Introduction
Hepatocellular carcinoma (HCC) is the most common
type of liver cancer, and is the third leading cause of cancer
deaths worldwide, causing nearly 745,000 deaths each year (1). HCC tends to invade vessels as it
progresses and is often associated with macroscopic vascular
invasion (MVI). Recent studies found that MVI including portal vein
tumor thrombosis (PVTT) and inferior vena cava tumor thrombosis
(IVCTT) were found in 12.5 to 39.7% of cases at the time of
diagnosis, respectively (2,3). The prognosis of HCC patients showing
MVI is extremely poor, and the median survival time (MST) of these
patients has been reported to be 2–3 months (2,4).
Sorafenib is the only evidence-based treatment option for patients
classified to be at an Advanced stage (C) in the Barcelona Clinic
Liver Cancer Staging System (BCLC) (5–7).
However, in a pooled analysis of 2 pivotal phase 3 trials,
sorafenib prolonged the median survival of these patients by only
47 days compared with placebo (7–9).
Prognosis improvement of advanced HCC is obtained by
combined modality therapy according to liver residual function and
progress (10,11). With recent technological advances,
external beam radiotherapy (RT) could be considered an alternative
treatment option for patients with HCC (12). Regarding the role of RT, the
effectiveness of three-dimensional conformal radiotherapy (3D-CRT)
has been recognized for treating PVTT/IVCTT (13). RT is a better initial therapy option
than sorafenib for patients who have advanced unresectable HCC with
PVTT (14). A combined treatment
consisting of transarterial chemoembolization (TACE) and RT has
shown promising radiologic response rates and improved overall
survival of HCC with MVI in observational studies (15,16).
Hepatic arterial infusion chemotherapy (HAIC) is
used more commonly than systemic chemotherapy, although no survival
advantage has been demonstrated. Randomized controlled studies are
currently underway to clarify the survival benefit of HAIC.
Moreover, various novel systemic chemotherapeutic agents are
currently under development in Japan, and further improvements in
the treatment outcomes are expected (17). High efficacy and safety of a new
combination therapy comprising of cisplatin-lipiodol suspension and
5-FU for HCC with PVTT, referred to as NewFP, has been reported; a
high response rate at 86.3% and a MST of 33 months has been
reported with this therapy regimen (18). In patients with advanced HCC and
major PVTT, survival was significantly longer in those treated with
HAIC combined with RT than it was with sorafenib (19).
This study aimed to investigate the efficacy and
safety of NewFP plus 3D-CRT for MVI as the initial treatment and
additionally that of sorafenib as the secondary treatment in
patients with advanced HCC showing MVI.
Patients and methods
Patients
This retrospective study enrolled patients from our
institute with unresectable advanced HCC who were treated with
NewFP plus 3D-CRT for MVI between January 2009 and December 2017.
In total, 32 HCC patients with MVI were registered retrospectively.
Of these patients, 18 were treated with NewFP plus 3D-CRT for MVI
and 14 were treated with sorafenib after NewFP plus 3D-CRT for
MVI.
The diagnosis of HCC was made on the basis of the
American Association for the Study of Liver Disease (AASLD)
guidelines (6). Inclusion criteria
were as follows: (i) HCC with PVTT in the first portal branch (Vp3)
or in the main portal trunk or contralateral portal branch (Vp4);
(ii) HCC with invasion to right or middle or left hepatic vein
(Vv2) or IVCTT (Vv3); (iii) removal of all detected tumors is
impossible with a sufficient hepatic functional reserve even if
thrombectomy using the peel-off technique is considered (20); (iv) Child-Pugh score of 5–8; (v) an
Eastern Cooperative Oncology Group (ECOG) performance status (PS)
of 0–2 (21); and (vi) no history of
radiotherapy to the liver or of sorafenib treatment.
After estimating the response to the initial
treatment with NewFP plus 3D-CRT for MVI, sorafenib was given to
patients who were not able to undergo curative therapies such as
hepatectomy and radiofrequency ablation (RFA) as a secondary
treatment.
This study was conducted in accordance with the 1975
Declaration of Helsinki after receiving approval from the
institutional review board of the Kagawa University, Kagawa, Japan
(approval no. Heisei29-192). The requirement for informed consent
from the participants was waived because of the retrospective
nature of the study.
Implantation of arterial catheter
An indwelling catheter (5-Fr W and G spiral
Catheter; Piolax, Tokyo, Japan) was inserted through the femoral
artery, with the distal end extended into the hepatic or
gastroduodenal artery with the proximal end connected to the port
system (P-U CELSITE PORT; TORAY, Tokyo, Japan), which was implanted
subcutaneously. The right gastric, gastroduodenal, and posterior
superior pancreaticoduodenal arteries were occluded with microcoils
to prevent gastroduodenal ulcers caused by anticancer agents.
Chemotherapy
HAIC involved cisplatin (50 mg fine powder in 5–10
ml lipiodol) and a continuous infusion of 5-FU (1,500 mg/5 days),
referred to as NewFP. On day 1 of treatment, cisplatin with
lipiodol was injected through the reservoir catheter, followed by
5-FU (250 mg). Following this, 5-FU (1,250 mg) was continuously
infused for 5 days using a balloon pump (SUREFUSER PUMP, Nipro
Pharma Corporation, Osaka, Japan). This regimen was administered
once per week during the first 2 weeks of admission. Subsequently,
a combination of 20–30 mg cisplatin with 2–6 ml lipiodol and
500–1,000 mg 5-FU was infused every 2 weeks at the out-patient
department for as long as possible (18). Treatment was discontinued in cases of
occurrence of grade 3 or higher adverse effects according to the
ECOG classification, with the exception of total bilirubin >3.0
mg/dl, platelet count <5×104/µl, and leukocyte count
<1,500/µl.
Radiotherapy
Planning computed tomography (CT) scans were
obtained under free-breathing conditions using scans with a scan
time of 3 sec per section. All patients underwent 3D-CRT, planned
using a radiation treatment planning system. The gross tumor volume
(GTV) consisted of the PVTT or IVCTT. A clinical target volume
(CTV) was defined as the GTV with or without the main tumor. If the
main tumor existed close to the GTV, the main tumor was included in
the CTV when possible. The planning target volume (PTV) consisted
of the CTV plus 8–10 mm margins. In principle, a prescribed dose at
an isocenter was 50 Gy in 25 fractions with 2 Gy per fraction once
daily using 6–10 MV photon beams delivered by a linear accelerator.
3D-CRT was started with the first cycle of HAIC.
Sorafenib treatment
Eligibility criteria for treatment with sorafenib
were as follows: (i) unresectable advanced HCC; (ii) no effect of
TACE; (iii) no previous sorafenib treatment for the liver tumor;
(iv) Child-Pugh class A or B (up to a score of 7 points) hepatic
function; (v) an ECOG performance status of 0–2 (21); and (vi) the following laboratory
findings: Leukocyte count >1,500/µl, platelet count
>7.5×104/µl, and serum hemoglobin level >8.5 g/dl.
Sorafenib was administered orally as a 400–800 mg dose daily per
the discretion of the chief physician. Dose reductions and
treatment interruptions were allowed according to drug-related
toxicity grades, as recommended.
Assessment of tumor response
To determine the therapeutic effect, baseline tumor
measurements were obtained within 1 month before treatment by
combining the largest diameters of selected target lesions in each
patient, as measured using CT or MRI. CT or MRI was performed 4–6
weeks after the initial treatment cycle and every 2–3 months
thereafter. The therapeutic effect was determined according to the
best overall response, which was defined by the Modified RECIST
(mRECIST) criteria (22). This was
as follows: Complete response (CR), the disappearance of any
intratumoral arterial enhancement in all target lesions; partial
response (PR), at least a 30% decrease in the sum of diameters of
viable (contrast enhancement in the arterial phase) target lesions,
taking as reference the baseline sum of the diameters of target
lesions; progressive disease (PD), an increase of at least 20% in
the sum of the diameters of viable (enhancing) target lesions,
taking as reference the smallest sum of the diameters of viable
(enhancing) target lesions recorded since treatment initiation; and
stable disease (SD), any case that does not qualify for either PR
or PD. Patients who died before their first radiographic assessment
were classified as having PD. Data from patients who died without
tumor progression were censored. The response rate was defined on
the basis of the independent radiologic review as the percentage of
patients whose best-response mRECIST rating of CR or PR was
maintained for at least 1 month after the first demonstration of
such a rating. The disease-control rate was defined on the basis of
independent radiologic review as the percentage of patients whose
best-response mRECIST rating of CR, PR, or SD was maintained for at
least 1 month after the first demonstration of such a rating.
Statistical analysis
All statistical analyses were performed using JMP
software (SAS Institute, Inc., Cary, NC, USA), version 13. Baseline
patient characteristics were analyzed using the Chi-square test,
the Welch's t test, or Fisher's exact probability test. Overall
survival rates and progression-free survival (PFS) were calculated
using the Kaplan-Meier method and compared using the log-rank test.
Changes in hepatic function using Child-Pugh scores before and
after 3D-CRT were analyzed using the Wilcoxon signed-rank test. The
Cox proportional-hazards model was used to evaluate the interaction
between baseline characteristics and overall survival or
therapeutic effect. All P values were two-tailed, and values less
than 0.05 were considered statistically significant.
Results
Patient characteristics
Of the 32 HCC patients, 18 were treated with NewFP
plus 3D-CRT for MVI (NewFP+3D-CRT group) and 14 were treated with
sorafenib after NewFP plus 3D-CRT for MVI (sorafenib after
NewFP+3D-CRT group). Comparisons of the clinical features between
the two groups are shown in Table I.
There were no significant differences in the baseline
characteristics between groups.
 | Table I.Patient characteristics (n=32).
Baseline patient characteristics were analyzed using the chi-square
test, Welch's t test, or Fisher's exact probability test. |
Table I.
Patient characteristics (n=32).
Baseline patient characteristics were analyzed using the chi-square
test, Welch's t test, or Fisher's exact probability test.
| Variables | NewFP+3D-CRT
(n=18) | Sorafenib after
NewFP+3D-CRT (n=14) | P-value |
|---|
| Age, median
(range) | 68 (37–83) | 68.5 (53–80) | 0.9130 |
| Sex, n (%) |
|
| 0.2379 |
|
Male | 15 (83.3) | 14 (100) |
|
|
Female | 3 (16.7) | 0 (0) |
|
| HBs antigen, n
(%) |
|
| 0.2302 |
|
Present | 6 (33.3) | 2 (14.3) |
|
|
Absent | 12 (66.7) | 12 (85.7) |
|
| HCV antibody, n
(%) |
|
| 0.1641 |
|
Present | 7 (38.9) | 9 (64.3) |
|
|
Absent | 11 (61.1) | 5 (35.7) |
|
| Child-Pugh score, n
(%) |
|
| 0.0590 |
| 5 | 1 (5.6) | 1 (7.1) |
|
| 6 | 6 (33.3) | 8 (57.1) |
|
| 7 | 7 (38.9) | 5 (35.7) |
|
| 8 | 4 (22.2) | 0 (0) |
|
| Tumor size,
maximum, median (mm) | 190, 66 | 137, 50 | 0.1587 |
| Number of tumors, n
(%) |
|
| 0.7120 |
|
<4 | 7 (38.9) | 4 (28.6) |
|
| ≥4 | 11 (61.1) | 10 (71.4) |
|
| Tumor extent, n
(%) |
|
| 0.7178 |
|
Unilobar involvement | 12 (66.7) | 8 (57.1) |
|
| Bilobar
involvement | 6 (33.3) | 6 (42.9) |
|
| AFP (ng/ml), n
(%) |
|
| 0.0993 |
|
<1,000 | 5 (27.8) | 8 (57.1) |
|
|
≥1,000 | 13 (72.2) | 6 (42.9) |
|
| DCP median
(mAU/ml), n (%) |
|
| 0.3893 |
|
<1,000 | 5 (27.8) | 6 (42.9) |
|
|
≥1,000 | 13 (72.2) | 8 (57.1) |
|
| Grade of portal
vein invasion, n (%) |
|
| 0.6744 |
|
Vp4 | 4 (22.2) | 3 (21.4) |
|
|
Vp3 | 14 (77.8) | 7 (50) |
|
| Hepatic vein
invasion, n (%) |
|
| 0.1103 |
|
Present | 2 (11.1) | 4 (28.6) |
|
|
Absent | 16 (88.9) | 10 (71.4) |
|
| Extra-hepatic
spread |
|
| 0.5828 |
|
Present | 4 (22.2) | 2 (14.3) |
|
|
Absent | 14 (77.8) | 12 (85.7) |
|
The NewFP+3D-CRT group (n=18) included 15 men
(83.3%) and 3 women (16.7%), with a mean age of 68 years (Table I). Chronic hepatitis C virus
infection was the predominant cause of HCC (n=7; 38.9%), followed
by chronic hepatitis B virus infection (n=6; 33.3%). Of the 18
patients, 7 patients (38.8%) had Child-Pugh class A hepatic
function and 11 patients (61.1%) had Child-Pugh class B hepatic
function. The median maximum tumor diameter was 190 mm. HCC showed
portal vein invasion, with 4 patients (22.2%) presenting with Vp4
and 14 patients (77.8%) with Vp3 type of invasion. Four patients
(22.2%) had extrahepatic spread (EHS).
The sorafenib after NewFP+3D-CRT group (n=14)
included 14 men (100%), with a mean age of 68.5 years (Table I). Chronic hepatitis C virus
infection was the predominant cause of HCC (n=9; 64.3%), followed
by chronic hepatitis B virus infection (n=2; 14.3%). Of the 14
patients, 9 patients (64.2%) had Child-Pugh class A hepatic
function and 5 patients (35.7%) had Child-Pugh class B hepatic
function. The median maximum tumor diameter was 137 mm. HCC showed
portal vein invasion, with 3 patients (21.4%) having Vp4 and 7
patients (50%) having Vp3. Two patients (14.3%) had EHS.
Overall response and efficacy
Table II shows the
results at the first radiologic assessment according to the
mRECIST. Of the 32 patients treated with NewFP plus 3D-CRT for MVI,
3 (9.4%), 16 (50%), and 7 (21.9%) patients had CR, PR, and SD,
respectively. The overall response rate was 59.4%, and the disease
control rate was 81.3%.
 | Table II.Therapeutic effects in all patients
(n=32). |
Table II.
Therapeutic effects in all patients
(n=32).
| Therapeutic
effects | NewFP+3D-CRT
(n=32) |
|---|
| CR | 3 |
| PR | 16 |
| SD | 7 |
| PD | 6 |
| ORR (CR+PR), n
(%) | 19 (59.4) |
| DCR (CR+PR+SD), n
(%) | 26 (81.3) |
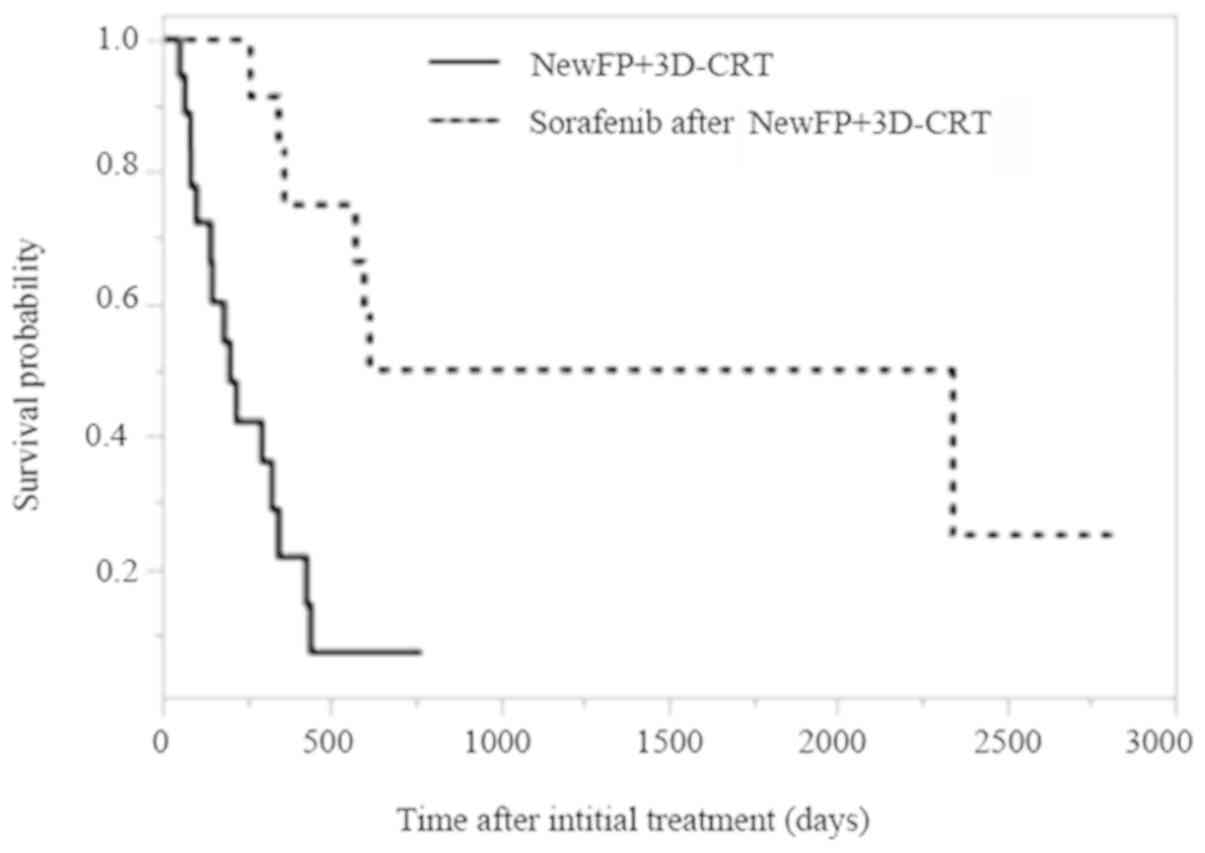
Cumulative overall survival curves of patients
treated with NewFP plus 3D-CRT for MVI or sorafenib after NewFP
plus 3D-CRT for MVI are shown in Fig.
1. The MST was 6.7 months for patients treated with NewFP plus
3D-CRT for MVI and 49.2 months for those treated with sorafenib
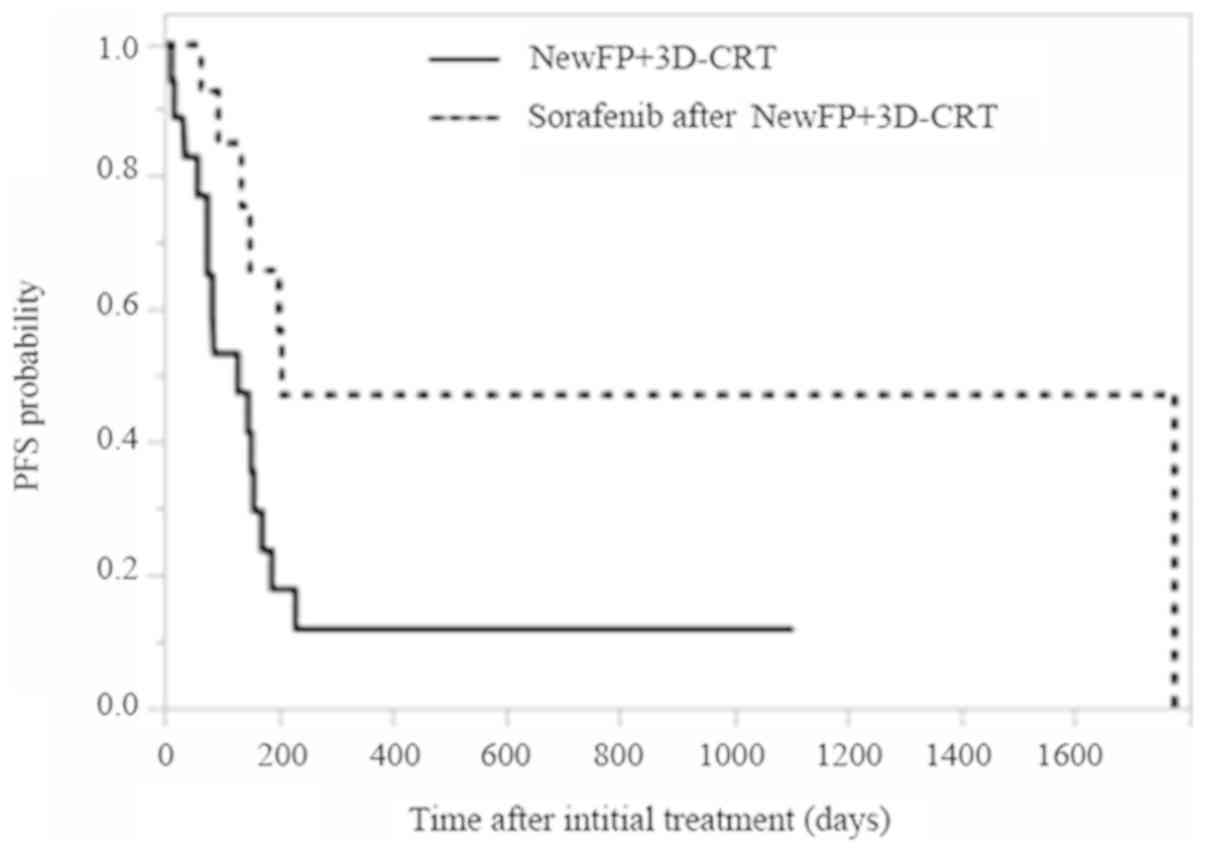
after NewFP plus 3D-CRT for MVI (P=0.0003). The PFS in patients
showing MVI who were treated either with NewFP plus 3D-CRT or with
sorafenib after NewFP plus 3D-CRT are shown in Fig. 2. The median PFS was 4.3 and 6.8
months for patients treated either with NewFP plus 3D-CRT for MVI
or those treated with sorafenib after NewFP plus 3D-CRT,
respectively (P=0.0219). Sorafenib, administered as the secondary
treatment after NewFP plus 3D-CRT for MVI was associated with a
significantly higher overall response rate, disease control rate,
and longer overall survival in HCC patients showing MVI.
Factors associated with survival
outcomes
The significant prognostic factors for overall
survival, according to univariate analysis, were response to
initial treatment with NewFP plus 3D-CRT (PR or CR, P=0.0479) and
the number of tumors (<4, P=0.0260). Multivariate analysis
confirmed that initial treatment with NewFP plus 3D-CRT (PR or CR,
hazard ratio, 0.2264; 95% confidence interval, 0.0737–0.6320;
P=0.0060) was an independent factor for overall survival (Table III).
 | Table III.Factors associated with overall
survival. The Cox proportional hazards model was used to evaluate
the interaction between baseline characteristics and overall
survival or therapeutic effect. Two-tailed values of P<0.05 were
considered to indicate a statistically significant result. |
Table III.
Factors associated with overall
survival. The Cox proportional hazards model was used to evaluate
the interaction between baseline characteristics and overall
survival or therapeutic effect. Two-tailed values of P<0.05 were
considered to indicate a statistically significant result.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | P-value | HR (95% CI) | P-value |
|---|
| Age (<75/75≤
years) | 0.3617 |
|
|
| Child-Pugh
(A/B) | 0.1069 |
|
|
| AFP
(<1,000/1,000≤ ng/ml) | 0.3891 |
|
|
| DCP
(<1,000/1,000≤ mAU/ml) | 0.6205 |
|
|
| Tumor number
(<4/4≤) | 0.0260a | 0.9731
(0.2995–3.0632) | 0.9628 |
| Tumor localization
(unilobar/bilobar) | 0.3699 |
|
|
| Vv2-3
(absent/present) | 0.1536 |
|
|
| Vp3-4
(absent/present) | 0.1536 |
|
|
| TACE-refractory
(abscent/present) | 0.5228 |
|
|
| Treatment response
to NewFP+3D-CRT (PR+CR/SD+PD) | 0.0479a | 0.2264
(0.0737–0.6320) | 0.0060b |
Safety and adverse events
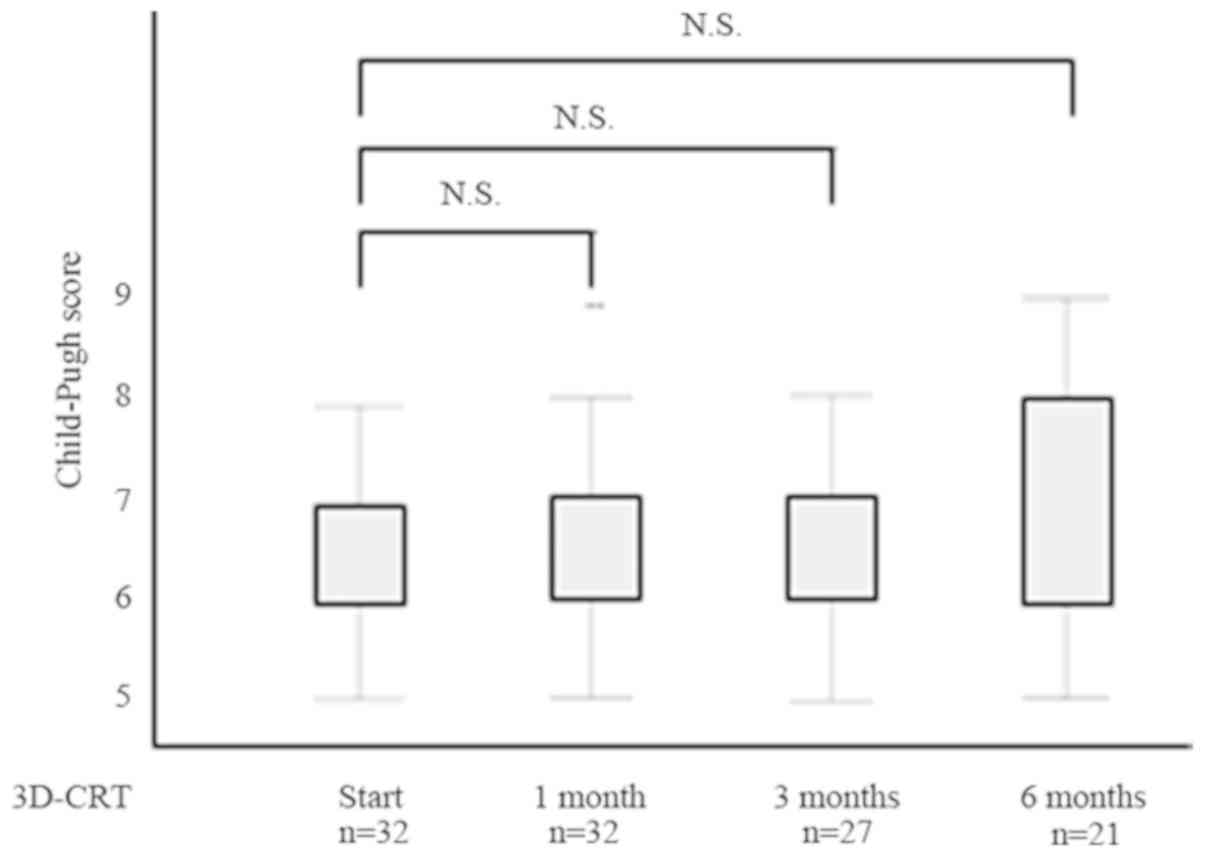
Changes in hepatic function using Child-Pugh scores
before and after 3D-CRT are shown in Fig. 3. In surviving cases, no significant
hepatic function decline was seen after 3D-CRT. On the other hand,
Child-Pugh scores decreased after 3D-CRT; hepatic function improved
in several patients.
Serious adverse events such as gastrointestinal
bleeding due to radiation gastritis were observed in 3 patients, 1
in the NewFP+3D-CRT group and 2 in the sorafenib after NewFP+3D-CRT
group. In all 3 cases, healing occurred using argon plasma
coagulation delivered via endoscopy. Treatment-related mortality
was not observed in the two groups.
Discussion
In the present study, for patients with advanced HCC
showing MVI, sorafenib after NewFP plus 3D-CRT for MVI was
associated with a significantly higher overall response and disease
control rate relative to sorafenib monotherapy. Furthermore,
patients administered sorafenib after NewFP plus 3D-CRT for MVI had
a good prognosis, with an MST of 49.2 months.
MVI is a prognostic factor for lower overall
survival among HCC patients. PVTT causes portal
hypertension-related complications such as varix or ascites, and is
associated with exacerbating factors such as a larger tumor size,
higher tumor grade, and alpha-fetoprotein elevation (23). Because blood from the inferior vena
cava flows into the pulmonary vessels, lung metastasis and
pulmonary embolism would be expected to be frequent in patients
with IVCTT. If the MVI cannot be reduced, it may lead to a decrease
in the portal vein blood flow; this may result in a further decline
in hepatic function, increasing the risk of sudden death.
Therefore, quick reduction of MVI is important to facilitate
subsequent treatment.
Sorafenib is recommended by BCLC guidelines as the
first-line therapy for advanced HCC, but its efficacy is limited
(24). In the SHARP trial, the MST
of patients with HCC showing MVI who were treated with sorafenib
was 8.1 months; the incidence of objective responses was low
(7). Several studies have reported
various combination strategies for HCC showing MVI (25). In a randomized clinical trial for
advanced HCC showing MVI, initial treatment with TACE plus RT was
well tolerated and conferred an improved progression-free survival,
objective response rate, time to progression, and overall survival,
compared with sorafenib treatment (26). On the other hand, HAIC combined with
RT was associated with a longer MST for HCC with PVTT compared with
sorafenib (19). In the present
study, immediate therapeutic response was obtained by using NewFP
(associated with a high response rate) plus 3D-CRT (associated with
a high local control rate) for MVI.
Although regorafenib and lenvatinib can currently be
administered as alternatives to sorafenib as molecular target drugs
for HCC patients showing MVI, they are limited to Child-Pugh class
A hepatic function (27–29). When sorafenib was withdrawn, the
introduction of secondary treatments was difficult, due to a
decline in hepatic functional reserve, fatigue, and a decline in
PS; as recorded in previous reports (30). In the present study, HCC patients
showing MVI of Child-Pugh class B hepatic function were safely able
to undergo NewFP plus 3D-CRT for MVI as the initial treatment. In
addition, several patients in whom hepatic function improved after
the initial treatment from Child-Pugh class B to A, were able to
receive sorafenib. Furthermore, in the multivariate analysis,
initial treatment was extracted as a significant factor associated
with overall survival. As the initial treatment, NewFP plus 3D-CRT
for MVI was well tolerated and provided a chance for secondary
treatment. On the other hand, we compared our study to a previous
open label, non-comparative, phase II trial in patients with
advanced HCC. Patients who received HAIC and were responders were
continued on HAIC and were expected to have good prognoses, while
the HAIC non-responders were switched to sorafenib (31). The MST of HCC patients showing MVI
was 25.4 months in this previous trial. Although there were few
differences between our study and the previous trial, the results
of sorafenib after NewFP plus 3D-CRT for MVI in our study was
superior to that of the previous trial. The reason for our
favorable results could be that immediate therapeutic response was
obtained by using NewFP (associated with a high response rate) plus
3D-CRT (associated with a high local control rate) for MVI. In
addition, avoiding the unnecessary stenosis of hepatic artery by
catheter therapy, the reduction of sensitivity to the drug,
deterioration of liver function, and appearance of collateral
arteries could also explain the favorable results (32,33). We
postulated that shifting to the secondary treatment promptly, while
maintaining hepatic function, would lead to improvement in
prognosis for advanced HCC showing MVI.
Sorafenib, an oral multikinase inhibitor that blocks
tumor cell proliferation and angiogenesis, significantly improved
overall survival compared with placebo in patients with advanced
HCC (7,9). In preclinical studies, sorafenib was
shown to exert a synergistic anticancer effect on cisplatin
(34). A recent study investigating
sorafenib combined with HAIC suggested that combining systemic
therapy and regional cytotoxic chemotherapy could enhance antitumor
activity (35). Therefore, we
speculate that the combination of NewFP and sorafenib
synergistically produced an antitumor effect, leading to the higher
overall response. Because a considerable proportion of HCC patients
showing MVI are unable to receive curative treatment, it is
important to explore multimodal strategies for such patients. To
the best of our knowledge, NewFP was associated with the longest
survival of HCC patients showing MVI in all studies reported so
far. No combined modality therapy of NewFP plus 3D-CRT and
sorafenib has yet to show a clear survival benefit. The present
study is the first reported to show that the combined modality
therapy of NewFP plus 3D-CRT and sorafenib is a feasible and
promising treatment option for HCC patients showing MVI.
There are several possible limitations to this
study. Since this investigation was a retrospective single-center
study, the possibility of unintentional selection bias during
patient selection cannot be fully excluded. Additionally, the
number of cases was small. Therefore, it is necessary to
investigate this treatment strategy at multiple centers via a
prospective study to confirm our findings.
In conclusion, for patients with advanced HCC
showing MVI, the initial treatment with NewFP plus 3D-CRT for MVI
was well tolerated, and administration of sorafenib as the
secondary treatment after NewFP plus 3D-CRT for MVI was associated
with a significantly higher overall response rate, disease control
rate, and longer overall survival. Our results suggest that a good
prognosis could be obtained by performing sorafenib treatment after
chemoradiotherapy for advanced HCC showing MVI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during this study are
available from the corresponding author on reasonable request.
Authors' contributions
TN and TM conceived and designed the study. TN
drafted the manuscript. TN, JT, AD, MN, KOu, TT, KF, SM, TSak, AM
and HY analyzed and interpreted the data. HK, TSan, YN, KOk, YS,
ST, TSh, KT, TH and TM interpreted the data and revised the
manuscript critically for important intellectual content. All
authors were involved in data interpretation and drafting the
manuscript and have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the 1975
Declaration of Helsinki after receiving approval from the
institutional review board of the Kagawa University, Kagawa, Japan
(approval no. Heisei29-192). The requirement for informed consent
from the participants was waived because of the retrospective
nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
MVI
|
macroscopic vascular invasion
|
|
FU
|
fluorouracil
|
|
3D-CRT
|
three-dimensional conformal
radiotherapy
|
|
MST
|
median survival time
|
|
PVTT
|
portal vein tumor thrombosis
|
|
IVCTT
|
inferior vena cava tumor
thrombosis
|
|
RT
|
radiotherapy
|
|
TACE
|
transarterial chemoembolization
|
|
HAIC
|
hepatic arterial infusion
chemotherapy
|
|
RFA
|
radiofrequency ablation
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Bustamante J, Castells A,
Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J and Bruix J: Natural
history of untreated nonsurgical hepatocellular carcinoma:
Rationale for the design and evaluation of therapeutic trials.
Hepatology. 29:62–67. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Minagawa M and Makuuchi M: Treatment of
hepatocellular carcinoma accompanied by portal vein tumor thrombus.
World J Gastroenterol. 12:7561–7567. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cabibbo G, Enea M, Attanasio M, Bruix J,
Craxi A and Camma C: A meta-analysis of survival rates of untreated
patients in randomized clinical trials of hepatocellular carcinoma.
Hepatology. 51:1274–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruix J, Reig M and Sherman M:
Evidence-based diagnosis, staging, and treatment of patients with
hepatocellular carcinoma. Gastroenterology. 150:835–853. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruix J, Cheng AL, Meinhardt G, Nakajima
K, De Sanctis Y and Llovet J: Prognostic factors and predictors of
sorafenib benefit in patients with hepatocellular carcinoma:
Analysis of two phase III studies. J Hepatol. 67:999–1008. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo M and Ueshima K: Positioning of a
molecular-targeted agent, sorafenib, in the treatment algorithm for
hepatocellular carcinoma and implication of many complete remission
cases in Japan. Oncology. 78 (Suppl 1):S154–S166. 2010. View Article : Google Scholar
|
|
11
|
Zhao JD, Liu J, Ren ZG, Gu K, Zhou ZH, Li
WT, Chen Z, Xu ZY, Liu LM and Jiang GL: Maintenance of Sorafenib
following combined therapy of three-dimensional conformal radiation
therapy/intensity-modulated radiation therapy and transcatheter
arterial chemoembolization in patients with locally advanced
hepatocellular carcinoma: A phase I/II study. Radiat Oncol.
5:122010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Citrin DE: Recent developments in
radiotherapy. N Engl J Med. 377:1065–1075. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang YJ, Hsu HC, Wang CY, Wang CJ, Chen
HC, Huang EY, Fang FM and Lu SN: The treatment responses in cases
of radiation therapy to portal vein thrombosis in advanced
hepatocellular carcinoma. Int J Radiat Oncol Biol Phys.
73:1155–1163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakazawa T, Hidaka H, Shibuya A, Okuwaki
Y, Tanaka Y, Takada J, Minamino T, Watanabe M, Kokubu S and Koizumi
W: Overall survival in response to sorafenib versus radiotherapy in
unresectable hepatocellular carcinoma with major portal vein tumor
thrombosis: Propensity score analysis. BMC Gastroenterol.
14:842014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park HC, Yu JI, Cheng JC, Zeng ZC, Hong
JH, Wang ML, Kim MS, Chi KH, Liang PC, Lee RC, et al: Consensus for
radiotherapy in hepatocellular carcinoma from the 5th Asia-pacific
primary liver cancer expert meeting (APPLE 2014): Current practice
and future clinical trials. Liver Cancer. 5:162–174. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang K, Guo WX, Chen MS, Mao YL, Sun BC,
Shi J, Zhang YJ, Meng Y, Yang YF, Cong WM, et al: Multimodality
treatment for hepatocellular carcinoma with portal vein tumor
thrombus: A large-scale, multicenter, propensity matching score
analysis. Medicine (Baltimore). 95:e30152016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikeda M, Mitsunaga S, Shimizu S, Ohno I,
Takahashi H, Okuyama H, Kuwahara A and Okusaka T: Current status of
hepatocellular carcinoma in Japan. Chin Clin Oncol.
2:402013.PubMed/NCBI
|
|
18
|
Nagamatsu H, Hiraki M, Mizukami N, Yoshida
H, Iwamoto H, Sumie S, Torimura T and Sata M: Intra-arterial
therapy with cisplatin suspension in lipiodol and 5-fluorouracil
for hepatocellular carcinoma with portal vein tumour thrombosis.
Aliment Pharmacol Ther. 32:543–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kodama K, Kawaoka T, Aikata H, Uchikawa S,
Nishida Y, Inagaki Y, Hatooka M, Morio K, Nakahara T, Murakami E,
et al: Comparison of outcome of hepatic arterial infusion
chemotherapy combined with radiotherapy and sorafenib for advanced
hepatocellular carcinoma patients with major portal vein tumor
thrombosis. Oncology. 94:215–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inoue Y, Hasegawa K, Ishizawa T, Aoki T,
Sano K, Beck Y, Imamura H, Sugawara Y, Kokudo N and Makuuchi M: Is
there any difference in survival according to the portal tumor
thrombectomy method in patients with hepatocellular carcinoma?
Surgery. 145:9–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, national cancer
institute of the United States, national cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan SL, Chong CC, Chan AW, Poon DM and
Chok KS: Management of hepatocellular carcinoma with portal vein
tumor thrombosis: Review and update at 2016. World J Gastroenterol.
22:7289–7300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song DS, Song MJ, Bae SH, Chung WJ, Jang
JY, Kim YS, Lee SH, Park JY, Yim HJ, Cho SB, et al: A comparative
study between sorafenib and hepatic arterial infusion chemotherapy
for advanced hepatocellular carcinoma with portal vein tumor
thrombosis. J Gastroenterol. 50:445–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu K, Chen J, Lai L Meng X, Zhou B, Huang
W, Cai M and Shan H: Hepatocellular carcinoma with portal vein
tumor thrombus: Treatment with transarterial chemoembolization
combined with sorafenib-a retrospective controlled study.
Radiology. 272:284–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH,
An JH, Lee HC and Lim YS: Efficacy and safety of transarterial
chemoembolization plus external beam radiotherapy vs sorafenib in
hepatocellular carcinoma with macroscopic vascular invasion: A
randomized clinical trial. JAMA Oncol. 4:661–669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kudo M: Lenvatinib in advanced
hepatocellular carcinoma. Liver Cancer. 6:253–263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kudo M: A new era of systemic therapy for
hepatocellular carcinoma with regorafenib and lenvatinib. Liver
Cancer. 6:177–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Iavarone M, Cabibbo G, Piscaglia F,
Zavaglia C, Grieco A, Villa E, Cammà C and Colombo M; SOFIA
(SOraFenib Italian Assessment) study group, : Field-practice study
of sorafenib therapy for hepatocellular carcinoma: A prospective
multicenter study in Italy. Hepatology. 54:2055–2063. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hatooka M, Kawaoka T, Aikata H, Inagaki Y,
Morio K, Nakahara T, Murakami E, Tsuge M, Hiramatsu A, Imamura M,
et al: Hepatic arterial infusion chemotherapy followed by sorafenib
in patients with advanced hepatocellular carcinoma (HICS55): An
open label, non-comparative phase II trial. BMC Cancer. 18:6332018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ikeda M, Mitsunaga S, Shimizu S, Ohno I,
Takahashi H, Okuyama H, Kuwahara A, Kondo S, Morizane C, Ueno H, et
al: Efficacy of sorafenib in patients with hepatocellular carcinoma
refractory to transcatheter arterial chemoembolization. J
Gastroenterol. 49:932–940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hatooka M, Kawaoka T, Aikata H, Morio K,
Kobayashi T, Hiramatsu A, Imamura M, Kawakami Y, Murakami E, Waki
K, et al: Comparison of outcome of hepatic arterial infusion
chemotherapy and Sorafenib in patients with hepatocellular
carcinoma refractory to Transcatheter arterial chemoembolization.
Anticancer Res. 36:3523–3529. 2016.PubMed/NCBI
|
|
34
|
Yang Q, Zhang S, Kang M, Dong R and Zhao
J: Synergistic growth inhibition by sorafenib and cisplatin in
human osteosarcoma cells. Oncol Rep. 33:2537–2544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ikeda M, Shimizu S, Sato T, Morimoto M,
Kojima Y, Inaba Y, Hagihara A, Kudo M, Nakamori S, Kaneko S, et al:
Sorafenib plus hepatic arterial infusion chemotherapy with
cisplatin versus sorafenib for advanced hepatocellular carcinoma:
Randomized phase II trial. Ann Oncol. 27:2090–2096. 2016.
View Article : Google Scholar : PubMed/NCBI
|