HK and KN
critically revised the manuscript and were involved in data
interpretation. MO and MK finalized the manuscript and submitted
the paper for publication. All authors have edited the manuscript
for intellectual content. All authors have read and approved the
final version of this manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient and her family provided written informed
consent for the publication of the case details and any associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gemba K, Fujimoto N, Aoe K, Kato K,
Takeshima Y, Inai K and Kishimoto T: Treatment and survival
analyses of malignant mesothelioma in Japan. Acta Oncol.
52:803–808. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boffetta P: Epidemiology of peritoneal
mesothelioma: A review. Ann Oncol. 18:985–990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Delgermaa V, Takahashi K, Park EK, Le GV,
Hara T and Sorahan T: Global mesothelioma deaths reported to the
World Health Organization between 1994 and 2008. Bull World Health
Organ. 89:716–724, 724A-724C. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim J, Bhagwandin S and Labow DM:
Malignant peritoneal mesothelioma: A review. Ann Transl Med.
5:2362017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Babcock TL, Powell DH and Bothwell RS:
Radiation-induced peritoneal mesothelioma. J Surg Oncol. 8:369–372.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antman KH, Corson JM, Li FP, Greenberger
J, Sytkowski A, Henson DE and Weinstein L: Malignant mesothelioma
following radiation exposure. J Clin Oncol. 1:695–700. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hofmann J, Mintzer D and Warhol MJ:
Malignant mesothelioma following radiotherapy. Am J Med.
97:379–382. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kinutani M, Nagai N, Kurihara K, Sakata K,
Tanimoto H, Murakami T, Takehara K, Takenaka M, Okamoto E and Ohama
K: A case of malignant mesothelioma arising from uterine serosa
after radiation therapy in uterine cervical cancer. Nihon Sanka
Fujinka Gakkai Zasshi. 46:911–914. 1994.(In Japanese). PubMed/NCBI
|
|
9
|
Cavazza A, Travis LB, Travis WD, Wolfe JT
III, Foo ML, Gillespie DJ, Weidner N and Colby TV: Post-irradiation
malignant mesothelioma. Cancer. 77:1379–1385. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sato F, Yamazaki H, Ataka K, Mashima I,
Suzuki K, Takahashi T, Umezu H and Gejyo F: Malignant peritoneal
mesothelioma associated with deep vein thrombosis following
radiotherapy for seminoma of the testis. Intern Med. 39:920–924.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beier KM, Gallup DG, Burgess R and Stock
RJ: Occurrence of malignant peritoneal mesothelioma after surgery
and irradiation for cervical cancer. Gynecol Oncol. 17:375–380.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Horie A, Hiraoka K, Yamamoto O, Haratake
J, Tsuchiya T and Sugimoto H: An autopsy case of peritoneal
malignant mesothelioma in a radiation technologist. Acta Pathol
Jpn. 40:57–62. 1990.PubMed/NCBI
|
|
13
|
Domènech-Vilardell A, Rasiej MJ, Taub RN
and Ichise M: Clinical utility of 18F-FDG positron emission
tomography in malignant peritoneal mesothelioma. Q J Nucl Med Mol
Imaging. 60:54–61. 2016.PubMed/NCBI
|
|
14
|
Husain AN, Colby TV, Ordonez NG, Allen TC,
Attanoos RL, Beasley MB, Butnor KJ, Chirieac LR, Churg AM, Dacic S,
et al: Guidelines for pathologic diagnosis of malignant
mesothelioma. 2017 Update of the consensus statements from the
International Mesothelioma Interest Group. Arch Pathol Lab Med.
142:89–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baker PM, Clement PB and Young RH:
Malignant peritoneal mesothelioma in women: A study of 75 cases
with emphasis on their morphologic spectrum and differential
diagnosis. Am J Clin Pahol. 123:724–737. 2005. View Article : Google Scholar
|
|
16
|
Takeshima Y, Inai K, Amatya VJ, Gemba K,
Aoe K, Fujimoto N, Kato K and Kishimoto T: Accuracy of pathological
diagnosis of mesothelioma cases in Japan: Clinicopathological
analysis of 382 cases. Lung Cancer. 66:191–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Farioli A, Ottone M, Morganti AG,
Compagnone G, Romani F, Cammelli S, Mattioli S and Violante FS:
Radiation-induced mesothelioma among long-term solid cancer
survivors: A longitudinal analysis of SEER database. Cancer Med.
5:950–959. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gemba K, Fujimoto N, Kato K, Aoe K,
Takeshima Y, Inai K and Kishimoto T: National survey of malignant
mesothelioma and asbestos exposure in Japan. Cancer Sci.
103:483–490. 2012. View Article : Google Scholar : PubMed/NCBI
|