Introduction
Thyroid carcinoma coexisting with hyperthyroidism is
an uncommon occurrence (1), as low
thyroid-stimulating hormone (TSH) levels can suppress the
development and growth of differentiated thyroid carcinoma cells.
The majority of nodules in patients with low TSH levels are
considered to be benign (NCCN, British Thyroid Association)
(1); however, an increasing number
of thyroid carcinoma cases are diagnosed in patients with Graves'
disease, toxic goiter and functioning thyroid adenoma (2). These thyroid carcinomas may be embedded
in or adjacent to a larger hot nodule, and the majority are
non-functional. However, previous studies have reported that
hyperfunctioning thyroid carcinoma may present as autonomous
functioning thyroid nodules (AFTN) within the thyroid gland, or as
functioning lesions in metastatic foci (3–5). In
addition, Als et al (3)
identified 19 patients with toxic thyroid carcinoma in 2002, while
Mirfakhraee et al (5)
identified a solitary hyperfunctioning thyroid nodule harboring
thyroid carcinoma and reported 76 cases of malignant hot thyroid
nodules based on a literature search. Hyperfunctioning thyroid
carcinomas are capable of absorbing iodine, as well as synthesizing
and releasing thyroxine. Patients with hyperfunctioning thyroid
carcinomas may therefore present with clinical thyrotoxicosis. It
is considered that this type of hyperthyroidism may be caused by
hyperfunctioning thyroid carcinoma. However, as the incidence of
hyperfunctioning thyroid carcinoma is very low, diagnosis may be
delayed and the subsequent choice of treatment may be unsuitable.
Therefore, the aim of the present study was to improve our
understanding of hyperfunctioning thyroid carcinoma in order to
prevent misdiagnosis and to identify the most effective treatment
strategies.
Materials and methods
Search strategy and selection
criteria
A literature search of PubMed for studies published
in English between January 1990 and July 2017 was performed using
the terms, ‘hyperfunctioning thyroid carcinoma/cancer’, ‘malignant
hot/toxic thyroid nodule’, or ‘hyperfunctioning
papillary/follicular/Hürthle cell thyroid carcinoma’, followed by a
review of the identified articles. Hyperfunctioning thyroid
carcinoma was divided into primary and metastatic. The inclusion
criteria for studies involving primary hyperfunctioning thyroid
carcinoma were as follows: i) Thyroid carcinoma, papillary thyroid
carcinoma (PTC), follicular thyroid carcinoma (FTC) or Hürthle cell
carcinoma (HCC); ii) clinical hyperthyroidism with symptomatically
or biochemically diagnosed thyrotoxicosis; iii) AFTN, hot or warm
nodules (as determined by scintigraphy) and other thyroid tissues
with suppressed uptake (99mTc, and/or 131I or
123I); iv) thyroid carcinomas of an identical size to
hot or warm nodules, or the absence of hyperplasia in non-cancerous
thyroid tissues on pathological analysis. Studies involving cases
where the size of the thyroid carcinoma was not identical to that
of the hot or warm nodules on scintigraphy, or those where this
information was not included, were excluded from the present study,
as these tumors may be embedded in hot benign nodules and be
non-functional. The inclusion criteria for studies involving
metastatic hyperfunctioning thyroid carcinoma were required to meet
aforementioned points i, ii and iii; or i, ii and iv; or a minimum
of points i, ii and vi of the following: i) Thyroid carcinoma, PTC,
FTC or HCC confirmed by bioptic analysis of the metastatic lesions
or thyroid nodule; ii) clinical hyperthyroidism; iii)
hyperthyroidism that persists or develops following total
thyroidectomy; iv) increased 99mTc, and/or
131I or 123I uptake in the metastatic lesion
as determined by scintigraphy. Studies involving cases of
persistent euthyroidism following total thyroidectomy were also
excluded, as this may indicate functioning but not hyperfunctioning
thyroid carcinoma.
Study selection
Since the incidence of hyperfunctioning thyroid
carcinoma is very low, the number of cases found on PubMed was
small, and the majority of the cases had incomplete data. Of the
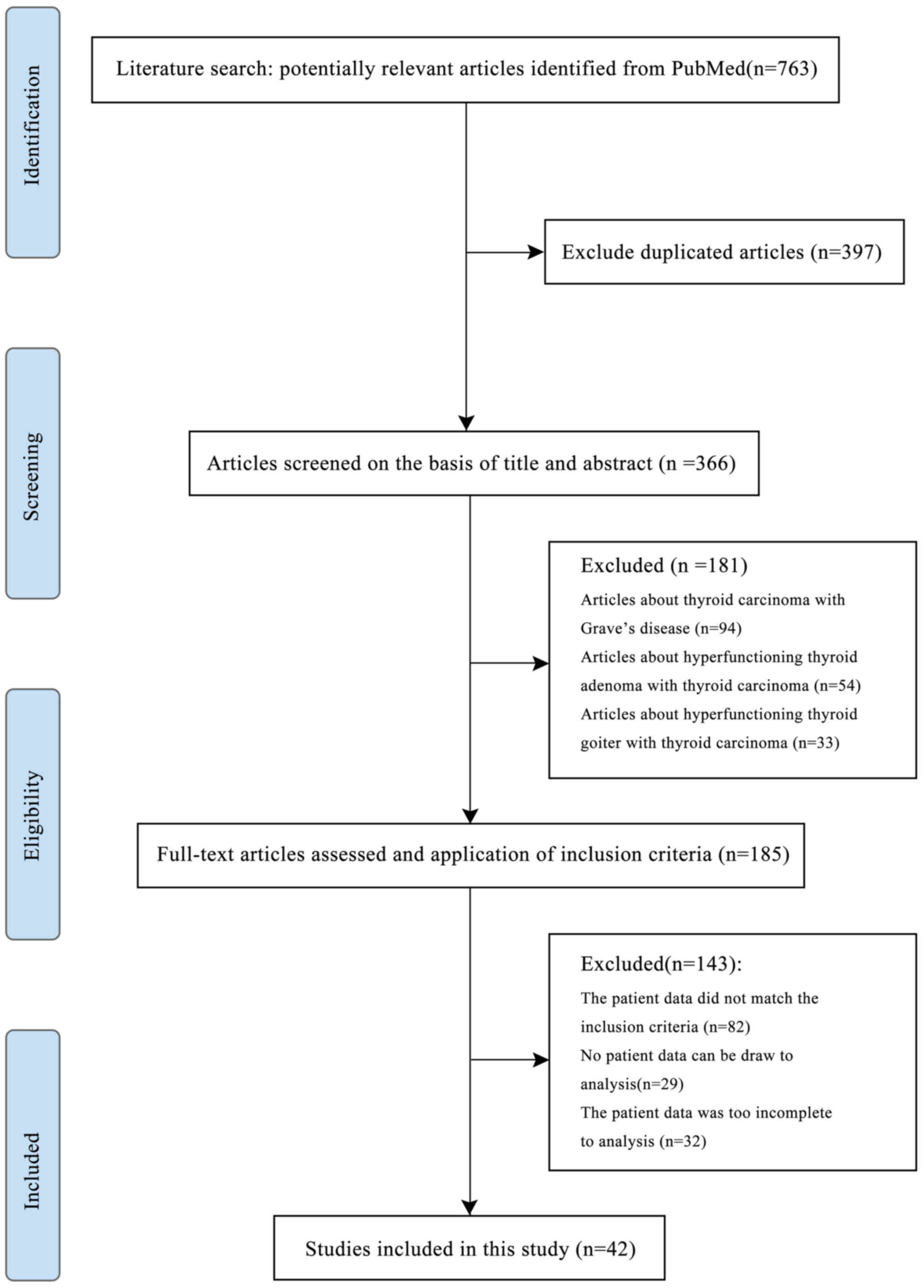
763 articles retrieved from PubMed using our search strategy, 397
were duplicated and 324 did not meet the inclusion criteria.
Finally, the remaining 42 articles were included in the present
study. A detailed flowchart of the study selection process is
presented in Fig. 1.
Results
Primary hyperfunctioning thyroid
carcinoma
The literature search identified 43 cases of primary
hyperfunctioning thyroid carcinoma between 1998 and 2017 (Table I) that fulfilled the inclusion
criteria (3,5–28); the
full-text versions of the majority of articles published before
1998 were unavailable. The mean age of patients was 50.1±19.0 years
(range, 11–79 years) and the female:male ratio was 2.31 (30:13).
All patients presented with clinical hyperthyroidism. Biochemical
thyrotoxicosis was confirmed in all patients, apart from 11 cases,
5 of which presented with low TSH and normal T3 and T4 levels, and
6 cases with incomplete information. Thyroid scintigraphy analysis
(99mTc and/or 131I or 123I) was
performed in all but 2 patients, and indicated the presence of hot
or warm nodules with suppressed uptake in the remainder of the
thyroid gland as AFTN. All 43 cases presented with at least one of
the following characteristics, indicating that the hyperfunctioning
nodule was in fact the thyroid carcinoma: i) Pathological tumor
size identical to the size of the nodule as determined by
preoperative thyroid scintigraphy analysis; or ii) the thyroid
tissue adjacent to the carcinoma was atrophic or normal. The
majority of the cases presented with a single hyperfunctioning
thyroid carcinoma, apart from 2 cases; patient 23 presented with
two hyperfunctioning FTCs, and patient 31 presented with 4
hyperfunctioning PTCs. The mean tumor size was 4.25±2.12 cm. A
total of 4.7% of the tumors were ≤1.0 cm in size, 11.6% were >1
to ≤2.0 cm, 39.5% were >2 to ≤4.0 cm and 44.2% were >4.0 cm.
Details on the preoperative ultrasound parameters were mostly
unavailable; however, based on the available information, there
were no characteristic findings indicative of thyroid carcinoma
(Table I). The results of
fine-needle aspiration (FNA) of the thyroid performed on 15
patients identified differentiated thyroid carcinoma (DTC) or
suspected DTC in 10 cases, no diagnosis by cytology in 4 cases, and
no malignant characteristics in 1 case. In terms of histological
subtype, 20 cases (46.5%) were FTC, 21 cases were PTC [including 7
follicular variant PTC (FVPTC)] and 2 cases were HCC.
 | Table I.Reported cases of primary
hyperfunctioning thyroid carcinoma. |
Table I.
Reported cases of primary
hyperfunctioning thyroid carcinoma.
| A, Study ID,
patient characteristics and findings on examination |
|---|
|
|---|
| No. | First author | Year | Age, years | Sex | Tumor growth | Pertechnetate | Chemical
thyrotoxicosis | FT3 (pmol/l) | FT4 (pmol/l) | TSH (uIU/ml) | AFTN size (cm) | Tumor size
(cm) | US | FNA | Pathology | (Refs.) |
|---|
| 1 | Appetecchia | 1998 | 23 | F | 3.5→ 4.0 cm | AFTN | Chemical | 11.55↑ | 25.52↑ | 0.11↓ | 3.5/4 | 4 | Inhomogeneous
nodule |
| Gross PTC, 4 PTC, 3
microfoci PTC | (6) |
| 2 | Mircescu | 2000 | 11 | F |
| Yes,
hyperfunctioning nodule | Chemical |
| 75↑ | 0.03↓ | 4.5 | 4 | Enlarged with
numerous cystic lesions |
| PTC 4.0 cm,
cystic | (7) |
| 3 | Bourasseau | 2000 | 47 | M |
| Yes, hot,
solitary | Chemical | ↑ | N | 0.05↓ | 3.5 | 3.5 | Solitary | Suspicious | PTC | (8) |
| 4 | Bourasseau | 2000 | 36 | M |
| Yes, hot,
solitary | No | N | N | 0.025↓ | 2.5 | 2.5 | Solitary | No diagnostic | FTC | (8) |
| 5 | Bourasseau | 2000 | 56 | M |
| Yes, hot,
solitary | Chemical |
| ↑ | 0.03↓ | 5.5 | 5.5 | Solitary | No | FTC | (8) |
| 6 | Bourasseau | 2000 | 39 | F |
| Yes, warm | Chemical | ↑ | ↑ | 0.004↓ | 1 | 1 | Multinodular | Suspicious | PTC | (8) |
| 7 | Bourasseau | 2000 | 33 | F |
| Yes, hot | No | N |
| 0.005↓ | 3 | 3 | Multinodular | Not diagnostic | PTC | (8) |
| 8 | Camacho | 2000 | 49 | F |
| Yes, Hot, AFTN | Chemical |
| 15.7↑ | 0.04↓ | 3.5 | 3.5 |
| No | FTC with
hemorrhagic central portion | (9) |
| 9 | Als | 2002 | 54 | M |
| Hot, AFTN | Uncertain |
|
|
| 8.5 | 8.5 |
| No | FTC | (3) |
| 10 | Als | 2002 | 62 | F |
| Uncertain | Uncertain |
|
|
|
|
|
| No | PTC | (3) |
| 11 | Als | 2002 | 50 | M |
| Hot, AFTN | Uncertain |
|
|
| 10 | 10 |
| No | FTC | (3) |
| 12 | Als | 2002 | 62 | M |
| Hot, AFTN | Chemical | ↑ | N | ↓ | 8 | 8 |
| No | FTC | (3) |
| 13 | Als | 2002 | 71 | F |
| Hot, AFTN | Chemical | ↑ | ↑ | ↓ | 4 | 4 |
| No | FTC | (3) |
| 14 | Als | 2002 | 69 | F |
| Hot, AFTN | Chemical | ↑ | N | ↓ | 6 | 6 |
| No | FTC | (3) |
| 15 | Als | 2002 | 55 | F |
| Hot, AFTN | Uncertain |
|
| ↓ | 5.5 | 5.5 |
| No | FTC | (3) |
| 16 | Als | 2002 | 79 | F |
| Hot, AFTN
(suppression) | Chemical | ↑ | ↑ | ↓ |
|
|
| No | FTC | (3) |
| 17 | Als | 2002 | 65 | M |
| Hot, AFTN | Chemical | ↑ | N | ↓ | 6.5 | 6.5 |
| No | FTC | (3) |
| 18 | Als | 2002 | 56 | M |
| Hot, AFTN | Chemical | ↑ | N |
|
|
|
| No | FVPTC | (3) |
| 19 | Als | 2002 | 75 | M |
| Hot, AFTN | Chemical | ↑ | N | ↓ | 5.5 | 5.5 |
| No | FTC | (3) |
| 20 | Als | 2002 | 77 | F |
| Hot, AFTN | Chemical | ↑ | N | ↓ | 4 | 4 |
| No | PTC | (3) |
| 21 | Als | 2002 | 71 | F |
| Hot, AFTN | Chemical | ↑ |
| ↓ | 6 | 6 |
| No | FTC | (3) |
| 22 | Als | 2002 | 74 | F |
| Hot, AFTN | Chemical | ↑ | ↑ | ↓ | 7 | 7 |
| No | FTC | (3) |
| 23 | Fuhrer | 2003 | 59 | M |
| Hot, AFTN right,
WBS: no uptake in lung | No | N | N | 0.01↓ | 3.5 | 3.5 | One solid with
calcification, one solid | Lung FNA: FTC | FTC ×2 | (10) |
| 24 | Wong | 2003 | 67 | F |
| Yes, hot, AFTN | Chemical |
| ↑ | ↓ | 2.5 | 3 |
| No feature of
carcinoma | Hürthle cell
carcinoma | (11) |
| 25 | Gozu | 2004 |
| F |
| Yes, hot 5.0 cm,
2.0 cm hypoactive | Chemical | 9.11↑ | 1.89↑ | 0.005↓ | 5 | 5 |
| No | PTC
(intracystic) | (12) |
| 26 | Majima | 2005 | 59 | F |
| AFTN | Chemical | 4.4↑ | 2.7↑ | 0.01↓ | 1.5 | 1.5 | Hypoechoic with
cystic degeneration, calcification | PTC | PTC | (13) |
| 27 | Bitterman | 2006 | 57 | F |
| Hot in right, cold
in left | Possibly | ? | ? | ? | 6 | 6 | Multinodular | Not diagnostic | FTC | (14) |
| 28 | Bitterman | 2006 | 59 | F |
| Hot, 5 cm AFTN | Possibly | ? | ? | ? | 5 | 5 | Solitary
nodule | No | FTC | (14) |
| 29 | Niepomniszcze | 2006 | 64 | F |
| Yes, AFTN | Chemical |
|
| 0.02↓ | 6 | 6 |
| no | FTC | (15) |
| 30 | Uludag | 2008 | 36 | M | 1.4→ 1.8 cm (11
months) | AFTN | No | N | N | 0.05↓ | 1.4 | 1.5 | Hypoechoic
nodule | PTC | PTC | (16) |
| 31 | Nishida | 2008 | 62 | F |
| 4 hot AFTN in both
lobes | Chemical | 5.2↑ | 2.39↑ | 0.007↓ | 2.0, 1.5,
0.6,1.5 | 2.0 | Multinodular | PTC | PTC ×4 | (17) |
| 32 |
Bommired-dipalli | 2010 | 63 | M | Enlarging (5
months) | Yes, AFTN
right | Chemical | N | 2.1↑ | 0.01↓ | 4 | 4 | Solid mass | FVPTC? | FVPTC, LN,
FVPTC | (18) |
| 33 | Azevedo | 2010 | 47 | F | Enlarging (2
years) | Yes, high iodine
uptake AFTN | Chemical |
| 2.75↑ | 0.05↓ | 2.6 | 3 | Solid nodule | PTC suggestive | FVPTC | (19) |
| 34 | Giovanella | 2010 | 68 | F |
| AFTN, no cold area
in nodule | Chemical | 7.6↑ | N | 0.006↓ | 5.3 | 5.3 | Hypoechoic
nodule | No | FTC | (20) |
| 35 | Tfayli | 2010 | 11 | F |
| Yes, predominant
AFTN | Chemical |
| 1.14 | ↓ | 3.5 | 3 | Non-homogenous
nodule | Not, diagnostic TC
not excluded | PTC | (21) |
| 36 | Karanchi | 2012 | 43 | F |
| AFTN | Chemical | 12.7↑ | 3.1↑ | 0.01↓ | 6.5 |
| Solid nodule | No | Hürthle cell
carcinoma | (22) |
| 37 | Nair | 2012 | 38 | M |
| Hot, AFTN right
lobe whole | Chemical | 6.12↑ | 2.9↑ | 0.003↓ | 3.8 | 3 | Hypoechoic with
scattered microcalcification | NO | PTC with multifocal
microPTC | (23) |
| 38 | Ruggeri | 2013 | 15 | F | 2.5→ 3.5 cm (6
months) | Yes, AFTN | Chemical | 5.0↑ | 20.15↑ | 0.001↓ | 3.5 | 3.5 | Isoechoic,
peripheral halo, blood flow, regular margin | No | FVPTC | (24) |
| 39 | Mirfakhraee | 2013 | 29 | F | 2.4→ 2.7 cm (2
years) | Yes, AFTN | No | N | N | 0.005↓ | 2.7 | 2.5 | Solid, isoechoic,
internal Hypervascularity | No | FTC | (5) |
| 40 | Gabalec | 2014 | 15 | F |
| Hot, AFTN | Chemical |
| 30.4↑ | 0.01↓ | 4.5 | 4 | Heterogenous,
well-demarcated nodule | Follicular
neoplasia? | FVPTC | (25) |
| 41 | Kuan | 2014 | 60 | F | No mention | Hot, AFTN right
lobe whole | Chemical | 7.71↑ | 7.75↓ | 0.005↓ | 8 | 8 | Hypoechoic,
avascular, nodule | Follicular
neoplasia, FTC? | FVPTC | (26) |
| 42 | Rees | 2015 | 16 | F |
| Yes, 2.6 cm
uptake | Chemical | 14.3↑ | 39.4↑ | 0.03↓ | 4 | 4 | Hyperechoic,
hypervascular nodule | DTC? | FVPTC | (27) |
| 43 | Kadia | 2016 | 37 | F | Enlarging (3
months) | – | Chemical |
| 23.3↑ | 0.13↓ | 3.6 | 3 | Isoechoic,
well-defined homogeneous solid nodule | NO | PTC encapsulated
variant | (28) |
|
| B, Treatment and
outcome |
|
| No. | First
author |
Treatment | Pretreatment
effect | Surgery
outcome | RAI effect and
prognosis |
Metastatic | (Refs.) |
|
| 1 | Appetecchia | Thyroidectomy +
bilateral neck dissection + RAI | Triamazole to
euthyroid | Hypothyroid, but
anterior cervical tumor residual | No thyrotoxicosis
or recurrence |
|
| (6) |
| 2 | Mircescu | Right
loboisthmectomy/total (2 months) + RAI | Methimazole +
blocker | No mention | 8 months, no
residual uptake |
|
| (7) |
| 3 | Bourasseau | Thyroidectomy | No mention | No mention | No |
|
| (8) |
| 4 | Bourasseau | Thyroidectomy | No mention | No mention | No |
|
| (8) |
| 5 | Bourasseau | Thyroidectomy | No mention | No mention | No |
|
| (8) |
| 6 | Bourasseau | Thyroidectomy | No mention | No mention | No |
|
| (8) |
| 7 | Bourasseau | Thyroidectomy | No mention | No mention | No |
|
| (8) |
| 8 | Camacho | Thyroidectomy +
RAI | No mention | No mention | 3 years, no
thyrotoxicosis or recurrence |
|
| (9) |
| 9 | Als | Surgery + RAI | Uncertain | Uncertain | 117 months | Yes | (3) |
| 10 | Als | Surgery + RAI +
PR | Uncertain | Uncertain | 82 months | Yes | (3) |
| 11 | Als | Surgery + RAI +
PR | carbimazole | Uncertain | 18 months | Yes | (3) |
| 12 | Als | Surgery + RAI | Uncertain | Uncertain | 190 months | Yes | (3) |
| 13 | Als | RAI + surgery +
RAI | Uncertain | Uncertain | 68 months | Yes | (3) |
| 14 | Als | Surgery + RAI +
PR | Uncertain | Uncertain | 28 months | Yes | (3) |
| 15 | Als | Surgery + RAI +
PR | Uncertain | Uncertain | 93 months | Yes | (3) |
| 16 | Als | RAI + surgery +
RAI | Uncertain | Uncertain | 46 months | Liver and
sacrum | (3) |
| 17 | Als | Surgery + RAI +
PR | Uncertain | Uncertain | 107 months | Yes | (3) |
| 18 | Als | Surgery + RAI | Uncertain | Uncertain | 208 months
alive | No | (3) |
| 19 | Als | Surgery + RAI | Uncertain | Uncertain | 181 months | No | (3) |
| 20 | Als | Surgery + RAI | Uncertain | Uncertain | 45 months | Uncertain | (3) |
| 21 | Als | Surgery + RAI +
PR | Uncertain | Uncertain | 44 months | Yes | (3) |
| 22 | Als | Surgery + RAI | Uncertain | Uncertain | 76 months
alive | Yes | (3) |
| 23 | Fuhrer | Total thyroidectomy
+ RAI - thoracic surgery (8 months) | Euthyroid | Hypothyroid with
RAI | Hypothyroid, 8
months thyrotoxicosis control good | Yes | (10) |
| 24 | Wong | Lobectomy/total
thyroidectomy + RAI | No mention | No mention | No mention |
|
| (11) |
| 25 | Gozu | Lobectomy/total
thyroidectomy + RAI | Euthyroid | Hypothyroid (6
weeks) | 1 year, no
thyrotoxicosis or recurrence |
|
| (12) |
| 26 | Majima | Lobectomy | No mention | Hypothyroid (3
months) | No |
|
| (13) |
| 27 | Bitterman | Loboisthmectomy +
nodule excision/total thyroidectomy | PTU, no clinical
improve | Disease-free 1.5
years | No |
|
| (14) |
| 28 | Bitterman | Left lobectomy | PTU, intolerance
several months | No mention | No |
|
| (14) |
| 29 | Niepomniszcze | Lobectomy/total
thyroidectomy + RAI | No mention | No mention | 6 months, no
thyrotoxicosis or recurrence |
|
| (15) |
| 30 | Yazici | RAI→total
thyroidectomy + CND | No mention | No recurrence or
residual disease | Thyrotoxicosis
control, but size increase |
|
| (16) |
| 31 | Nishida | Total
thyroidectomy | Thiamazole, 5
months to euthyroid | No recurrence and
residual disease (1 year) | No |
|
| (17) |
| 32 |
Bommireddipalli | Total thyroidectomy
+ RAI | No | No mention | 1 year, TG↑LN +,
1.5 year, LN biopsy + | Yes | (18) |
| 33 | Azevedo | Total thyroidectomy
+ RAI | Methimazole 2
months to euthyroid | True hypothyroidism
(2 months) then RAI | 3 years + 2 years
no thyrotoxicosis or recurrence |
|
| (19) |
| 34 | Giovanella | Right
loboisthmectomy + RAI | No | Hypothyroid | 3.4 years,
negative |
|
| (20) |
| 35 | Tfayli | Lobectomy/total
thyroidectomy + RAI | No mention | No mention | 1 year, no
thyrotoxicosis or recurrence |
|
| (21) |
| 36 | Karanchi |
Hemithyroidectomy/total thyroidectomy (1
year) + RAI | No control | Euthyroid (2
weeks) | No |
|
| (22) |
| 37 | Nair | Total thyroidectomy
+ CND + LND + RAI (4 weeks + 6 months) | Carbimazole to
euthyroid | No mention | TG 1 year high, LN
metastases | Yes | (23) |
| 38 | Ruggeri | Surgery | Methimazole to
euthyroid | No mention | No |
|
| (24) |
| 39 | Mirfakhraee | Left lobectomy | No mention | Euthyroid (6
months) with no recurrence of cancer | No |
|
| (5) |
| 40 | Gabalec |
Hemithyroidectomy/total thyroidectomy +
CND + RAI | Triamazole | No mention | To hypothyroid
state |
|
| (25) |
| 41 | Kuan | Total
thyroidectomy | No mention | No mention | No |
|
| (26) |
| 42 | Rees | Left lobectomy +
RAI | Carbimazole to
euthyroid | No mention | Wel-controlled |
|
| (27) |
| 43 | Kadia | Left lobectomy | methimazole +
blocker | Euthyroid (2–4
weeks) | No |
|
| (28) |
Of the 15 patients pretreated with anti-thyroid
drugs, the results indicated disease control to euthyroid in 8
patients, unknown outcome for 4 patients, no disease control in 2
patients and drug intolerance in 1 patient. Thyroid surgery was
performed in all patients. In all patients with available
information on disease outcome (n=9), thyrotoxicosis was
well-controlled by surgery. Radioactive iodine (RAI) treatment was
performed preoperatively in 3 patients who had been initially
diagnosed with benign AFTN, and postoperatively in 20 patients. As
long-term follow-up data were absent for the majority of the
patients, and as patients were treated with RAI within a short time
period following surgery, it was difficult to evaluate the effect
of RAI alone on those patients. However, the available data
indicated that only few (14 cases in 43 cases) suffered recurrence
of thyrotoxicosis or carcinoma within a short follow-up period
[44.5 months (6–208 months)] following thyroid surgery and RAI.
Metastatic hyperfunctioning thyroid
carcinoma
Following a literature search, a total of 28 cases
of metastatic hyperfunctioning thyroid cancer were identified
(Table II) (3,4,29–44)
according to the aforementioned inclusion criteria. All patients
had either clinical thyrotoxicosis with biochemical data indicating
hyperthyroidism, or been diagnosed as thyrotoxicosis. In addition,
all cases (apart from case 56) had a high 99mTc, and/or
131I or 123I uptake in distant lesions, as
demonstrated by whole-body scanning. All patients presented with
multiple or large metastases to the bone, lungs, liver or
mediastinum. The largest metastatic lesion was observed in the
liver of patient 62 (17.0 cm). The mean patient age was 61.2±10.8
years, and the female:male ratio was 1.8 (18:10). Histopathological
examination revealed that 20 cases were FTC, 5 cases were PTC
(including 1 FVPTC), 1 case was insular TC, and 1 case was an
unknown type of DTC. A total of 14 patients with metastatic
hyperfunctioning thyroid carcinoma had undergone thyroidectomy,
while the remaining 14 patients had no history of thyroidectomy.
Thyroid scans were performed in 13 of the 14 cases with no
thyroidectomy history, and the results of 6 cases indicated none to
normal uptake, cold regions in the thyroid gland and the presence
or absence of cold nodules. The remaining 7 cases were diagnosed
with AFTN. Thyroid FNAs were performed in 5 cases with no history
of thyroidectomy: DTC (1 PTC and 1 FTC) was diagnosed in 2 cases,
follicular cells were identified in another 2 cases, and no
malignant cells were detected in the remaining case. Biopsies of
the metastatic lesions were performed in 3 cases, and the results
indicated metastatic DTC. Therefore, of the 14 patients without
thyroidectomy, 4 were diagnosed with metastatic hyperfunctioning
thyroid cancer, 2 as suspicious and 8 as uncertain.
 | Table II.Reported cases of metastatic
hyperfunctioning thyroid carcinoma. |
Table II.
Reported cases of metastatic
hyperfunctioning thyroid carcinoma.
| A, Study ID,
patient characteristics and findings on examination |
|---|
|
|---|
| No. | First author | Year | Age, years | Sex | Thyroid-ectomy
history | Thyroid scan | Whole body
scan |
Thyroto-xicosis | FT3 (pmol/l) | FT4 (pmol/l) | TSH (uIU/ml) | TG (ng/ml) | Metastatic
location | Thyroid FNA | Biopsy on
metastasis | Pathology | (Refs.) |
|---|
| 44 | Girelli | 1990 | 66 | F | No | Normal, uptake cold
nodules | High uptake in
distant lesions | Clinical |
|
| 0.01↓ | 5,300 | Bone | PTC | Metastatic | PTC PTC | (29) |
| 45 | Mizukami | 1994 | 64 | F | No | Hot AFTN 4.0 | High uptake in
distant lesions | Clinical |
|
| ↓ |
| Bone |
Microfo-llicular | No | FTC | (30) |
| 46 | Russo | 1997 | 60 | F | No | Hot AFTN two | High uptake in
distant lesions (after surgery) | Clinical | 2.8↑ | N | 0.06↓ | 513 | Lung | No | No | Insular TC | (31) |
| 47 | Salvatori | 1993 | 79 | F | No | Cold areas | High uptake in
distant lesions | Clinical | 10.4↑ | 3.8↑ | 0.06↓ | 382 | Lung | FTC | No | FTC | (32) |
| 48 | Als | 2002 | 61 | M | No | Hot AFTN | High uptake in
distant lesions | Clinical |
|
|
|
| Uncertain | No | No | FTC | (3) |
| 49 | Als | 2002 | 65 | F | No | Hot AFTN | High uptake in
distant lesions | Clinical |
|
|
|
| Uncertain | No | No | FTC | (3) |
| 50 | Als | 2002 | 71 | F | No | Hot AFTN | High uptake in
distant lesions | Clinical |
| ↑ | ↓ |
| Uncertain | No | No | FTC | (3) |
| 51 | Als | 2002 | 62 | F | No | Hot AFTN | High uptake in
distant lesions | Clinical |
|
| ↓ |
| Uncertain | No | No | FTC | (3) |
| 52 | Als | 2002 | 63 | M | No | Hot AFTN | High uptake in
distant lesions | Clinical |
| N | ↓ |
| Uncertain | No | No | PTC | (3) |
| 53 | Sundaraiya | 2009 | 68 | M | No | Cold nodule | High uptake in
distant lesions | Clinical | 42.6↑ | 100↑ | ↓ |
| Rib | No | Rib FTC | FTC multifocal | (33) |
| 54 | Damle | 2012 | 65 | M | No | – | High uptake in
distant lesions | Clinical |
|
| 0.03↓ | 300 | Lung, bone | Follicular
neoplasm | No | FTC | (34) |
| 55 | Damle | 2012 | 62 | M | No | No uptake | High uptake in
distant lesions | Clinical | ↑ | ↑ | ↓ | 300 | Bone | No | Metastatic FTC |
| (34) |
| 56 | Gardner | 2014 | 66 | F | No | Diffuse
reduction | No WBS | Clinical | 25.1↑ | 37.9↑ | 0.006↓ |
| Lung, bone | No malignant
cells |
| FVPTV | (35) |
| 57 | Kunawudhi | 2016 | 43 | F | No | Cold | High uptake in
distant lesions | Clinical | 32.55↑ | 6.34↑ | 0.026↓ |
| Bone, liver | No | No | FTC | (36) |
| 58 | Abs | 1991 | 57 | F | Partial
thyroid-ectomy | Normal | High uptake in
distant lesions | Clinical |
|
| 0.6↓ | 640 | Mediasti-num |
|
| FTC | (37) |
| 59 | Lorberb-oym | 1996 | 67 | F | Total
thyroid-ectomy |
| High uptake in
distant lesions | Clinical | 273↑ | 15.7↑ | 0.1↓ |
| Hemipelvis |
|
| FTC | (38) |
| 60 | Yoshimura | 1997 | 61 | M | Total thyroidectomy
+ RAI + hip replacement |
| High uptake in
distant lesions | Clinical | 46.1↑ | 105.3↑ | 0.05↓ | 329 | Pelvis |
|
| FTC | (39) |
| 61 | Salvatori | 1993 | 69 | F | Partial
thyroidectomy | Low uptake | High uptake in
distant lesions | Clinical | 3.8↑ | 10.4↑ | 0.06↓ | 48,680 | Lung |
|
| DTC | (32) |
| 62 | Guglielmi | 1999 | 58 | F | Subtotal
thyroidectomy |
| High uptake in
distant lesions | Clinical | 18.4↑ | 44.5↑ | 0.1↓ | 3,686 | Liver, lung |
| Liver FTC | FTC | (40) |
| 63 | Basaria | 2002 | 74 | M | Total thyroidectomy
8 years |
| High uptake in
distant lesions | Clinical | ↑ | ↑ | ↓ | 2,280 | Mediastinum and
lung |
|
| PTC | (41) |
| 64 | Orsolon | 2008 | 66 | M | Total
thyroidectomy |
| High uptake in
distant lesions | Clinical | 4.5↑ | 1.6 | <0.1↓ | >10,000 | Bone, lung |
|
| FTC | (42) |
| 65 | Tan | 2009 | 39 | F | Total thyroidectomy
+ hip replacement + RAI |
| High uptake in
distant lesions (FDG) | Clinical | 27.9↑ | 4.41↑ | 0.01↓ | 1,000 | Pelvic mass |
|
| FTC | (43) |
| 66 | Nishihara | 2010 | 59 | F | Total thyroidectomy
+ EBRT |
| High uptake in
distant lesions | Clinical | ↑ | ↑ | 0.01↓ | 8,000 | Multiple bone and
lung |
|
| FTC | (44) |
| 67 | Qiu | 2015 | 45 | M | Total
thyroidectomy |
| High uptake in
distant lesions | Clinical | 13.42↑ | 33.9↑ | 0.04↓ |
| Bone |
|
| FTC | (4) |
| 68 | Qiu | 2015 | 75 | M | Total
thyroidectomy |
| High uptake in
distant lesions | Clinical | 9.35↑ | 27.18↑ | 0.24↓ |
| Lung |
|
| PTC | (4) |
| 69 | Qiu | 2015 | 43 | F | Total
thyroidectomy |
| High uptake in
distant lesions | Clinical | 7.23↑ | 29.14↑ | 0.22↓ |
| Bone |
|
| FTC | (4) |
| 70 | Qiu | 2015 | 51 | F | Total
thyroidectomy |
| High uptake in
distant lesions | Clinical | 9.51↑ | 31.73↑ | 0.02↓ |
| Bone, lung |
|
| FTC | (4) |
| 71 | Qiu | 2015 | 54 | F | Total
thyroidectomy |
| High uptake in
distant lesions | Clinical | 7.83↑ | 32.15↑ | 0.01↓ |
| Bone |
|
| FTC | (4) |
|
| B, Treatment and
outcome |
|
| No. | First
author | Pretreatment
antithyroid drug |
Treatment | Surgery
outcome | Response to
RAI | (Refs.) |
|
| 44 | Girelli | Thyrotoxicosis to
subhyperthyroidism | Total thyroidectomy
+ RAI | Persistent | Hyperthyroidism
persisting 6 months after RAI | (29) |
| 45 | Mizukami | Unknown | RAI | – | Persistent after 2
RAI | (30) |
| 46 | Russo | Unknown | Subtotal
thyroidectomy/1 year total + RAI 2 | Mild
hyperthyroidism | Hypothyroid, TG
remains high (2 years) | (31) |
| 47 | Salvatori | Effect not
shown | Total thyroidectomy
+ RAI | Improved only 1
month | Hyperthyroidism
persisting 4 months after RAI | (32) |
| 48 | Als | Unknown | RAI + surgery +
RAI | Persistent | 27 months,
died | (3) |
| 49 | Als | Unknown | RAI + surgery +
RAI | Persistent | 39 months,
died | (3) |
| 50 | Als | Unknown | Surgery + RAI | Persistent | 229 months,
died | (3) |
| 51 | Als | Unknown | Surgery + RAI | Persistent,
possible improvement | 10 months,
died | (3) |
| 52 | Als | Unknown | Surgery + RAI | Persistent | 71 months,
died | (3) |
| 53 | Sundaraiya | Effect not
shown | Total thyroidectomy
+ RAI | Persistent | 3 months RAI
hypothyroid with tumor control | (33) |
| 54 | Damle | Thyrotoxicosis
difficult to control | Subtotal
thyroidectomy + RAI | Improved only 2
months | 5 years
of no recurrence of thyrotoxicosis | (34) |
| 55 | Damle | Thyrotoxicosis
difficult to control | RAI | – | 3 years of no
recurrence of thyrotoxicosis | (34) |
| 56 | Gardner | Thyrotoxicosis
difficult to control | Total thyroidectomy
+ RAI | Died of thyroid
storm 12 days postoperatively | – | (35) |
| 57 | Kunawudhi | Effect not
shown | Total thyroidectomy
+ right LND + RAI + EBRT | Persistent | 2 years,
progressive disease | (36) |
| 58 | Abs | Thyrotoxicosis
difficult to control | Rib
biopsy + RAI, good |
|
|
|
| RAI 9 years, no
metastases | (37) |
| 59 | Lorberboym | Pretreatment to
euthyroid +EBRT to hypothyroid | Pretreatment + EBRT
+ RAI |
|
|
|
| 4 weeks RAI
hypothyroid | (38) |
| 60 | Yoshimura | No mention | RAI +
pretreatment |
|
|
|
| Rapid improvement
1.5 years survival | (39) |
| 61 | Salvatori | Effect not
shown | RAI |
|
|
|
| Hyperthyroidism
persisting 6 months after RAI | (32) |
| 62 | Guglielmi | Failure to control
thyrotoxicosis | ILP + RAI |
|
|
|
| 1.5 years good
control | (40) |
| 63 | Basaria | Good control | Pretreatment +
RAI |
|
|
|
| 3 months
hypothyroid | (41) |
| 64 | Orsolon | Unknown | Unknown |
|
|
|
| Unknown | (42) |
| 65 | Tan | Worsening | Removal of pelvis
mass and partial bone | Thyrotoxicosis
disappeared | Resistant to
RAI | (43) |
| 66 | Nishihara | Unknown | RAI low
multiple |
|
|
|
| 10 months after
RAI, toxicosis control, but tumor progression, 8 years of
survival | (44) |
| 67 | Qiu | Unknown | RAI + palliative
resection |
|
|
|
| Effect not clearly
shown |
|
| 68 | Qiu | Unknown | RAI |
|
|
|
| Effect not clearly
shown | (4) |
| 69 | Qiu | Unknown | RAI + palliative
resection |
|
|
|
| Effect not clearly
shown | (4) |
| 70 | Qiu | Unknown | RAI + palliative
resection |
|
|
|
| Effect not clearly
shown | (4) |
| 71 | Qiu | Unknown | RAI |
|
|
|
| Effect not clearly
shown | (4) |
The results demonstrated that, of the 13 patients
who underwent pretreatment with anti-thyroid medication, 6
experienced difficulties or were unable to control thyrotoxicosis,
while only 3 patients became euthyroid. The outcome of
thyrotoxicosis in the remaining 4 patients was uncertain. Total or
subtotal thyroidectomy was performed in 12 of 14 cases without a
history of thyroidectomy. One patient succumbed to thyroid crisis
at 12 days post-surgery. Following surgery, thyrotoxicosis
persisted in 8 patients, while a transient improvement was observed
in 3 patients. All patients underwent multi-dose RAI, apart from 2
patients (patient 56 succumbed to the disease and the outcome of
patient 64 is unknown). Following RAI, the majority of the patients
exhibited a significant improvement in hyperthyroidism and good
cancer control; however, thyrotoxicosis in patient 44 persisted for
up to 6 months. Patients 55 and 58 experienced no recurrence of
thyrotoxicosis or cancer during a follow-up period of 3 or 9 years,
respectively following RAI treatment. Of particular note, patient
65 developed RAI resistance 4 years after the first dose of RAI.
This patient's thyrotoxicosis was caused by pelvic metastasis,
which was cleared following surgical removal of the pelvic
mass.
Discussion
Thyroid carcinoma coexisting with hyperthyroidism is
rare and is more commonly encountered among younger, female
patients (5). Diagnosis relies on
clinical and histopathological correlation. On histopathological
examination, the lack of hyperplastic thyroid tissue often suggests
a hyperfunctioning thyroid cancer (28).
The results of the present study have several
implications, as discussed below. First, the prevalence of
different histological subtypes of hyperfunctioning thyroid
carcinoma was investigated in the present study. The results
indicated that 46.5% of primary hyperfunctioning thyroid carcinomas
and 71.4% (20/28) of metastatic hyperfunctioning thyroid carcinomas
were of the FTC subtype. Mirfakhraee et al (5) reported that 36.4% (28/77) of solitary
hyperfunctioning thyroid nodules harboring a thyroid carcinoma, in
which the majority are primary hyperfunctioning thyroid carcinomas,
were of the FTC subtype. Qiu et al (4) reported that the prevalence of FTC in
functioning metastatic thyroid carcinoma was 60.5% (23/38), of
which 5 cases were hyperfunctioning. By comparison, the
Surveillance, Epidemiology and End Results (SEER) cancer registry
program (1974–2013) (45), which
records all histological thyroid cancer cases as a single group,
indicates that the prevalence of FTC is 10.8% and that of PTC is
83.6%. Therefore, there appears to be a higher prevalence of FTC
among patients with hyperfunctioning thyroid carcinoma, and a
particularly high prevalence among patients with metastatic
disease. This suggests that hyperfunctioning thyroid carcinoma may
be more likely to occur in either primary or metastatic FTC when
compared with PTC. The reason for this is unknown. The results
presented by Qiu et al (4)
indicate that the prognosis of patients with metastatic
hyperfunctioning FTC is worse compared with that for patients with
PTC.
Tumor size is an additional important factor to
consider for hyperfunctioning thyroid carcinoma. In the present
study, the mean tumor size of primary hyperfunctioning thyroid
carcinoma was observed to be 4.25±2.12 cm. These results are
consistent with those presented by Mirfakhraee et al
(5), who reported a mean tumor size
of 4.13±1.68 cm in malignant hot nodules (the majority of which
were hyperfunctioning thyroid carcinomas). By comparison, the SEER
cancer registry program (1974–2013) (45) reports that 28.6% of thyroid
carcinomas are ≤1.0 cm in size, 26.0% are >1.0 to ≤2.0 cm, 23.0%
are >2.0 to ≤4.0 cm, 9.6% are >4.0 cm and 13.0% are unknown.
Pazaitou-Panayiotou et al (2)
conducted a well-organized review, which demonstrated that the
majority of non-functioning thyroid carcinomas that coexist with
Graves' disease, toxic nodule goiter or hyperfunctioning adenoma,
are microcarcinomas (35.0–88.0%). In addition, similar
characteristics were observed in these metastatic hyperfunctioning
thyroid carcinoma patients. It is considered that large primary or
metastatic tumors may synthesize excessive thyroid hormones more
readily, which may cause hyperthyroidism. Somatic mutations in TSH
receptor genes may explain the hyperthyroidism caused by thyroid
cancer. These mutations activate the intracellular cAMP cascade,
induce hormone production and, ultimately, lead to hyperthyroidism
(28,46). Pringle et al (47) observed that thyroid-specific knockout
of PrkarIa leads to hyperthyroidism and thyroid cancer in
mice. Moreover, they suggested that another genetic mutation may be
implicated in metastasis, apart from PrkarIa mutation in the
thyroid (47). As DTC cells have
similar functions to normal thyroid follicular cells, such as
TSH-dependence, absorption of iodine and secretion of
thyroglobulin, DTC cells may also secrete thyroxine. When
autoregulation mechanisms are impeded, such as in Graves' disease,
large DTCs may secrete excessive amounts of thyroxine resulting in
hyperthyroidism. These results also indicate that debulking surgery
may play a key role in the treatment of this rare disease.
As regards the diagnosis of hyperfunctioning thyroid
carcinoma, it is difficult to distinguish malignant from benign
AFTN, as they share common characteristics, such clinical
thyrotoxicosis with hot nodules on thyroid scintigraphy. However,
the following factors may help determine whether thyrotoxicosis is
the result of primary hyperfunctioning thyroid carcinoma: i) No
improvement in thyrotoxicosis following RAI treatment (patient 30
in the present systematic review) (16); ii) ultrasound results indicating the
presence of hypoechoic solid nodules with microcalcifications
(patients 23 and 37 in the present systematic review) (10,23); and
iii) tumor growth over a short time period (patient 32 and 43 in
the present systematic review) (18,28).
Additional risk factors for malignancy were also reported, such as
age (<20 or >60 years), male sex, a family history of DTC, a
previous history of head or neck irradiation, tumor fixation to
adjacent structures and symptoms of tumor invasion (3,5). Most
importantly, AFTN should not be considered to rule out the
possibility of malignant thyroid tumor. The applicability of
thyroid FNA in differentiating malignant from benign AFTN is
limited. This is because ~50% of primary hyperfunctioning thyroid
carcinomas are FTCs, which are difficult to distinguish from
follicular adenoma by FNA. However, if follicular neoplasms in the
thyroid nodule are detected by FNA, combined with high uptake in
distant lesions on whole-body scan images and thyrotoxicosis, a
diagnosis of metastatic hyperfunctioning thyroid carcinoma, FTC or
FVPTC should be considered. Of the 5 metastatic hyperfunctioning
thyroid carcinoma patients who underwent FNA, 2 cases were DTC (1
PTC and 1 FTC) and 2 cases were follicular neoplasms; therefore,
these 4 patients were diagnosed with metastatic hyperfunctioning
thyroid carcinoma. FNA may therefore facilitate the diagnosis of
hyperfunctioning metastatic thyroid carcinoma. In 13 of 14 patients
with no history of thyroidectomy who underwent thyroid scans, 6
cases demonstrated no increased uptake in the thyroid gland. For
these patients, and for patients who develop thyrotoxicosis
following total/subtotal thyroidectomy, a diagnosis of metastatic
hyperfunctioning thyroid carcinoma should be considered and a
whole-body scan should be performed with other additional imaging
methods in order to identify metastatic lesions. Core needle
aspiration and pathological analysis by H&E staining may also
facilitate the diagnosis of primary or metastatic thyroid
carcinoma. Hyperfunctioning thyroid carcinoma will require
diagnosis by FNA or core needle aspiration and whole-body scanning,
as well as confirmation of clinical thyrotoxicosis.
Drug management is considered more suitable for
primary hyperthyroidism with Graves' disease. However, based on our
clinical experience, favorable clinical benefits may be achieved
with early surgery in cases with secondary hyperthyroidism caused
by nodular goiter or thyroid adenoma. Furthermore, surgery can
effectively cure patients with hyperthyroidism with non-functioning
thyroid carcinomas. For the treatment of hyperfunctioning thyroid
carcinoma, the primary aim is to control hyperthyroidism, as well
as the cancer itself. Therefore, surgery, particularly total
thyroidectomy, is the first-line treatment option for patients with
primary hyperfunctioning thyroid carcinoma, as it does not only
confirm the diagnosis following pathological examination, but also
resolves thyrotoxicosis and cures the cancer. Of the 43 patients in
the present study, all except 4 patients diagnosed preoperatively
by FNA, were diagnosed with thyroid carcinoma following thyroid
surgery. In addition, all 43 patients developed
euthyroidism/hypothyroidism within a short time-period following
surgery. However, total thyroidectomy may not be the optimal
first-line treatment option for patients with hyperfunctioning
metastatic lesions with non-functioning primary thyroid carcinoma
(as indicated by no increased uptake on thyroid scintigraphy). This
is because a total thyroidectomy is unable to control
thyrotoxicosis and may even lead to deterioration, as the majority
of hormones are produced by metastatic lesions. Of the 5 cases who
had undergone total or subtotal thyroidectomy, postoperative
thyrotoxicosis persisted in 3 patients, transient improvements were
observed in 1 patient, and the remaining patient succumbed to
thyroid crisis 12 days after surgery. In addition, the significance
of total thyroidectomy in terms of 131I therapy was
markedly lower in patients with low thyroid bed 131I
uptake and intense 131I uptake in distant metastatic
lesions. However, for patients with functional primary and
metastatic tumors, total thyroidectomy may be the optimal primary
treatment option, as it eliminates the hot primary thyroid
carcinoma, which produces a certain amount of thyroid hormones,
removes the thyroid gland and reduces the 131I dose
required to treat the metastatic lesions. In addition, total
thyroidectomy and subsequent pathological diagnosis may be
particularly useful for patients who have not undergone a
preoperative FNA.
RAI is necessary for treating hyperfunctioning
metastatic lesions in patients with thyroid carcinoma (4); it is a first-line treatment option for
patients with a history of thyroidectomy or for those with no
increased uptake in the thyroid gland. To avoid a possible thyroid
storm, pretreatment with antithyroid medication is required.
Fractionated RAI (as for patient 66 in the present systematic
review) (44), or minimal invasive
local ablation may also be considered (as for patient 62 in the
present systematic review) (40). If
the metastatic lesion is resistant to RAI and the functioning
lesion resectable, surgery may be considered as a treatment option.
This was demonstrated in patient 65 (43), whose thyrotoxicosis disappeared
following surgical removal of the functioning pelvic mass. However,
it is difficult to evaluate the efficiency of RAI following surgery
in patients with primary functioning thyroid carcinoma without
metastasis. As the majority of primary hyperfunctioning thyroid
tumors were large, and metastasis was reported during follow-up
post-surgery, RAI was considered as a treatment option following
surgery in patients with primary hyperfunctioning thyroid carcinoma
(12,19).
In conclusion, the results of the present study
indicated that the size of hyperfunctioning thyroid tumors is
markedly larger, and primary or metastatic FTC is more commonly
hyperfunctioning compared with PTC. FNA or core needle aspiration
together with whole-body scanning may play a key role in the
diagnosis of clinical thyrotoxicosis. In addition, surgery and RAI
are the preferred treatments for primary and metastatic
hyperfunctioning thyroid carcinoma, respectively. However, there
were certain limitations to the present study: We evaluated studies
using the Newcastle-Ottawa Scale and the scores of the studies
ranged 2–4. Considering that the number of hyperfunctioning thyroid
carcinomas is small and most studies are published as case reports,
a risk of bias may exist and the results must be interpreted with
caution.
Acknowledgements
JL gratefully acknowledges the support of Shanghai
Jiao Tong University K.C. Wong Medical Fellowship Fund (2017) and
Program of Foreign Visiting Studies of Young Teachers in Shanghai
Colleges and Universities (2017). The authors would like to thank
Xiaoyun Xu for proofreading the article.
Funding
The present study was funded by the Shanghai Jiao
Tong University K.C. Wong Medical Fellowship Fund (2017) and the
Program of Foreign Visiting Studies of Young Teachers in Shanghai
Colleges and Universities (2017).
Availability of data and materials
All the datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
JL conceived the study and drafted and wrote the
manuscript, YW and DD collected the data, MZ analyzed and
interpreted the data and provided the clinical suggestion. All the
authors have read and approved the final version of this manuscript
for publication.
Ethics approval and consent to
participate
This article does not contain any studies with human
participants or animals performed by any of the authors.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests to disclose.
References
|
1
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pazaitou-Panayiotou K, Michalakis K and
Paschke R: Thyroid cancer in patients with hyperthyroidism. Horm
Metab Res. 44:255–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Als C, Gedeon P, Rösler H, Minder C,
Netzer P and Laissue JA: Survival analysis of 19 patients with
toxic thyroid carcinoma. J Clin Endocrinol Metab. 87:4122–4127.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiu ZL, Shen CT and Luo QY: Clinical
management and outcomes in patients with hyperfunctioning distant
metastases from differentiated thyroid cancer after total
thyroidectomy and radioactive iodine therapy. Thyroid. 25:229–237.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mirfakhraee S, Mathews D, Peng L, Woodruff
S and Zigman JM: A solitary hyperfunctioning thyroid nodule
harboring thyroid carcinoma: Review of the literature. Thyroid Res.
6:72013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Appetecchia M and Ducci M:
Hyperfunctioning differentiated thyroid carcinoma. J Endocrinol
Invest. 21:189–192. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mircescu H, Parma J, Huot C, Deal C,
Oligny LL, Vassart G and Van Vliet G: Hyperfunctioning malignant
thyroid nodule in an 11-year-old girl: Pathologic and molecular
studies. J Pediatr. 137:585–587. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bourasseau I, Savagner F, Rodien P,
Duquenne M, Reynier P, Guyetant S, Bigorgne JC, Malthièry Y and
Rohmer V: No evidence of thyrotropin receptor and G(s alpha) gene
mutation in high iodine uptake thyroid carcinoma. Thyroid.
10:761–765. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Camacho P, Gordon D, Chiefari E, Yong S,
DeJong S, Pitale S, Russo D and Filetti S: A Phe 486 thyrotropin
receptor mutation in an autonomously functioning follicular
carcinoma that was causing hyperthyroidism. Thyroid. 10:1009–1012.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Führer D, Tannapfel A, Sabri O, Lamesch P
and Paschke R: Two somatic TSH receptor mutations in a patient with
toxic metastasising follicular thyroid carcinoma and non-functional
lung metastases. Endocr Relat Cancer. 10:591–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wong CP, AuYong TK and Tong CM:
Thyrotoxicosis: A rare presenting symptom of Hurthle cell carcinoma
of the thyroid. Clin Nucl Med. 28:803–806. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gozu H, Avsar M, Bircan R, Claus M, Sahin
S, Sezgin O, Deyneli O, Paschke R, Cirakoglu B and Akalin S: Two
novel mutations in the sixth transmembrane segment of the
thyrotropin receptor gene causing hyperfunctioning thyroid nodules.
Thyroid. 15:389–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Majima T, Doi K, Komatsu Y, Itoh H, Fukao
A, Shigemoto M, Takagi C, Corners J, Mizuta N, Kato R and Nakao K:
Papillary thyroid carcinoma without metastases manifesting as an
autonomously functioning thyroid nodule. Endocr J. 52:309–316.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bitterman A, Uri O, Levanon A, Baron E,
Lefel O and Cohen O: Thyroid carcinoma presenting as a hot nodule.
Otolaryngol Head Neck Surg. 134:888–889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niepomniszcze H, Suárez H, Pitoia F,
Pignatta A, Danilowicz K, Manavela M, Elsner B and Bruno OD:
Follicular carcinoma presenting as autonomous functioning thyroid
nodule and containing an activating mutation of the TSH receptor
(T620I) and a mutation of the Ki-RAS (G12C) genes. Thyroid.
16:497–503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uludag M, Yetkin G, Citgez B, Isgor A and
Basak T: Autonomously functioning thyroid nodule treated with
radioactive iodine and later diagnosed as papillary thyroid cancer.
Hormones (Athens). 7:175–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishida AT, Hirano S, Asato R, Tanaka S,
Kitani Y, Honda N, Fujiki N, Miyata K, Fukushima H and Ito J:
Multifocal hyperfunctioning thyroid carcinoma without metastases.
Auris Nasus Larynx. 35:432–436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bommireddipalli S, Goel S, Gadiraju R,
Paniz-MondolFi A and DePuey EG: Follicular variant of papillary
thyroid carcinoma presenting as a toxic nodule by I-123
scintigraphy. Clin Nucl Med. 35:770–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Azevedo MF and Casulari LA:
Hyperfunctioning thyroid cancer: A five-year follow-up. Arq Bras
Endocrinol Metabol. 54:78–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giovanella L, Fasolini F, Suriano S and
Mazzucchelli L: Hyperfunctioning solid/trabecular follicular
carcinoma of the thyroid gland. J Oncol. 2010:2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tfayli HM, Teot LA, Indyk JA and Witchel
SF: Papillary thyroid carcinoma in an autonomous hyperfunctioning
thyroid nodule: Case report and review of the literature. Thyroid.
20:1029–1032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karanchi H, Hamilton DJ and Robbins RJ:
Hürthle cell carcinoma of the thyroid presenting as thyrotoxicosis.
Endocr Pract. 18:e5–e9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nair CG, Jacob P, Babu M and Menon R:
Toxic thyroid carcinoma: A new case. Indian J Endocrinol Metab.
16:668–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruggeri RM, Campenni A, Giovinazzo S,
Saraceno G, Vicchio TM, Carlotta D, Cucinotta MP, Micali C,
Trimarchi F, Tuccari G, et al: Follicular variant of papillary
thyroid carcinoma presenting as toxic nodule in an adolescent:
Coexistent polymorphism of the TSHR and Gsα genes. Thyroid.
23:239–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gabalec F, Svilias I, Plasilova I,
Hovorkova E, Ryska A and Horacek J: Follicular variant of papillary
carcinoma presenting as a hyperfunctioning thyroid nodule. J
Pediatr Hematol Oncol. 36:e94–e96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuan YC and Tan FH: Thyroid papillary
carcinoma in a ‘hot’ thyroid nodule. QJM. 107:475–476. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rees DO, Anthony VA, Jones K and Stephens
JW: Follicular variant of papillary thyroid carcinoma: An unusual
cause of thyrotoxicosis. BMJ Case Rep. 2015:2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kadia BM, Dimala CA, Bechem NN and Aroke
D: Concurrent hyperthyroidism and papillary thyroid cancer: A
fortuitous and ambiguous case report from a resource-poor setting.
BMC Res Notes. 9:3692016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Girelli ME, Casara D, Rubello D, Pelizzo
MR, Busnardo B and Ziliotto D: Severe hyperthyroidism due to
metastatic papillary thyroid carcinoma with favorable outcome. J
Endocrinol Invest. 13:333–337. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mizukami Y, Michigishi T, Nonomura A,
Yokoyama K, Noguchi M, Hashimoto T, Nakamura S and Ishizaki T:
Autonomously functioning (hot) nodule of the thyroid gland. A
clinical and histopathologic study of 17 cases. Am J Clin Pathol.
101:29–35. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Russo D, Tumino S, Arturi F, Vigneri P,
Grasso G, Pontecorvi A, Filetti S and Belfiore A: Detection of an
activating mutation of the thyrotropin receptor in a case of an
autonomously hyperfunctioning thyroid insular carcinoma. J Clin
Endocrinol Metab. 82:735–738. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salvatori M, Rufini V, Corsello SM,
Saletnich I, Rota CA, Barbarino A and Troncone L: Thyrotoxicosis
due to ectopic retrotracheal adenoma treated with radioiodine. J
Nucl Biol Med. 37:69–72. 1993.PubMed/NCBI
|
|
33
|
Sundaraiya S, Dizdarevic S, Miles K, Quin
J, Williams A, Wheatley T and Zammitt C: Unusual initial
manifestation of metastatic follicular carcinoma of the thyroid
with thyrotoxicosis diagnosed by technetium Tc 99m pertechnetate
scan: Case report and review of literature. Endocr Pract.
15:458–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Damle NA, Bal C, Kumar P, Soundararajan R
and Subbarao K: Incidental detection of hyperfunctioning thyroid
cancer metastases in patients presenting with thyrotoxicosis.
Indian J Endocrinol Metab. 16:631–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gardner D and Ho SC: A rare cause of
hyperthyroidism: Functioning thyroid metastases. BMJ Case Rep.
2014.(pii): bcr2014206468. 2014.PubMed/NCBI
|
|
36
|
Kunawudhi A, Promteangtrong C and
Chotipanich C: A case report of hyperfunctioning metastatic thyroid
cancer and rare I-131 avid liver metastasis. Indian J Nucl Med.
31:210–214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abs R, Verhelst J, Schoofs E and De Somer
E: Hyperfunctioning metastatic follicular thyroid carcinoma in
Pendred's syndrome. Cancer. 67:2191–2193. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lorberboym M and Mechanick JI: Accelerated
thyrotoxicosis induced by iodinated contrast media in metastatic
differentiated thyroid carcinoma. J Nucl Med. 37:1532–1535.
1996.PubMed/NCBI
|
|
39
|
Yoshimura Noh J, Mimura T, Kawano M,
Hamada N and Ito K: Appearance of TSH receptor antibody and
hyperthyroidism associated with metastatic thyroid cancer after
total thyroidectomy. Endocr J. 44:855–859. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guglielmi R, Pacella CM, Dottorini ME,
Bizzarri GC, Todino V, Crescenzi A, Rinaldi R, Panunzi C, Rossi Z,
Colombo L and Papini E: Severe thyrotoxicosis due to
hyperfunctioning liver metastasis from follicular carcinoma:
Treatment with (131)I and interstitial laser ablation. Thyroid.
9:173–177. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Basaria S and Salvatori R: Thyrotoxicosis
due to metastatic papillary thyroid cancer in a patient with
Graves' disease. J Endocrinol Invest. 25:639–642. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Orsolon P, Giachetti M, Lupi A, Salgarello
M, Malfatti V and Zanco P: Pre-therapy hyperfunctioning follicular
thyroid carcinoma evaluation with I-131 whole-body scan and with
F-18 FDG PET/CT. Clin Nucl Med. 33:882–886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tan J, Zhang G, Xu W, Meng Z, Dong F,
Zhang F, Jia Q and Liu X: Thyrotoxicosis due to functioning
metastatic follicular thyroid carcinoma after twelve I-131
therapies. Clin Nucl Med. 34:615–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nishihara E, Amino N and Miyauchi A:
Fractionated radioiodine therapy for hyperthyroidism caused by
widespread metastatic follicular thyroid carcinoma. Thyroid.
20:569–570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lim H, Devesa SS, Sosa JA, Check D and
Kitahara CM: Trends in thyroid cancer incidence and mortality in
the United States, 1974–2013. JAMA. 317:1338–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Salih AM, Kakamad FH and Nihad H:
Hyperfunctioning papillary thyroid carcinoma: A case report with
literature review. Int J Surg Case Rep. 26:202–204. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pringle DR, Yin Z, Lee AA, Manchanda PK,
Yu L, Parlow AF, Jarjoura D, La Perle KM and Kirschner LS:
Thyroid-specific ablation of the Carney complex gene, PRKAR1A,
results in hyperthyroidism and follicular thyroid cancer. Endocr
Relat Cancer. 19:435–446. 2012. View Article : Google Scholar : PubMed/NCBI
|