Introduction
Lobectomy has been the standard procedure for lung
cancer resection since the widespread acceptance of the 1995 Lung
Cancer Study Group (LCSG) randomized trial of lobectomy compared
with limited resection for stage IA non-small-cell lung cancer
(NSCLC) (1). However, lung cancer
screening with low-dose computed tomography (CT) and widespread use
of spiral CT imaging has contributed to the identification and
diagnosis of early-stage NSCLC (2,3). In the
past decade, the number of patients presenting with very small and
peripheral lung cancers has markedly increased. Meanwhile, a
growing population of older patients with significant medical
comorbidities that preclude major operations are being diagnosed
with early lung cancer. These factors have led to the popularity of
sublobar resection in recent years.
Early lung cancer presents as a wide area of
ground-glass opacity (GGO) on CT scans, which is likely to be less
invasive adenocarcinoma associated with a good prognosis (4–6).
Therefore, these patients are considered to be feasible candidates
for sublobar resections, i.e. segmentectomy or wedge resection, as
confirmed by the prospective JCOG 0201 study in Japan (4).
However, radiologically solid-dominant lung cancer
has been regarded as a different, highly invasive category of lung
cancer (7,8). Thus, sublobar resection for lung cancer
with a solid-dominant appearance on thin-section CT scans, namely
invasive lung cancer, remains controversial (9,10). A
total of three multi-center, prospective, randomized studies
focused on this issue are currently ongoing, and the data have not
yet been published (11–13).
Segmentectomy, rather than wedge resection, is
preferred for patients with stage IA NSCLC as it is an anatomical
resection involving more extensive lymph node dissection (14–16).
Whether sublobar resection, particularly segmentectomy, is
comparable to lobectomy in terms of oncologic outcomes in
radiologically solid (i.e. invasive) NSCLC remains unknown.
This meta-analysis investigated whether sublobar
resection has comparable oncologic outcomes to lobectomy in lung
cancer with a solid-dominant appearance. The evaluated outcomes
were local recurrence, distant recurrence, recurrence-free survival
(RFS) and overall survival (OS).
Materials and methods
Search strategies
Systematic computerized searches of the PubMed,
Embase and Cochrane Library databases and Google Scholar were
performed from their dates of inception to April 2017. The
following search terms were used: ‘non-small-cell lung cancer
(NSCLC)/lung cancer’ and
‘lobectomy/sublobectomy/segmentectomy/limited resection/sublobar
resection’ and ‘recurrence/prognosis/survival’ and ‘solid’. The
search was limited to English and the Abstract/Title. The citation
lists of all retrieved articles were scanned to identify other
potentially relevant publications.
Study selection
The following criteria were used for study
inclusion. i) Either completed randomized controlled trials (RCTs)
or retrospective observational studies that compared sublobar
resection/segmentectomy with lobectomy in treating patients with
clinical stage IA NSCLC, according to the 7th edition of TNM
classification (17). ii) Nodules
were peripheral with ‘solid or solid-dominant appearance’ on
thin-section CT. The solid component was defined as an area of
increased opacification that completely obscured the underlying
vascular markings. GGO was defined as an area of slight,
homogeneous increase in density that did not obscure the underlying
vascular markings (9). In the
current study, a solid tumor was defined as a tumor exhibiting only
consolidation without GGO, while a solid dominant tumor was defined
as a tumor in which the ratio of the maximum diameter of
consolidation to the maximum tumor diameter was >50% on
thin-section CT. iii) The primary outcomes of interest in this
study were OS, disease-free survival (DFS)/RFS, and local or
distant recurrence rate. Only studies that reported at least one of
the outcomes were included. iv) The most recent or completed study
was chosen if the studies were based on overlapping patients.
The exclusion criteria were as follows: i) Stage IA
lung cancer was characterized as GGO-dominant or its nature was not
described on CT; ii) papers that were not published in English; and
iii) case reports, abstracts, conference reports, reviews and
experiments.
Statistical analysis
The meta-analysis was performed in accordance with
the recommendations of the Cochrane Collaboration and the Quality
of Reporting of Meta-analyses (QUORUM) guidelines (18,19). The
hazard ratio (HR) was used as a summary statistic for censored
outcomes (OS and RFS), as previously described (20). An HR >1 represented a survival
benefit favoring the sublobar resection/segmentectomy group. The
odds ratio (OR) was used as the summary statistic for dichotomous
variables. An OR <1 favored the sublobar resection/segmentectomy
group. The pooled OR/HR and 95% confidence intervals (CIs) were
graphically presented as forest plots. The effect measure OR/HR was
calculated by a fixed effects model (using the Mantel-Haenszel
method) or a random effects model (using the DerSimonian and Laird
method) based on the heterogeneity among studies (21,22).
The heterogeneity of the included
studies was detected using the Cochran test
Random effects models were used if high
heterogeneity was detected among the studies (P<0.1 or
I2 >50%). Otherwise, fixed effects models were
used. To combine the data, an HR with 95% CI was used, which were
directly obtained from the original articles. When the HR was not
directly reported in the original articles, it was estimated, as
previously described (23). A funnel
plot and the Egger test were used to investigate possible
publication bias (24). Statistical
significance was set at P<0.05.
Analyses were performed using STATA version 13.0
software (Stata Corporation).
Results
Literature Search and Study
Characteristics
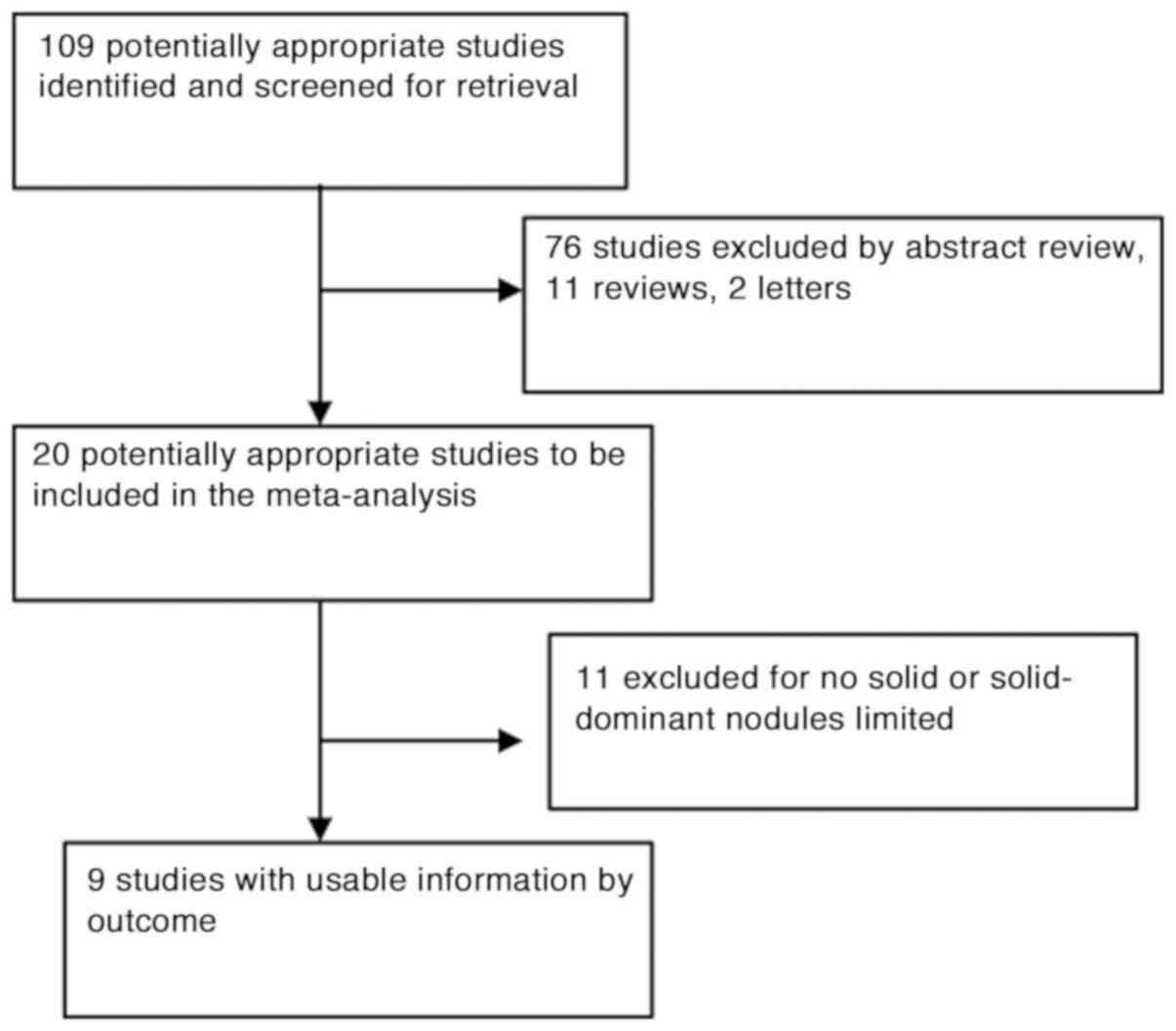
A total of 109 publications were identified using
the predefined search strategy (Fig.
1). Eighty-nine studies were excluded after screening the
titles and abstracts, and full texts of the remaining 20 studies
were retrieved. These included 11 reviews, two letters and 76
studies, which were either wedge resection/sublobar resection
versus lobectomy or insufficient data for the specified endpoints.
Eleven were excluded due to lack of definite solid or
solid-dominant nodules reporting. Finally, nine studies that met
the inclusion criteria were included in the meta-analysis (Fig. 1). Study characteristics are
summarized in Table I. The combined
study population from the included studies was 2,265 patients with
1,728 lobectomies, 425 segmentectomies and 112 wedge resections.
All studies were retrospective studies. Seven studies reported
several pathological types of NSCLC, whereas two studies only
included adenocarcinoma. In four studies, sublobar resection
involved segementectomy and wedge resection, but in the other five
studies, sublobar resection only referred to segmentectomy. Five
studies described intentional sublobar resection, three compromised
procedure and one had both categories.
 | Table I.General characteristics of the
enrolled studies. |
Table I.
General characteristics of the
enrolled studies.
|
|
|
|
|
|
| Extent of
resection |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Author, year | Refs. | Institution | Study period | Total patients | Including
criteria | Lob | Seg | WR | Stagea | Tool of
staging | Outcomes | Selection category
of sub |
|---|
| Hattori et
al, 2017 | (25) | Juntendo University
School of Medicine | 2008-2014 | 353 | Solid-dominant | 270 | 83 |
| cT1aN0M0 | PET-CT | RFS OS | Intentional |
| Hattori et
al, 2016 | (26) | Juntendo University
School of Medicine | 2008-2013 | 154 | Solid-dominant and
pure solid | 123 | 31 |
| cT1bN0M0 | PET-CT | RFS OS | Intentional |
| Altorki et
al, 2014 | (27) | I-ELCAP
database | 1993-2011 | 347 | Solid nodules | 294 | 16 | 37 | cIA | PET-CT | 10-year
Kaplan-Meier | Intentional |
| Tsutani et
al, 2014 | (28) | Hiroshima
University, Kanagawa Cancer Center, Cancer Institute Hospital, and
Hyogo Cancer Center, Japan | 2005-2010 | 327 | ≥50% solid
component | 286 | 41 |
| cIA | PET-CT | RFS | Intentional |
| Koike et al,
2016 | (29) | Niigata University
Hospital and Niigata Cancer Center Hospital | 1998-2009 | 251 | Without GGO | 151 | 100 |
| cT1aN0M0 | CT | OS DFS | Intentional (n=74)
and compromised (n=26) |
| Fiorelli et
al, 2016 | (30) | Multicenter | 2006-2016 | 239 | Solid-dominant and
pure solid | 149 | 39 | 51 | cT1a,b-2aN0M0 | PET-CT | OS DFS | Compromised |
| Kodama et
al, 2016 | (31) | Osaka Medical
Center cancer registry database | 1997-2010 | 312 | Solid and
part-solid | 232 | 80 |
| cT1aN0M0 | CT, PET-CT | OS RFS | Intentional |
| Jeon et al,
2014 | (32) | Seoul St. Mary's
Hospital | 2001-2010 | 164 | Without GGO | 133 | 12 | 19 | cIA | CT, PET-CT | OS DFS | Compromised |
| Inoue et al,
2010 | (33) | Osaka University
Hospital, Osaka, Japan | 1992-2007 | 118 | Solid or solid
dominant | 90 | 23 | 5 | cIA | CT, PET-CT | DFS | Compromised |
Primary outcome measures
Local and Distant Recurrence
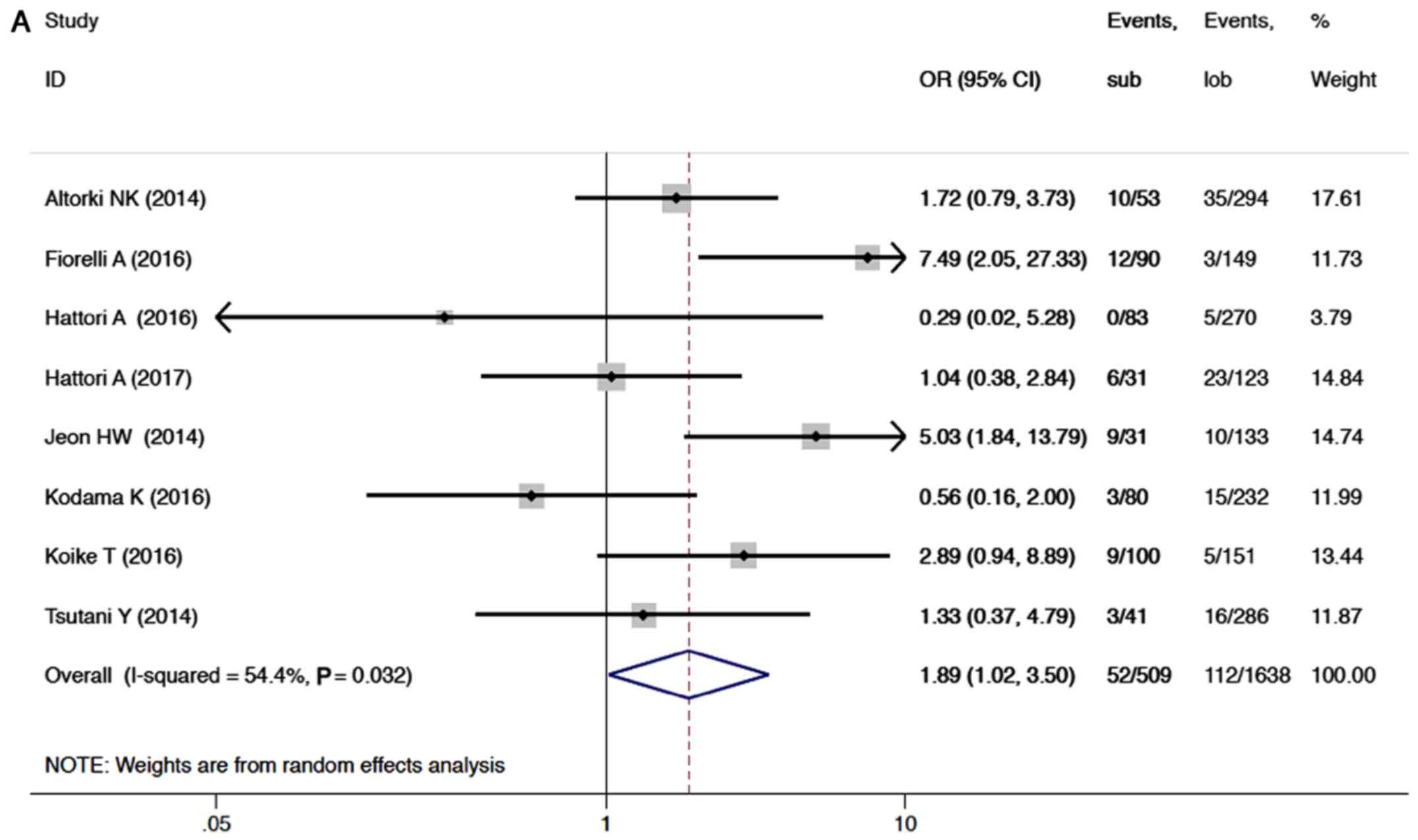
Eight studies reported local recurrence in the
sublobar resection and lobectomy groups, providing a total sample
size of 2,147 patients for evaluation. Meta-analysis of the data
showed that patients treated with a sublobar resection were
inferior to patients treated with lobectomy, with a pooled OR of
1.89 (95% CI, 1.02–3.50; P=0.04; Fig.
2A). There was moderate inter-study heterogeneity (P=0.03;
I2=54%).
However, in subgroup analysis of anatomic
segmentectomy versus lobectomy, there was no obvious difference in
local recurrence between the two groups (OR=1.19; 95% CI,
0.68–2.10; P=0.61; Fig. 2B).
Inter-study heterogeneity was not significant in subgroup analysis
(P=0.316; I2=15.5%).
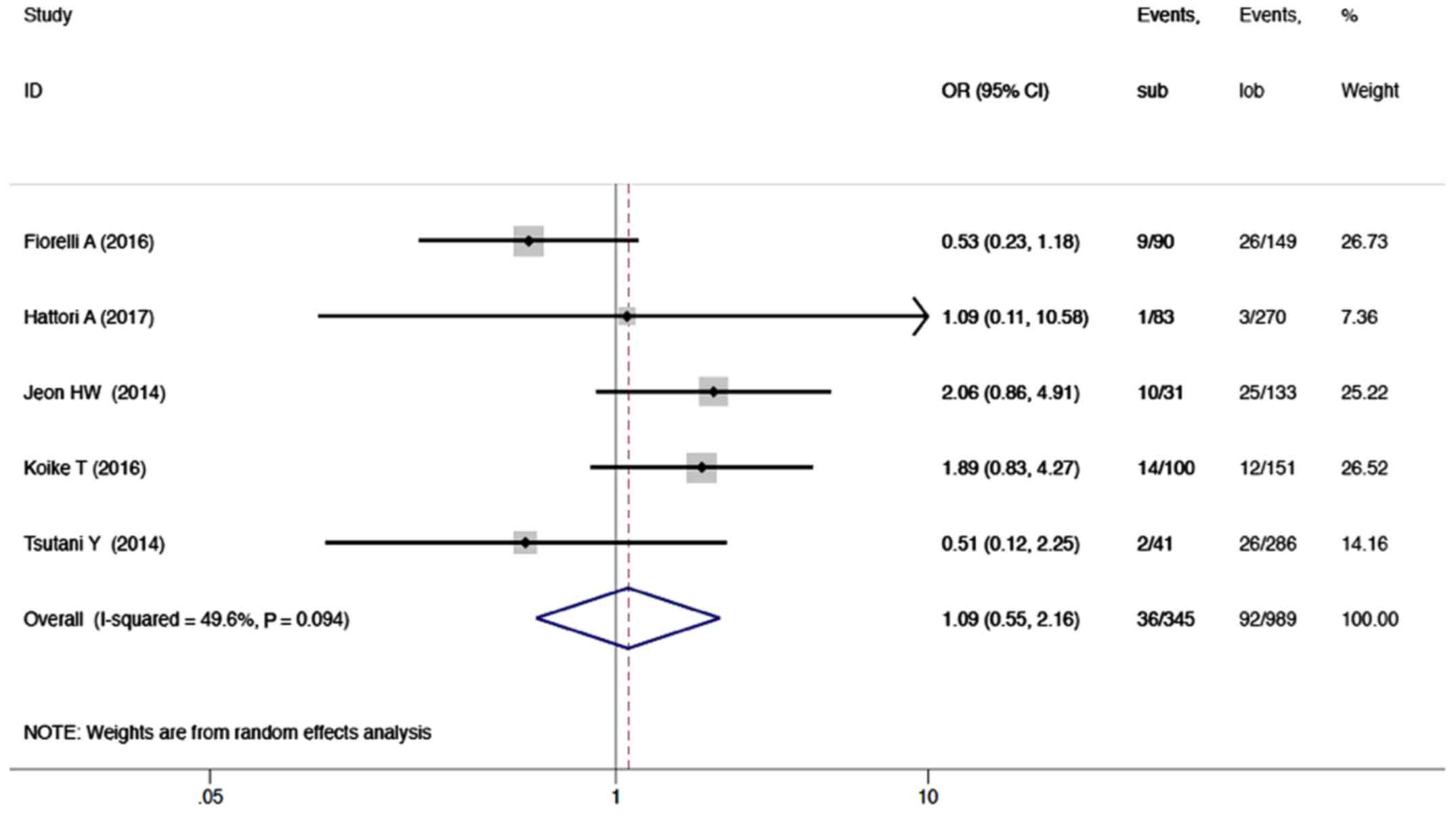
Sublobar resection was not associated with a
significant negative impact on distant recurrence based on five
reports (1,334 patients) as compared to lobectomy. The OR was 1.09
(95% CI, 0.55–2.16; P=0.796; I2=49.6%; Fig. 3).
RFS and OS
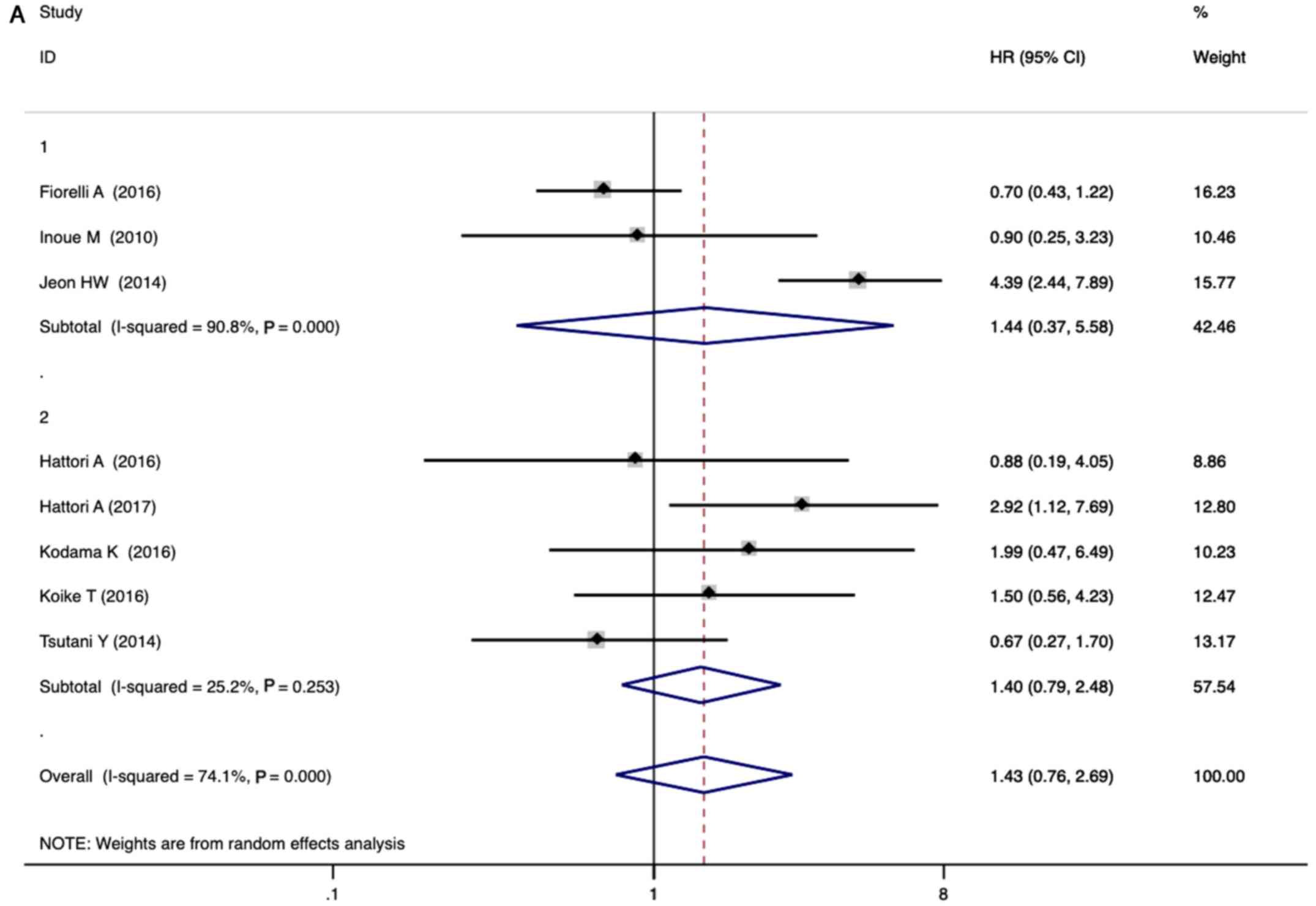
For RFS, eight eligible studies that included a
total of 1918 patients were pooled. Given the significant
heterogeneity among studies (I2=74.1%), a
random-effects model was used to pool the HR of the studies. As
seen in Fig. 4A, the combined HR for
the eight studies was 1.43 (95% CI, 0.76–2.69; P=0.27). Both in
intentional and compromised sublobar resection groups, there are
similar combined HR (1.40 vs. 1.44) in RFS. Accordingly, there was
no statistical difference in RFS between sublobar resection (both
intentional and compromised sublobar resection) and lobectomy
group. In subgroup analysis of anatomic segmentectomy versus
lobectomy, there was no statistical difference in RFS (HR=1.40; 95%
CI, 0.79–2.48; P=0.244; Fig. 4B).
Furthermore, inter-study heterogeneity was not obvious in subgroup
analysis (P=0.253; I2=25.2%).
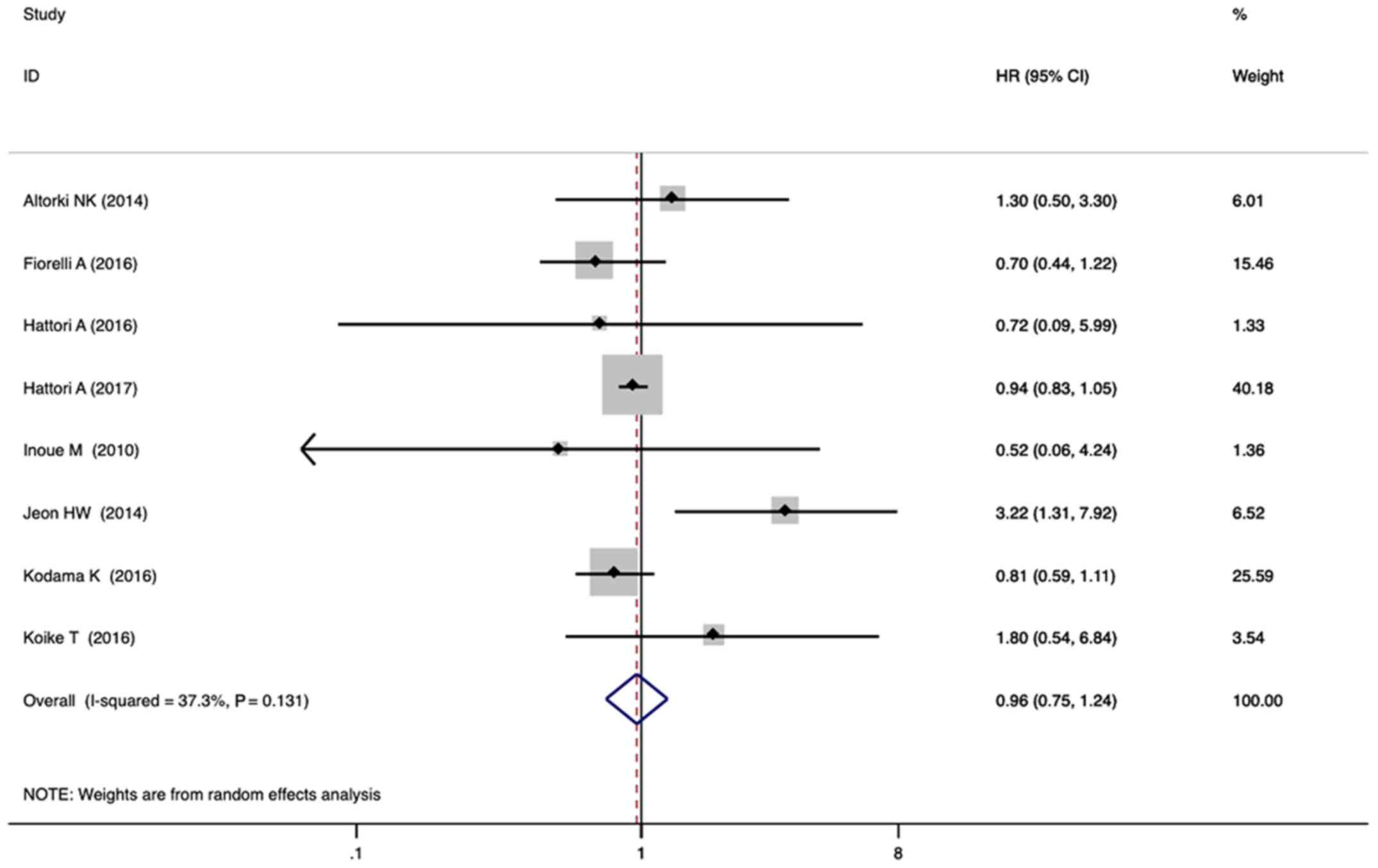
For OS, eight studies with a total of 1938 patients
were included in the quantitative analysis. The combined HR for the
eight studies was 0.96 (95% CI, 0.75–1.24; P=0.77; Fig. 5) with a random-effects model,
indicating that sublobar resection was associated with similar OS
as lobectomy. There was minor heterogeneity between studies
(I2=37.3%; P=0.13).
Publication Bias
The results of the Egger test did not suggest any
evidence of publication bias in local and distant recurrence
(P=0.933, P=0.699; respectively). For RFS and OS, the funnel plots
provided no evidence of overt publication bias. The Egger test also
showed no significant publication bias in RFS (P=0.774) and OS
(P=0.557).
Discussion
Lobectomy is widely recommended as the standard
treatment for patients with stage IA NSCLC who can tolerate the
procedure (1). Recently, sublobar
resection (including wedge resection and segmentectomy) was
suggested as an alternative surgical treatment for early-stage
NSCLC (34–45). A number of meta-analyses have
reported that sublobar resection or segmentectomy have comparable
oncologic outcomes to lobectomy in patients with stage IA NSCLC
(46–48). The most favorable subset consists of
radiologically non-invasive lung cancer, usually defined as a
consolidation/tumor (C/T) ratio <0.5 on thin-section CT
(4,49,50).
Recently, several studies have compared the efficacy of sublobar
resection with lobectomy for treating solid-dominant stage IA
NSCLC, which is conventionally an unfavorable indication for
sublobar resection (51,52). Therefore, this meta-analysis
involving nine studies and 2,265 patients was conducted to examine
the efficacy of sublobar resection for the treatment of
solid-dominant stage IA NSCLC.
According to this meta-analysis, sublobar resection
had a higher local recurrence rate than lobectomy in solid-dominant
NSCLC. However, there was no significant difference between the
local recurrence rates of segmentectomy and lobectomy. Moreover,
the distant recurrence risk was comparable between sublobar
resection and lobectomy. The RFS and OS values of patients with
solid-dominant tumors who underwent sublobar resection were similar
to those of patients who underwent lobectomy. Despite the obvious
heterogeneity between studies in RFS, studies with intentional
sublobar resection had no obvious heterogeneity. The subgroup
analysis showed that segmentectomy was equivalent to lobectomy with
respect to RFS, with no obvious heterogeneity. The results
indicated that anatomic segmentectomy with systemic lymph node
dissection may be an alternative surgical procedure for
solid-dominant stage IA NSCLC. These findings should be confirmed
in prospective studies, such as JCOG 0802/WJOG4607L (11,12) and
NCT00499330 (13).
In comparing with other meta-analyses, the present
study selected patients with radiologically solid-dominant early
stage NSCLC. Up to now, the use of segmentectomy in radiologically
solid-dominant early stage NSCLC has been controversial. Given the
lack of solid evidence, it is necessary to summarize the relevant
studies prior to the results of several large RCTs being delivered.
This analysis suggested segmentectomy had comparable oncologic
efficacy to lobectomy for solid-dominant stage IA NSCLC. This may
be a novel concept.
The present meta-analysis had several limitations.
First, the meta-analysis was based on retrospective cohort studies
and the level of evidence was relatively low compared with that for
RCTs. The number of studies was limited. In addition, not all
studies were of high quality, which introduced a potential bias.
Second, even though subgroup analyses were conducted, heterogeneity
persisted in the meta-analysis, primarily due to the use of wedge
resection in four studies. Numerous studies have highlighted the
technical and oncological differences between wedge resection and
segmentectomy (14–16). Segmentectomy is an anatomic resection
frequently accompanied by hilar and mediastinal lymph node sampling
or dissection. It is inappropriate to combine the two
oncologically-distinct procedures of wedge resection and
segmentectomy in radiologically-invasive stage IA lung cancer.
Moreover, heterogeneity was also observed in solid part ratio (pure
solid or solid-dominant type), tumor size (T1a or T1b) and the
accuracy of clinical staging. Third, publication bias is a major
concern in all meta-analyses. Although the present analysis did not
show publication bias, it should be noted that this meta-analysis
could not completely exclude biases. For example, intentional
sublobar resection had a different compromised selection criteria,
which may lead to bias. Finally, some of the included studies
reported a relatively short follow-up duration. Therefore, RCTs
with longer follow-up durations are needed to precisely compare
segmentectomy (not including wedge resection) with lobectomy in
solid-dominant stage IA NSCLC.
The current meta-analysis suggested that lobectomy
and sublobar resection for solid-dominant stage IA NSCLC were
equivalent with respect to distant recurrence, RFS and OS, but the
outcome for local recurrence with sublobar resection was inferior
to that with lobectomy. Nevertheless, segmentectomy had comparable
oncologic efficacy to lobectomy for solid-dominant stage IA NSCLC.
Therefore, segmentectomy with systemic node dissection/sampling may
be a feasible alternative in selected solid-dominant (not
pure-solid) stage IA NSCLC cases, such as smaller peripheral
neoplastic nodules (53), air
bronchogram (54) and lower SUVmax
(10,55,56).
However, these findings should be confirmed by prospective
randomized controlled trials in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG participated in the literature search, performed
the statistical analysis, and drafted the manuscript. ZR, JL and BW
participated in the literature search. YL and CL participated in
the study design. XT conceived the study, participated in its
design and coordination, and helped to draft the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small-cell lung cancer
|
|
HR
|
hazard ratio
|
|
OR
|
odds ratio
|
|
RFS
|
recurrence-free survival
|
|
OS
|
overall survival
|
|
CT
|
computer tomography
|
|
GGO
|
ground-glass opacity
|
|
DFS
|
disease-free survival
|
|
RCT
|
randomized control trial
|
References
|
1
|
Ginsberg RJ and Rubinstein LV; Lung Cancer
Study Group, : Randomized trial of lobectomy versus limited
resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg.
60:615–622; discussion 622–623. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Church TR, Black WC, Aberle DR, Berg CD,
Clingan KL, Duan F, Fagerstrom RM, Gareen IF, Gierada DS, Jones GC,
et al National Lung Screening Trial Research Team, : Results of
initial low-dose computed tomographic screening for lung cancer. N
Engl J Med. 368:1980–1991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horeweg N, Scholten ET, de Jong PA, van
der Aalst CM, Weenink C, Lammers JW, Nackaerts K, Vliegenthart R,
ten Haaf K, Yousaf-Khan UA, et al: Detection of lung cancer through
low-dose CT screening (NELSON): A prespecified analysis of
screening test performance and interval cancers. Lancet Oncol.
15:1342–1350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asamura H, Hishida T, Suzuki K, Koike T,
Nakamura K, Kusumoto M, Nagai K, Tada H, Mitsudomi T, Tsuboi M, et
al Japan Clinical Oncology Group Lung Cancer Surgical Study Group,
: Radiographically determined noninvasive adenocarcinoma of the
lung: Survival outcomes of Japan Clinical Oncology Group 0201. J
Thorac Cardiovasc Surg. 146:24–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho JH, Choi YS, Kim J, Kim HK, Zo JI and
Shim YM: Long-term outcomes of wedge resection for pulmonary
ground-glass opacity nodules. Ann Thorac Surg. 99:218–222. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsutani Y, Miyata Y, Nakayama H, Okumura
S, Adachi S, Yoshimura M and Okada M: Appropriate sublobar
resection choice for ground glass opacity-dominant clinical stage
IA lung adenocarcinoma: Wedge resection or segmentectomy. Chest.
145:66–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsutani Y, Miyata Y, Yamanaka T, Nakayama
H, Okumura S, Adachi S, Yoshimura M and Okada M: Solid tumors
versus mixed tumors with a ground-glass opacity component in
patients with clinical stage IA lung adenocarcinoma: Prognostic
comparison using high-resolution computed tomography findings. J
Thorac Cardiovasc Surg. 146:17–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsunaga T, Suzuki K, Takamochi K and Oh
S: What is the radiological definition of part-solid tumour in lung
cancer? Eur J Cardiothorac Surg. 51:242–247. 2017.PubMed/NCBI
|
|
9
|
Hattori A, Matsunaga T, Hayashi T,
Takamochi K, Oh S and Suzuki K: Prognostic impact of the findings
on thin-section computed tomography in patients with subcentimeter
non-small cell lung cancer. J Thorac Oncol. 12:954–962. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hattori A, Suzuki K, Matsunaga T, Miyasaka
Y, Takamochi K and Oh S: What is the appropriate operative strategy
for radiologically solid tumours in subcentimetre lung cancer
patients? Eur J Cardiothorac Surg. 47:244–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura K, Saji H, Nakajima R, Okada M,
Asamura H, Shibata T, Nakamura S, Tada H and Tsuboi M: A phase III
randomized trial of lobectomy versus limited resection for
small-sized peripheral non-small cell lung cancer
(JCOG0802/WJOG4607L). Jpn J Clin Oncol. 40:271–274. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang F, Sui X, Chen X, Zhang L, Wang X,
Wang S and Wang J: Sublobar resection versus lobectomy in Surgical
Treatment of Elderly Patients with early-stage non-small cell lung
cancer (STEPS): Study protocol for a randomized controlled trial.
Trials. 17:1912016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
ClinicalTrials.gov, . Comparison of
different types of surgery in treating patients with stage IA
non-small cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT00499330?term2018
|
|
14
|
Tamura M, Matsumoto I, Takata M, Yoshida S
and Saito D: Sublobar resections in stage IA non-small-cell lung
cancer: Segmentectomy versus wedge resection. Indian J Thorac
Cardiovasc Surg. 30:264–271. 2014. View Article : Google Scholar
|
|
15
|
Sienel W, Dango S, Kirschbaum A, Cucuruz
B, Hörth W, Stremmel C and Passlick B: Sublobar resections in stage
IA non-small cell lung cancer: Segmentectomies result in
significantly better cancer-related survival than wedge resections.
Eur J Cardiothorac Surg. 33:728–734. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith CB, Swanson SJ, Mhango G and
Wisnivesky JP: Survival after segmentectomy and wedge resection in
stage I non-small-cell lung cancer. J Thorac Oncol. 8:73–78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L;
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions. The
IASLC Lung Cancer Staging Project, : proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jadad AR, Cook DJ, Jones A, Klassen TP,
Tugwell P, Moher M and Moher D: Methodology and reports of
systematic reviews and meta-analyses: A comparison of Cochrane
reviews with articles published in paper-based journals. JAMA.
280:278–280. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of observational studies in epidemiology: A
proposal for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siannis F, Barrett JK, Farewell VT and
Tierney JF: One-stage parametric meta-analysis of time-to-event
outcomes. Stat Med. 29:3030–3045. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
23
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hattori A, Matsunaga T, Takamochi K, Oh S
and Suzuki K: Locoregional recurrence after segmentectomy for
clinical-T1aN0M0 radiologically solid non-small-cell lung
carcinoma. Eur J Cardiothorac Surg. 51:518–525. 2017.PubMed/NCBI
|
|
26
|
Hattori A, Matsunaga T, Takamochi K, Oh S
and Suzuki K: The oncological outcomes of segmentectomy in
clinical-T1b lung adenocarcinoma with a solid-dominant appearance
on thin-section computed tomography. Surg Today. 46:914–921. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Altorki NK, Yip R, Hanaoka T, Bauer T, Aye
R, Kohman L, Sheppard B, Thurer R, Andaz S, Smith M, et al I-ELCAP
Investigators, : Sublobar resection is equivalent to lobectomy for
clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc
Surg. 147:754–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsutani Y, Miyata Y, Nakayama H, Okumura
S, Adachi S, Yoshimura M and Okada M: Segmentectomy for clinical
stage IA lung adenocarcinoma showing solid dominance on radiology.
Eur J Cardiothorac Surg. 46:637–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koike T, Kitahara A, Sato S, Hashimoto T,
Aoki T, Koike T, Yoshiya K, Toyabe S and Tsuchida M: Lobectomy
versus segmentectomy in radiologically pure solid small-sized
non-small cell lung cancer. Ann Thorac Surg. 101:1354–1360. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fiorelli A, Caronia FP, Daddi N, Loizzi D,
Ampollini L, Ardò N, Ventura L, Carbognani P, Potenza R, Ardissone
F, et al: Sublobar resection versus lobectomy for stage I non-small
cell lung cancer: An appropriate choice in elderly patients? Surg
Today. 46:1370–1382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kodama K, Higashiyama M, Okami J, Tokunaga
T, Imamura F, Nakayama T, Inoue A and Kuriyama K: Oncologic
outcomes of segmentectomy versus lobectomy for clinical T1a N0 M0
non-small cell lung cancer. Ann Thorac Surg. 101:504–511. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jeon HW, Kim YD, Kim KS, Sung SW, Park HJ
and Park JK: Sublobar resection versus lobectomy in solid-type,
clinical stage IA, non-small cell lung cancer. World J Surg Oncol.
12:2152014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Inoue M, Minami M, Sawabata N, Utsumi T,
Kadota Y, Shigemura N and Okumura M: Clinical outcome of resected
solid-type small-sized c-stage IA non-small cell lung cancer. Eur J
Cardiothorac Surg. 37:1445–1449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
El-Sherif A, Gooding WE, Santos R,
Pettiford B, Ferson PF, Fernando HC, Urda SJ, Luketich JD and
Landreneau RJ: Outcomes of sublobar resection versus lobectomy for
stage I non-small cell lung cancer: A 13-year analysis. Ann Thorac
Surg. 82:408–416. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hamatake D, Yoshida Y, Miyahara S,
Yamashita S, Shiraishi T and Iwasaki A: Surgical outcomes of lung
cancer measuring less than 1 cm in diameter. Interact Cardiovasc
Thorac Surg. 15:854–858. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang W, Pang X, Xi J, Chen X, Wang Q,
Qian C and Fan H: Clinical outcome of subcentimeter non-small cell
lung cancer after surgical resection: Single institution experience
of 105 patients. J Surg Oncol. 110:233–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kates M, Swanson S and Wisnivesky JP:
Survival following lobectomy and limited resection for the
treatment of stage I non-small cell lung cancer<=1 cm in size: A
review of SEER data. Chest. 139:491–496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kilic A, Schuchert MJ, Pettiford BL,
Pennathur A, Landreneau JR, Landreneau JP, Luketich JD and
Landreneau RJ: Anatomic segmentectomy for stage I non-small cell
lung cancer in the elderly. Ann Thorac Surg. 87:1662–1668. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koike T, Koike T, Sato S, Hashimoto T,
Aoki T, Yoshiya K, Yamato Y, Watanabe T, Akazawa K, Toyabe SI, et
al Niigata Chest Surgery Research Group, : Lobectomy and limited
resection in small-sized peripheral non-small cell lung cancer. J
Thorac Dis. 8:3265–3274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Landreneau RJ, Normolle DP, Christie NA,
Awais O, Wizorek JJ, Abbas G, Pennathur A, Shende M, Weksler B,
Luketich JD, et al: Recurrence and survival outcomes after anatomic
segmentectomy versus lobectomy for clinical stage I non-small-cell
lung cancer: A propensity-matched analysis. J Clin Oncol.
32:2449–2455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okada M, Koike T, Higashiyama M, Yamato Y,
Kodama K and Tsubota N: Radical sublobar resection for small-sized
non-small cell lung cancer: A multicenter study. J Thorac
Cardiovasc Surg. 132:769–775. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Okami J, Ito Y, Higashiyama M, Nakayama T,
Tokunaga T, Maeda J and Kodama K: Sublobar resection provides an
equivalent survival after lobectomy in elderly patients with early
lung cancer. Ann Thorac Surg. 90:1651–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsutani Y, Miyata Y, Nakayama H, Okumura
S, Adachi S, Yoshimura M and Okada M: Oncologic outcomes of
segmentectomy compared with lobectomy for clinical stage IA lung
adenocarcinoma: Propensity score-matched analysis in a multicenter
study. J Thorac Cardiovasc Surg. 146:358–364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yano M, Yoshida J, Koike T, Kameyama K,
Shimamoto A, Nishio W, Yoshimoto K, Utsumi T, Shiina T, Watanabe A,
et al Japanese Association for Chest Surgery, : Survival of 1737
lobectomy-tolerable patients who underwent limited resection for
cStage IA non-small-cell lung cancer. Eur J Cardiothorac Surg.
47:135–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao ZR, Situ DR, Lau RW, Mok TSK, Chen
GG, Underwood MJ and Ng CS: Comparison of Segmentectomy and
Lobectomy in Stage IA Adenocarcinomas. J Thorac Oncol. 12:890–896.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang L, Li M, Yin R, Zhang Q and Xu L:
Comparison of the oncologic outcomes of anatomic segmentectomy and
lobectomy for early-stage non-small cell lung cancer. Ann Thorac
Surg. 99:728–737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fan J, Wang L, Jiang GN and Gao W:
Sublobectomy versus lobectomy for stage I non-small-cell lung
cancer, a meta-analysis of published studies. Ann Surg Oncol.
19:661–668. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cao C, Chandrakumar D, Gupta S, Yan TD and
Tian DH: Could less be more?-A systematic review and meta-analysis
of sublobar resections versus lobectomy for non-small cell lung
cancer according to patient selection. Lung Cancer. 89:121–132.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Suzuki K, Koike T, Asakawa T, Kusumoto M,
Asamura H, Nagai K, Tada H, Mitsudomi T, Tsuboi M, Shibata T, et al
Japan Lung Cancer Surgical Study Group (JCOG LCSSG), : A
prospective radiological study of thin-section computed tomography
to predict pathological noninvasiveness in peripheral clinical IA
lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol.
6:751–756. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Razi SS, John MM, Sainathan S and
Stavropoulos C: Sublobar resection is equivalent to lobectomy for
T1a non-small cell lung cancer in the elderly: A Surveillance,
Epidemiology, and End Results database analysis. J Surg Res.
200:683–689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sawabata N: Is segmentectomy suitable for
solid-type lung cancer? J Thorac Cardiovasc Surg. 146:728–729.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Baisi A, Raveglia F, De Simone M and
Cioffi U: Do tumor size and carcinoembryonic antigen level affect
surgical management of partially solid early-stage lung cancer? Ann
Thorac Surg. 103:10362017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hattori A, Maeyashiki T, Matsunaga T,
Takamochi K, Oh S and Suzuki K: Predictors of pathological
non-invasive lung cancer with pure-solid appearance on computed
tomography to identify possible candidates for sublobar resection.
Surg Today. 46:102–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hattori A, Suzuki K, Maeyashiki T, Fukui
M, Kitamura Y, Matsunaga T, Miyasaka Y, Takamochi K and Oh S: The
presence of air bronchogram is a novel predictor of negative nodal
involvement in radiologically pure-solid lung cancer. Eur J
Cardiothorac Surg. 45:699–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Okada M, Mimae T, Tsutani Y, Nakayama H,
Okumura S, Yoshimura M and Miyata Y: Segmentectomy versus lobectomy
for clinical stage IA lung adenocarcinoma. Ann Cardiothorac Surg.
3:153–159. 2014.PubMed/NCBI
|
|
56
|
Okada M, Nakayama H, Okumura S, Daisaki H,
Adachi S, Yoshimura M and Miyata Y: Multicenter analysis of
high-resolution computed tomography and positron emission
tomography/computed tomography findings to choose therapeutic
strategies for clinical stage IA lung adenocarcinoma. J Thorac
Cardiovasc Surg. 141:1384–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|