Introduction
Lung cancer is the leading incident cancer and cause
of cancer mortality worldwide. There were about 2.1 million new
lung cancer cases and 1.8 million deaths predicted in 2018
(1). Unfortunately, there is
currently no standard third-line treatment for advanced
non-small-cell lung cancer (NSCLC). So there is still a need to
develop innovative, more effective, and safer anticancer drugs.
Anlotinib is a novel multi-target tyrosine kinase
inhibitor that is designed to primarily inhibit vascular
endothelial growth factor receptor (VEGFR)2/3, fibroblast growth
factor receptor (FGFR)1-4, platelet-derived growth factor receptors
(PDGFR)α/β, c-Kit, and Ret (2).
ALTER 0303 Phase 3 Randomized Clinical Trial showed that anlotinib
as third-line therapy for advanced NSCLC can prolong progress free
survival (PFS) for 4 months (5.4 vs. 1.4, P<0.0001) and OS for
3.3 months (9.6 vs. 6.3, P=0.002) compared with placebo among
Chinese patients. A total of 437 patients were randomized 2:1 to
receive either oral anlotinib or placebo (12 mg QD from days 1 to
14 of a 21-day cycle). Treatment continued until tumor progression
or discontinuation due to toxicity. An exploratory subgroup
analysis of the ALTER0303 trial showed that anlotinib significantly
improved progress free survival (PFS) and overall survival (OS) in
patients with both sensitive EGFR mutations and wild-type EGFR and
it can prolong PFS for 2.93 months (P=0.001) and OS for 2 months
(P=0.0932) for squamous lung cancer. It is the first oral
multi-target tyrosine kinase inhibitor drug can prolong PFS for
squamous lung cancer (3). Moreover,
The China Food and Drug Administration (CFDA) approved single agent
anlotinib as a third-line treatment for patients with advanced
NSCLC. Clinical trials have confirmed that anlotinib is well
tolerated. Wu et al (4)
assessed the efficacy and toxicity of anlotinib. They found that
anlotinib plays a significant role in the salvage treatment of
advanced NSCLC.
Anlotinib can inhibit both tumor angiogenesis and
tumor cell proliferation (5).
Compared with other RTK inhibitors, such as sorafenib and
sunitinib, anlotinib can inhibit more targets. Studies have shown
that anlotinib significantly inhibits VEGF/PDGF-BB/FGF-2-induced
angiogenesis in vitro and in vivo (2). Thence, it had broader and better
antitumor efficacy than did other RTK inhibitors in vivo.
There are also many studies on the anti-tumor mechanism of
anlotinib. One study showed that anlotinib could induce protective
autophagy, related to anti-angiogenic property of multikinase
inhibitor anlotinib (6). Lu et
al (7) believe that
anlotinib-induced serum CCL2 level decreases were associated with
the benefits of PFS and OS in refractory advanced NSCLC
patients.
Compared to the mono-target agents, it may cause
more AEs in patients. Whether side effects occur dependent on the
individual physical characteristics of the patients (8). The most common adverse events (AEs) of
anlotinib included hypertension, fatigue, lack or loss of appetite,
thyroid-stimulating hormone elevation, anorexia
hypertriglyceridemia, hand-foot syndrome and hypercholesterolemia
(3). However, bronchopleural fistula
as a result of treatment with anlotinib or other TKIs have not been
reported. Here, we present the first report of a bronchopleural
fistula caused by multi-target tyrosine kinase inhibitor
therapy.
Case report
A 69-year-old male never-smoker was admitted to the
hospital in April 2011 with a cough and hemoptysis, which had been
ongoing for 3 months. The vital signs of the patient were stable.
Enhanced computed tomography (CT) scans of the patient's chest
revealed tumors on the lower lobe of the right lung and on the
upper lobe of the left lung. Then he underwent radical resection of
the right lung cancer and nodule wedge resection of left lung.
Postoperative pathology: Poorly differentiated squamous cell
carcinoma in the right lung and left lung. The patient was
diagnosed as having stage IA cancer (cT1cN0M0) according to
postoperative pathology, and began receiving chemotherapy
consisting of gemcitabine and cisplatin for four cycles. However, a
chest CT scan demonstrated disease progression with metastases in
the lungs in December 2016. Therefore, he received first-line
chemotherapy including paclitaxel liposome (210 mg) and nedaplatin
(100 mg) for 6 cycles from July 1, 2017 to October 24, 2017. In
January 3th, 2018, efficacy evaluation is disease progression (PD,
RECIST1.1). To control the disease second-line chemotherapy GP
regimen (gemcitabine 1.4 g d1, d8+ nedaplatin 100 mg d1) treatment
was given for 2 months. He received ‘gemcitabine 1.4 g d1, d8+
nedaplatin 100 mg d1’ second-line chemotherapy for 2 cycles from
January 2018 to March 2018. Efficacy evaluation is stable disease
(SD, RECIST1.1) in this time, but patients refused to continue to
receive chemotherapy due to gastrointestinal reaction (NCI CTC3.0
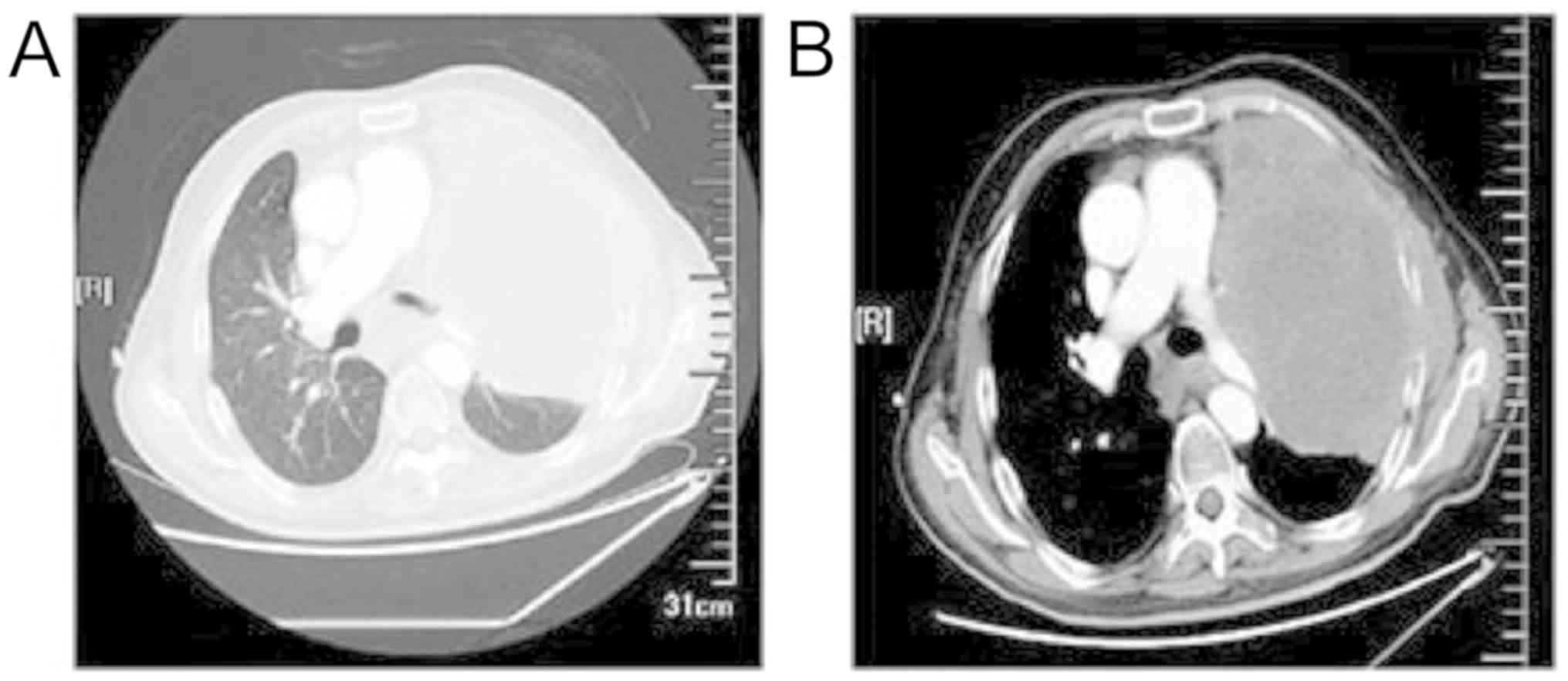
grade III). In June 2018, the CT of the chest was obtained. This
revealed the two lungs metastasized, the left lung lesions
increased, the two lung metastases were larger than the front, and
the mediastinal multiple lymph node metastasis lesions were both
larger than the previous one. The left 4 and 5 ribs were invaded by
tumor, no abnormality in the abdominal pelvic CT (Fig. 1A-B). Efficacy evaluation is disease
progression (PD, RECIST1.1). We recommended he to biopsy again for
genetic testing to see if there were sensitive mutations, but he
refused. Considered the poor general condition of this patient (PS
score 2), anlotinib was planned as third-line therapy (12 mg, po,
qd, d1-14, q21 days). Initially the patient was well tolerated.
After the second cycle of oral targeted therapy, the patient
presented to hospital with pain in the oral mucosa and tongue,
which affected eating. He also felt pleuritic chest pain, shortness
of breath, and nonproductive cough which was significantly worse
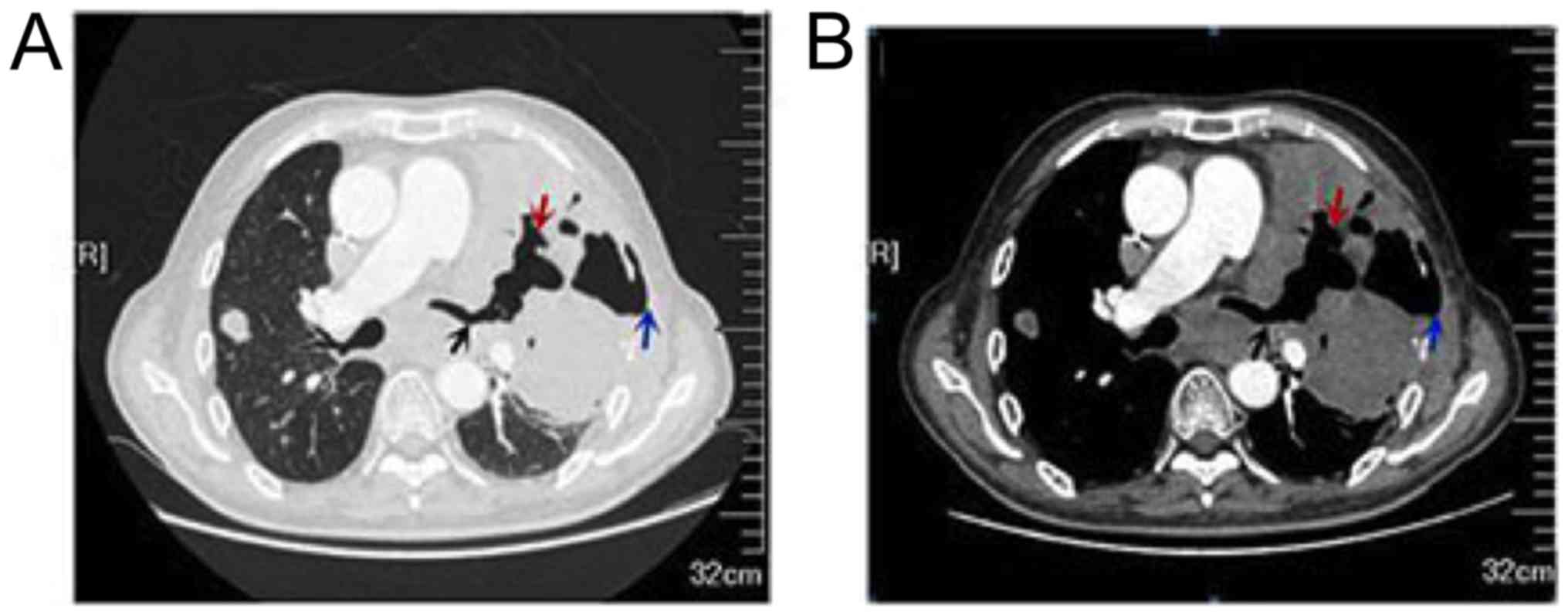
than before. Chest CT demonstrated left lung lesions appeared
necrotic cavity with left chest wall emphysema and the left upper
lobe and the lower lobe of the two lungs showed a exudative change
(Fig. 2A-B), the right upper lobe
subpleural lesions was smaller than before. Efficacy evaluation is
stable disease (SD, RECIST1.1). The cardiothoracic surgery was
consulted, BPF caused by targeted treatment was considered
comprehensively, and then brought about secondary infection.
Anlotinib was discontinued. However, the symptoms cannot be
relieved after anti-infective treatment and supportive therapies.
Finally, the patient died of respiratory failure on August 28,
2018. The overall survival time was 88 months and it was found to
survive for 22 months after lung metastasis.
Discussion
Anlotinib is a newly developed multiple receptor
tyrosine kinase (RTKs) oral small molecule inhibitor, and is
recommended in the Chinese Society of Clinical Oncology Guidelines
for the Diagnosis and Treatment of Primary Lung Cancer (2018
Edition) for the same indication (9). It inhibits both neoplastic angiogenesis
and tumor growth pathways and is well tolerated.
Bronchopleural fistula (BPF) is defined as an
abnormal channel that develops as a result of connection between
the bronchus and the pleura. It may be caused by necrotizing
pneumonia or empyema, lung tumor, lung injury, chest drainage and
chest puncture, radiotherapy and chemotherapy. It is most commonly
occur after pneumonectomy. Residual tumor in the bronchial stump
after resection, inflammation of the residual site, or combined
with tuberculosis, follow by chemotherapy and radiotherapy will
increase the incidence of bronchopleural fistula. In addition,
there is a meta analysis show that diabetes mellitus is an
independent risk factor of bronchopleural fistula after pulmonary
resection (10). Bronchopleural
fistula (BPF) is a rare but severe complication, it has a higher
mortality rate. It is reported that the mortality rate of BPF after
pneumonectomy is 18–50% (11). Chang
et al reported 1 patient (1.6%) complicated with bronchial
fistula in 64 cases of patients with unresectable III stage
non-small cell lung cancer after proton beam radiotherapy (12). Zheng et al (13) reported 2 of 682 patients (0.29%) with
lung cancer after pulmonary ablation complicated with BPF (13). At present, there is no relevant
literature report about the occurrence of BPF after targeted
therapy in patients with primary lung cancer. In the ALTER 0303
study, no patient experienced adverse events of bronchopleural
fistula. Only a small proportion of subjects reported grades 3/4
event (3). Thus, BPF may be rare in
the general population. No cases have been reported.
The mechanism by which targeted drugs cause
perforation of lung cancer is not clear. There are more reports of
targeted drugs causing gastrointestinal perforation (14–17).
However, reports of targeted drug-induced perforation of the lungs
are rare, especially in primary lung cancer. Gennatas et al
(18) reported a case of
pneumothorax during crizotinib therapy in a patient with with a
Stage IV, TTF1 positive, EGFR wild-type adenocarcinoma of the lung.
Pneumothorax occurring in the setting of targeted therapy for
pulmonary metastatic disease is a well-described clinical situation
that has been reviewed by previous authors (19–22). Chi
et al (23) reported 166
patients with Refractory Metastatic Soft-Tissue Sarcoma were
treated with anlotinib. What needs to be noticed is that mong
those, there were 4 patients (2.4%) with grades 3 pneumothorax. But
no treatment-related death occurred. We think there are two reasons
of BPF for this patient. Firstly, the tumor was very large,
connected with the main bronchus and invaded the pleura, which
provided anatomical and pathological basis for the formation of
BPF. Direct invasion of tumor or extension of cavitary tumor
lesions could be the probable causes. Secondly, anlotinib can
inhibit tumor angiogenesis and tumor growth. We also found
clinically in other patients that the probability of tumor necrosis
after treatment with erlotinib was significantly higher than other
TKI-targeted drugs. The tumor of this patient shrank, and the
necrosis inside the tumor was obviously and accompanied by
cavitation after oral administration of anlotinib, which eventually
led to the occurrence of bronchopleural fistula.
To the best of our knowledge, this is the first
known case report of a bronchopleural fistula caused by
multi-target tyrosine kinase inhibitor therapy. Despite there is no
bronchopleural fistula complication found in the patient in ALTER
0303 study, we should be careful for patients with non-small-cell
lung cancer, especially centrally located squamous cell carcinoma,
large tumor, close to trachea and/or pleura, and combined with
diabetes mellitus, when we use the drugs with antiangiogenic
effect, especially for squamous cell lung cancer. Because they have
the potential to cause life-threatening complications, like
bronchopleural fistula.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of
Nanjing Science and Technology Commission of Jiangsu Province
(grant no. 201605064) and Jiangsu Provincial Department of
Education (grant no.SJCX-0374).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JGH conceived and designed the study. DL and GLW
wrote the manuscript. GLW, LCL, JM, XFH, FXQ and ZG have
contributed to the clinical management of the patient. All authors
critically reviewed the manuscript and approved the final
manuscript.
Ethics approval and consent to
participate
The China Food and Drug Administration (CFDA)
approved single agent anlotinib as a third-line treatment for
patients with advanced NSCLC. The current study was approved by the
Institutional Ethics Review Board of Jiangsu Province Academy of
Traditional Chinese Medicine, China Nanjing 220000. The patient and
his family agreed to participate in this study. This study is
normative.
Patient consent for publication
The patient and his family agreed to the publication
of the case details.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin B, Song X, Yang D, Bai D, Yao Y and Lu
N: Anlotinib inhibits angiogenesis via suppressing the activation
of VEGFR2, PDGFRβ and FGFR1. Gene. 654:77–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han BH, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of Anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu D, Nie J, Dai L, Hu W, Zhang J, Chen X,
Ma X, Tian G, Han J, Han S, et al: Salvage treatment with anlotinib
for advanced non-small cell lung cancer. Thorac Cancer.
10:1590–1596. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y
and Lou L: Preclinical characterization of anlotinib, a highly
potent and selective vascular endothelial growth factor receptor-2
inhibitor. Cancer Sci. 109:1207–1219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang L, Hui K, Hu C, Wen Y, Yang S, Zhu
P, Wang L, Xia Y, Qiao Y, Sun W, et al: Autophagy inhibition
potentiates the anti-angiogenic property of multikinase inhibitor
anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung
cancer cells. J Exp Clin Cancer Res. 38:712019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu J, Zhong H, Chu T, Zhang X, Li R, Sun
J, Zhong R, Yang Y, Alam MS, Lou Y, et al: Role of
anlotinib-induced CCL2 decrease in anti-angiogenesis and response
prediction for nonsmall cell lung cancer therapy. Eur Respir J.
53:2019. View Article : Google Scholar
|
|
8
|
Wang J, Zhao Y, Wang Q, Zhang L, Shi J,
Wang Z, Cheng Y, He J, Shi Y, Yu H, et al: Prognostic factors of
refractory NSCLC patients receiving anlotinib hydrochloride as the
third- or further-line treatment. Cancer Biol Med. 15:443–451.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
No authors listed, . Chinese guidelines
for diagnosis and treatment of primary lung cancer 2018 (English
version). Chin J Cancer Res. 31:1–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li SJ, Fan J, Zhou J, Ren YT, Shen C and
Che GW: Diabetes mellitus and risk of bronchopleural fistula after
pulmonary resections: A meta-analysis. Ann Thorac Surg.
102:328–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okuda M, Go T and Yokomise H: Risk factor
of bronchopleural fistula after general thoracic surgery: Review
article. Gen Thorac Cardiovasc Surg. 65:679–685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang JY, Verma V, Li M, Zhang W, Komaki
R, Lu C, Allen PK, Liao Z, Welsh J, Lin SH, et al: Proton beam
radiotherapy and concurrent chemotherapy for unresectable stage III
non-small cell lung cancer: Final results of a phase 2 study. JAMA
Oncol. 3:e1720322017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng A, Yang X, Ye X, Huang G, Wei Z,
Wang J, Han X, Ni X and Meng M: Bronchopleural fistula after lung
ablation: Experience in two cases and literature review. Indian J
Cancer. 52 (Suppl 2):e41–e46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peters NA, Richel DJ, Verhoeff JJ and
Stalpers LJ: Bowel perforation after radiotherapy in a patient
receiving sorafenib. J Clin Oncol. 26:2405–2406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inoue T, Kinoshita H, Komai Y, Kawabata T,
Kawa G, Uemura Y and Matsuda T: Two cases of gastrointestinal
perforation after radiotherapy in patients receiving tyrosine
kinase inhibitor for advanced renal cell carcinoma. World J Surg
Oncol. 10:1672012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li XF, Tan YN, Cao Y, Xu JH, Zheng S and
Yuan Y: A case report of gastrointestinal hemorrhage and
perforation during apatinib treatment of gastric cancer. Medicine
(Baltimore). 94:e16612015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ou SH, Ahn JS, De Petris L, Govindan R,
Yang JC, Hughes B, Lena H, Moro-Sibilot D, Bearz A, Ramirez SV, et
al: Alectinib in crizotinib-refractory ALK-rearranged
non-small-cell lung cancer: A phase II global study. J Clin Oncol.
34:661–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gennatas S, Stanway SJ, Thomas R, Min T,
Shah R, O'Brien ME and Popat S: Early pneumothorax as a feature of
response to crizotinib therapy in a patient with ALK rearranged
lung adenocarcinoma. BMC Cancer. 13:2072013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katta A, Fesler MJ, Tan A, Vuong G and
Richart JM: Spontaneous bilateral pneumothorax in metastatic renal
cell carcinoma on sunitinib therapy. Cancer Chemother Pharmacol.
66:409–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang SH, Lin JK, Chen WS, Lin TC, Yang SH,
Jiang JK, Chang SC, Lan YT, Chao TC, Yen CC, et al: Pneumothorax
after bevacizumab-containing chemotherapy: A case report. Jpn J
Clin Oncol. 41:269–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Yang H, Zhao M and He X:
Bilateral pneumothorax after bevacizumab-containing chemotherapy in
fibrosarcoma. J Thorac Dis. 4:229–231. 2012.PubMed/NCBI
|
|
22
|
Makino T, Kudo S and Ogata T: Pneumothorax
after treatment with bevacizumab-containing chemotherapy for breast
cancer-a case report. Gan To Kagaku Ryoho. 41:233–235. 2014.(In
Japanese). PubMed/NCBI
|
|
23
|
Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang
G, Du F, Sun Y, Wu Q, Qu G, et al: Safety and efficacy of
anlotinib, a multikinase angiogenesis inhibitor, in patients with
refractory metastatic soft-tissue sarcoma. Clin Cancer Res.
24:5233–5238. 2018.PubMed/NCBI
|