Introduction
Neoadjuvant chemotherapy (NAC) followed by surgery
is a standard treatment of resectable stage II or III esophageal
squamous cell carcinoma in Japan. A prospective randomized trial
comparing postoperative adjuvant chemotherapy with cisplatin and
5-fluorouracil (CF) regimen vs. preoperative chemotherapy
(JCOG9907), showed a significant survival advantage of NAC
(1). However, significant weight
loss and malnutrition may occur during NAC, which usually has a
number of weeks duration (2), and
are of concern especially in patients with tumors that obstruct
passage of liquids and solid food. Treatment of patients with
obstructing tumors may be restricted to surgery without NAC, or
with NAC with inadequate enteral nutrition. Laparoscopic
jejunostomy (Lap-J) is easy to perform and safely provides
nutritional support of patients with cervical and esophageal
obstructing tumors and those with advanced gastric cancer (3). This study evaluated the nutritional
benefit of Lap-J in patients receiving NAC with a CF regimen for
obstructing, locally advanced esophageal cancer.
Materials and methods
This study retrospectively evaluated 91 patients who
were given NAC for locally-advanced esophageal cancer at the
National Defense Medical College Hospital between 2009 and 2017.
The study included 14 women and 77 men with a median age of 71
(range 43–86) years. Fifteen patients underwent Lap-J prior to NAC
(Lap-J group), and 76 patients received NAC without Lap-J (control
group). The patient characteristics are shown in Table I. Lap-J was indicated in patients
with dysphagia because of stenosis that could not be passed by a
standard gastroscopy and nasogastric tube. The contraindications
for Lap-J were unsuitability for general anesthesia and
gastrointestinal disease. Lap-J was not indicated in patients with
adequate oral intake of food or liquid supplements. Chemotherapy
was administered twice every four weeks, including cisplatin 80
mg/m2 by intravenous drip infusion for 2 h on day 1 and
5-FU 800 mg/m2 by continuous infusion on days 1–5
(1). Nutritional quality was
monitored by body weight, body mass index (BMI), total blood
protein and albumin concentrations, total lymphocyte count,
hemoglobin, prognostic nutritional index (PNI) (4), modified Glasgow prognostic score (mGPS)
(5), and psoas muscle index (PI)
before and after NAC. The blood tests were measured using
biochemical analyzer (LABOSPECT 008, Hitachi High-Technologies,
Tokyo, Japan) and blood cell analyzer (XE5000, Sysmex, Hyogo,
Japan). Computed tomography (CT) scanning was undergone before and
after NAC, in the supine position, from neck to pelvis, with a
64-multidetector CT scanner (Aquilion 64, Toshiba, Tokyo, Japan).
The PI (cm2/m2) was calculated from CT images
and the psoas muscle cross-sectional area at the L3 level
normalized against length (6). The
mean total observation period was 722 days (95% confidence interval
565 to 880). The study protocol was reviewed and approved by the
Institutional Review Board of the National Defense Medical College.
Written informed consent was obtained from each patient prior to
study inclusion.
 | Table I.Clinicopathological characteristics of
Lap-J and control patients. |
Table I.
Clinicopathological characteristics of
Lap-J and control patients.
|
| Lap-J group | Control |
|
|---|
|
|
|
|
|
|---|
|
| N=15 | N=76 | P-value |
|---|
| Patient's
characteristics |
| Age (years) | 69 (52–75) | 71 (43–86) | 0.84 |
| Sex |
|
| 0.60 |
| Male | 12 | 65 |
|
|
Female | 3 | 11 |
|
| Tumor
characteristics |
| Location |
|
|
|
| Ce | 0 (0%) | 2 (3%) | 0.25 |
| Ut | 3 (20%) | 8 (11%) |
|
| Mt | 8 (53%) | 30 (39%) |
|
| Lt | 4 (27%) | 28 (37%) |
|
| Ae | 0 (0%) | 8 (11%) |
|
| T stage |
|
|
|
| T1 | 1 (7%) | 8 (11%) | 0.58 |
| T2 | 3 (20%) | 9 (12%) |
|
| T3 | 8 (53%) | 51 (67%) |
|
| T4 | 3 (20%) | 8 (11%) |
|
| N stage |
|
|
|
| N0 | 4 (26%) | 15 (20%) | 0.70 |
| N1 | 6 (40%) | 23 (30%) |
|
| N2 | 3 (20%) | 23 (30%) |
|
| N3 | 1 (7%) | 12 (16%) |
|
| N4 | 1 (7%) | 3 (4%) |
|
| Preoperative
chemotherapy |
|
|
|
| Two
cycles | 13 (87%) | 63 (83%) | 0.77 |
| One
cycle | 2 (13%) | 13 (17%) |
|
| Nutritional supports
during NAC |
| None | 0 (0%) | 48 (63%) | <0.01 |
|
Nutritional oral
supplements | 0 (0%) | 26 (34%) |
|
|
Nasogastric tube | 0 (0%) | 2 (3%) |
|
|
Lap-J | 15 (100%) | 0 (0%) |
|
Lap-J procedure
Lap-J was performed as previously described
(3). In brief, under general
anesthesia and after laparoscopic exploration of the peritoneal
cavity, the jejunum 20–30 cm distant from the Treitz ligament was
pulled out through the umbilical trocar incision. A serosal suture
was placed, and a jejunostomy was performed by the Witzel technique
using a needle jejunostomy kit (Covidien, Tokyo, Japan). The
jejunal loop was gently returned to the abdomen and the feeding
tube was drawn through the abdominal wall via the left lower trocar
incision. The jejunum was then laparoscopically sutured to the
anterior abdominal wall with approximately six sutures.
Statistical analysis
Normally-distributed continuous data were reported
as means and standard error (SE). Non-normally distributed
continuous data were reported as medians and interquartile range
(IQR). Categorical variables were reported as numbers and
percentages. Differences in normally-distributed clinical variables
were compared using the student t-test. Pearson's chi-square test
or Fisher's exact test were used for categorical data. The
Mann-Whitney U test was used to analyze non-normally distributed
continuous and ranked data, as appropriate. Overall (OS) and
disease-free (DFS) survival were estimated by the Kaplan-Meier
method. The significance of differences in survival was compared
with the log-rank test. The significance of the association of
clinical variables and patient outcomes was tested by univariate
analysis. Variables with a P-value <0.10 were included in a
subsequent multivariate (logistic regression or Cox proportional
hazards regression) analysis. All tests were two-sided, and
P-values <0.05 were considered statistically significant. The
statistical analysis was performed using JMP statistics version
11.0 (SAS Institute Inc., Carey, NC, USA).
Results
There were no significant differences in tumor
characteristics (location, depth, or nodal involvement) in the 2
groups. Forty-eight patients had no nutritional support during NAC,
26 required nutritional liquid supplements, and two required
nasogastric tube feeding. All patients in the Lap-J group were able
to stand, walk, and begin enteral nutrition via the jejunostomy on
postoperative day 1. Fourteen of the 15 patients (93.3%) did not
experience any procedure-related complications. One had
subcutaneous abscess necessitating the removal of jejunostomy tube
during NAC. NAC began at a median of seven days after Lap-J surgery
(range, 3–19 days). No mortalities were associated with Lap-J.
The nutritional characteristics in the two groups
before the start of NAC are shown in Table II. Body weight, BMI, PI, serum
albumin, and PNI were significantly lower in the Lap-J group in
comparison with the control group. Two score of mGPS occurred more
frequently in the Lap-J group than in the control group. Changes in
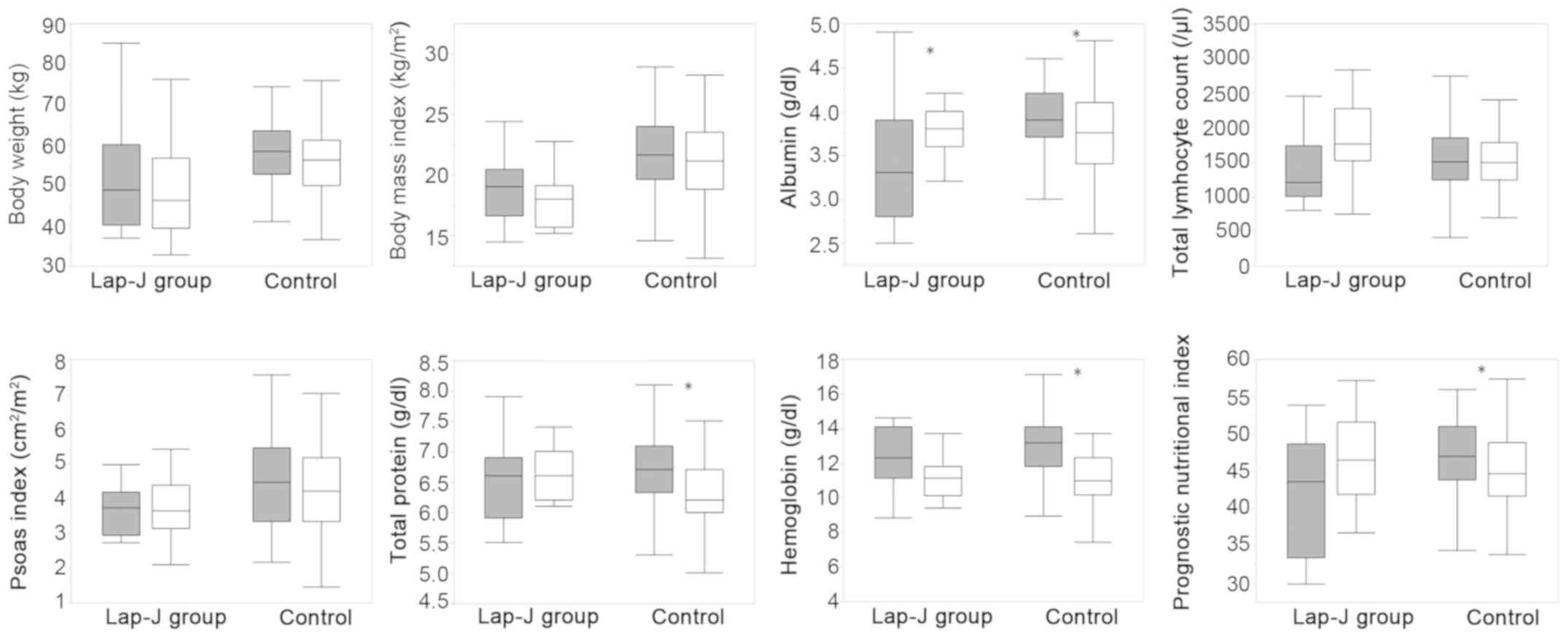
the values of nutritional variables during NAC are shown in
Fig. 1. Significant decreases in
serum total protein, albumin, and hemoglobin and in PNI occurred
during NAC in the control group but not in the Lap-J group. Serum
albumin was significantly higher in the Lap-J group after NAC than
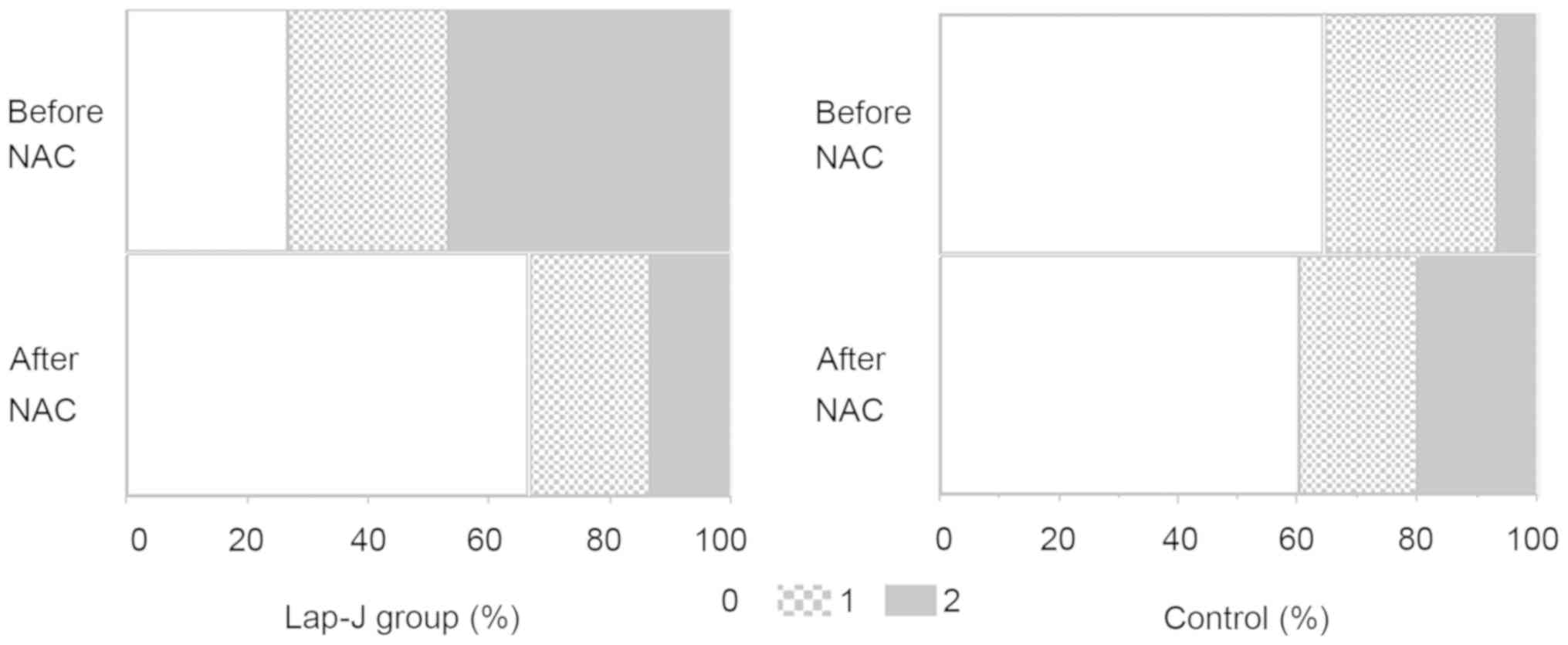
in the control group. The number of patients with a 2 score of mGPS
in the control group increased after NAC, but decreased in the
Lap-J group (Fig. 2). There were no
differences in the occurrence of postoperative complications, i.e.,
anastomotic leakage (Lap-J 27% vs. control 24%) and pneumonia
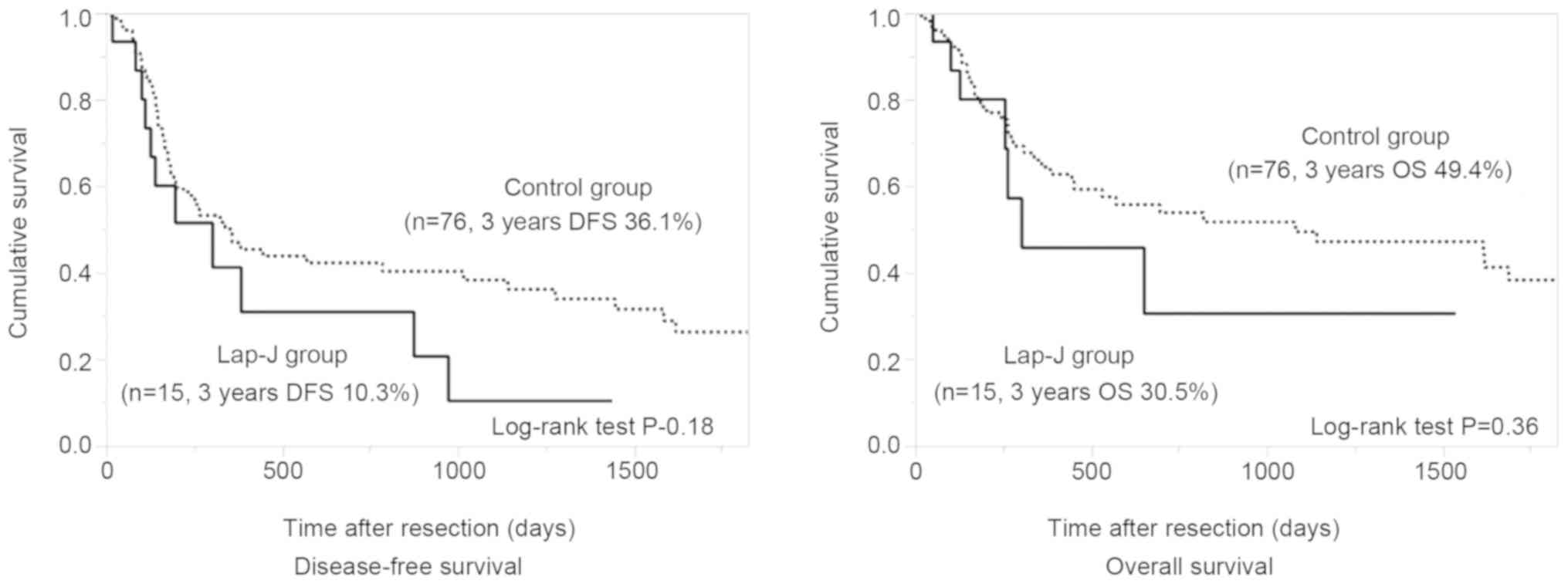
(Lap-J 27% vs. control 38%). The differences in DFS and OS of the
two groups were not significant (Fig.
3).
 | Table II.Nutritional variables prior to
neoadjuvant chemotherapy. |
Table II.
Nutritional variables prior to
neoadjuvant chemotherapy.
|
| Lap-J group | Control | P-value |
|---|
| Age, years | 69 (52–75) | 71 (43–86) | 0.84 |
| Sex male/female | 12/3 | 65/11 | 0.60 |
| Body weight (kg) | 50.5±13.0 | 57.9±8.4 | <0.01 |
| BMI
(kg/m2) | 19.4±4.3 | 21.9±3.1 | <0.05 |
| PMI
(cm2/m2) | 3.7±0.7 | 4.6±1.4 | <0.05 |
| Total protein
(g/dl) | 6.5±0.7 | 6.7±0.5 | 0.16 |
| Albumin (g/dl) | 3.5±0.7 | 3.9±0.4 | <0.01 |
| Total lymphocyte
count (/µl) | 1398±521 | 1572±509 | 0.23 |
| Hemoglobin
(g/dl) | 12.1±1.9 | 13.0±1.7 | 0.06 |
| Prognostic
nutritional index | 41.6±8.0 | 46.9±4.9 | <0.01 |
| Modified Glasgow
prognostic score |
| 0 | 4 (26.7%) | 49 (64.5%) |
|
| 1 | 4 (26.7%) | 22 (28.9%) | <0.01 |
| 2 | 7 (46.7%) | 5 (6.6%) |
|
Discussion
Despite progress in perioperative management and
operative procedures, esophagectomy for esophageal cancer is still
a highly-invasive procedure associated with serious postoperative
complications, including pneumonia and anastomotic leakage
(7,8). Combination chemotherapy for esophageal
carcinoma has improved local tumor control and reduced the
incidence of distant treatment failure after surgery. However,
esophageal cancer is still one of the most refractory cancers
(1). Poor nutrition increases the
risk of poor short- and long-term outcomes in patients with
esophageal cancer (9,10). Preoperative chemotherapy in itself
induces malnutrition because of treatment-related anorexia and
nausea. Patients with advanced esophageal cancer frequently
experience dysphagia from stenosis caused by the tumor. Appropriate
nutritional support during NAC is essential for the improvement of
surgical outcomes in patients with advanced esophageal cancer.
Among the nutritional interventions that are
available during preoperative therapy, enteral feeding by a
nasogastric tube is the easiest and least invasive intervention.
However, in addition to discomfort, nasogastric tubes are subject
to accidental extubation, aspiration risk, and the need of
hospitalization, all of which compromise patients' quality of life.
Temporary endoscopic stent placement provides instant resolution of
dysphagia, but often causes clinically significant complications
such as stent migration and esophago-tracheo-bronchial fistulas
(11,12). The use of esophageal stents in the
neoadjuvant setting may lead to complications that can compromise
the opportunity for curative surgery in a small proportion of
patients (13). Percutaneous
endoscopic gastrostomy has been described as an option for
provision of nutrition (14), but it
might interfere with the subsequent use of a gastric conduit for
reconstruction. Feeding jejunostomies are effective and reasonable
options for providing perioperative nutritional support, and
prolonged tube feeding can be continued after radical esophagectomy
(15).
In this study, Lap-J feeding effectively maintained
serum total protein, albumin, and hemoglobin, and the PNI during
NAC compared with the control group. In addition to maintaining
nutritional status, Lap-J contributed to improved quality of life
in patients with obstructing esophageal cancer because they could
live at home except for receiving chemotherapy infusions. The study
did not demonstrate any benefits of Lap-J associated with
infectious complications, hospital stay, or long-term outcome after
radical esophagectomy compared with the control group. That might
have been a consequence of the small number of patients undergoing
Lap-J and the presence of more advanced disease in patients with
obstructing esophageal tumors than in those patients with
non-obstructing esophageal tumors. The results support the conduct
of a randomized controlled trial with a larger patient population
to evaluate the impact of Lap-J on the perioperative management of
obstructing esophageal cancer. In addition, the superiority of
Lap-J over conventional nutritional supports such as nasogastric
tube and temporary stent should be clarified in near future to
demonstrate the efficacy of Lap-J.
Lap-J has several advantages over conventional open
procedure. Firstly, lap-J can explore the whole abdominal cavity,
which may detect small liver and peritoneal metastases (16). Secondly, Lap-J possesses the minimal
invasive procedure as compared to open procedure, which makes it
possible to start the following therapy (17,18).
In conclusion, we recommend Lap-J for safe and
effective perioperative nutritional support during NAC in patients
with obstructing esophageal cancer. However, the survival advantage
of Lap-J during NAC should be clarified in the prospective
randomized study with sufficient number of patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data used and/or analyzed during this published
article are available from the corresponding author on reasonable
request.
Authors' contributions
KN, HT and HU collaborated in the conception and
design of the study. KN, HN, MH, NI, SN and HH acquired the data.
KN, HT, SH, SA, KH and HU performed data analysis and
interpretation. All authors were involved in writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ando N, Kato H, Igaki H, Shinoda M, Ozawa
S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun J, Wang D, Mei Y, Jin H, Zhu K, Liu X,
Zhang Q and Yu J: Value of the prognostic nutritional index in
advanced gastric cancer treated with preoperative chemotherapy. J
Surg Res. 209:37–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsujimoto H, Hiraki S, Takahata R, Nomura
S, Ito N, Kanematsu K, Horiguchi H, Aosasa S, Yamamoto J and Hase
K: Laparoscopic jejunostomy for obstructing upper gastrointestinal
malignancies. Mol Clin Oncol. 3:1307–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smale BF, Mullen JL, Buzby GP and Rosato
EF: The efficacy of nutritional assessment and support in cancer
surgery. Cancer. 47:2375–2381. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, Fletcher CD, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC:
A comparison of inflammation-based prognostic scores in patients
with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer.
47:2633–2641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hiraoka A, Aibiki T, Okudaira T, Toshimori
A, Kawamura T, Nakahara H, Suga Y, Azemoto N, Miyata H, Miyamoto Y,
et al: Muscle atrophy as pre-sarcopenia in Japanese patients with
chronic liver disease: Computed tomography is useful for
evaluation. J Gastroenterol. 50:1206–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsujimoto H, Takahata R, Nomura S, Yaguchi
Y, Kumano I, Matsumoto Y, Yoshida K, Horiguchi H, Hiraki S, Ono S,
et al: Video-assisted thoracoscopic surgery for esophageal cancer
attenuates postoperative systemic responses and pulmonary
complications. Surgery. 151:667–673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeuchi H, Miyata H, Gotoh M, Kitagawa Y,
Baba H, Kimura W, Tomita N, Nakagoe T, Shimada M, Sugihara K and
Mori M: A risk model for esophagectomy using data of 5354 patients
included in a Japanese nationwide web-based database. Ann Surg.
260:259–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mariette C, Taillier G, Van Seuningen I
and Triboulet JP: Factors affecting postoperative course and
survival after en bloc resection for esophageal carcinoma. Ann
Thorac Surg. 78:1177–1183. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Law S, Wong KH, Kwok KF, Chu KM and Wong
J: Predictive factors for postoperative pulmonary complications and
mortality after esophagectomy for cancer. Ann Surg. 240:791–800.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langer FB, Schoppmann SF, Prager G,
Tomaselli F, Pluschnig U, Hejna M, Schmid R and Zacherl J:
Temporary placement of self-expanding oesophageal stents as
bridging for neo-adjuvant therapy. Ann Surg Oncol. 17:470–475.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jones CM and Griffiths EA: Should
oesophageal stents be used before neo-adjuvant therapy to treat
dysphagia in patients awaiting oesophagectomy? Best evidence topic
(BET). Int J Surg. 12:1172–1180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bower M, Jones W, Vessels B, Scoggins C
and Martin R: Nutritional support with endoluminal stenting during
neoadjuvant therapy for esophageal malignancy. Ann Surg Oncol.
16:3161–3168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhatti AB, Rizvi FH, Waheed A, Raza SH,
Syed AA, Khattak S and Aasim Yusuf M: Does prior percutaneous
endoscopic gastrostomy alter post-operative outcome after
esophagectomy. World J Surg. 39:441–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gupta V: Benefits versus risks: A
prospective audit. Feeding jejunostomy during esophagectomy. World
J Surg. 33:1432–1438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grondona P, Andreani SM, Barr N and Singh
KK: Laparoscopic feeding jejunostomy technique as part of staging
laparoscopy. Surg Laparosc Endosc Percutan Tech. 15:263–266. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han-Geurts IJ, Lim A, Stijnen T and Bonjer
HJ: Laparoscopic feeding jejunostomy: A systematic review. Surg
Endosc. 19:951–957. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siow SL, Mahendran HA, Wong CM, Milaksh NK
and Nyunt M: Laparoscopic T-tube feeding jejunostomy as an adjunct
to staging laparoscopy for upper gastrointestinal malignancies: The
technique and review of outcomes. BMC Surg. 17:252017. View Article : Google Scholar : PubMed/NCBI
|