Introduction
Chronic infection with hepatitis C virus (HCV) is a
well-recognized risk factor for liver cirrhosis and hepatocellular
carcinoma (HCC), which is one of the most common malignancies
worldwide (1,2). HCV infection is a leading cause of HCC;
the prevalence rate of HCV infection among HCC patients is around
70% in Japan (3,4). The recurrence risk of patients with HCC
is extremely high compared to that of other malignancies and this
is associated with poor survival (5,6).
Therefore, anti-virus therapy for the eradication of HCV is very
important to prevent the development and recurrence of HCC and
prolong the prognosis of patients with this malignancy (7-10).
A meta-analyses including 30 studies analyzing the association
between response to HCV interferon (IFN)-based therapy and
development of HCC demonstrated that eradication of HCV infection
dramatically reduced the risk of HCC (relative risk for all persons
0.24; 95% confidence interval [CI] 0.18 to 0.31) (10).
The recent introduction of IFN-free direct-acting
antivirals (DAAs) led to revolutionary progress in HCV treatment
because of its higher tolerability and sustained virological
response (SVR) rate than conventional IFN-based therapy (11). Therefore, IFN-based therapy has been
replaced by DAA therapy and the number of patients who achieved SVR
with this therapy is increasing steadily. Several studies suggest
that eradication of HCV by DAA therapy is associated with improved
liver function and quality of life as well as reduced risk of
decompensated liver disease (7,12,13).
Moreover, it has gradually been determined that the suppressive
effects of DAA therapy on HCC development are similar to those of
IFN-based therapy (8,9,14).
However, some conflicting data has described unexpected high rates
of HCC occurrence and recurrence after successful DAA treatment
(15-17).
Therefore, it is important to examine whether eradication of HCV by
DAA therapy reduces the risk of HCC.
The purpose of the present study was to evaluate the
suppressive effect of DAA therapy on the recurrence of HCV-related
HCC after curative treatment. The anti-liver tumorigenesis effects
in the DAA therapy group were compared not only to a non-antiviral
therapy group but also to an IFN-based therapy group.
Patients and methods
Patients, treatment and determination
of HCC recurrence
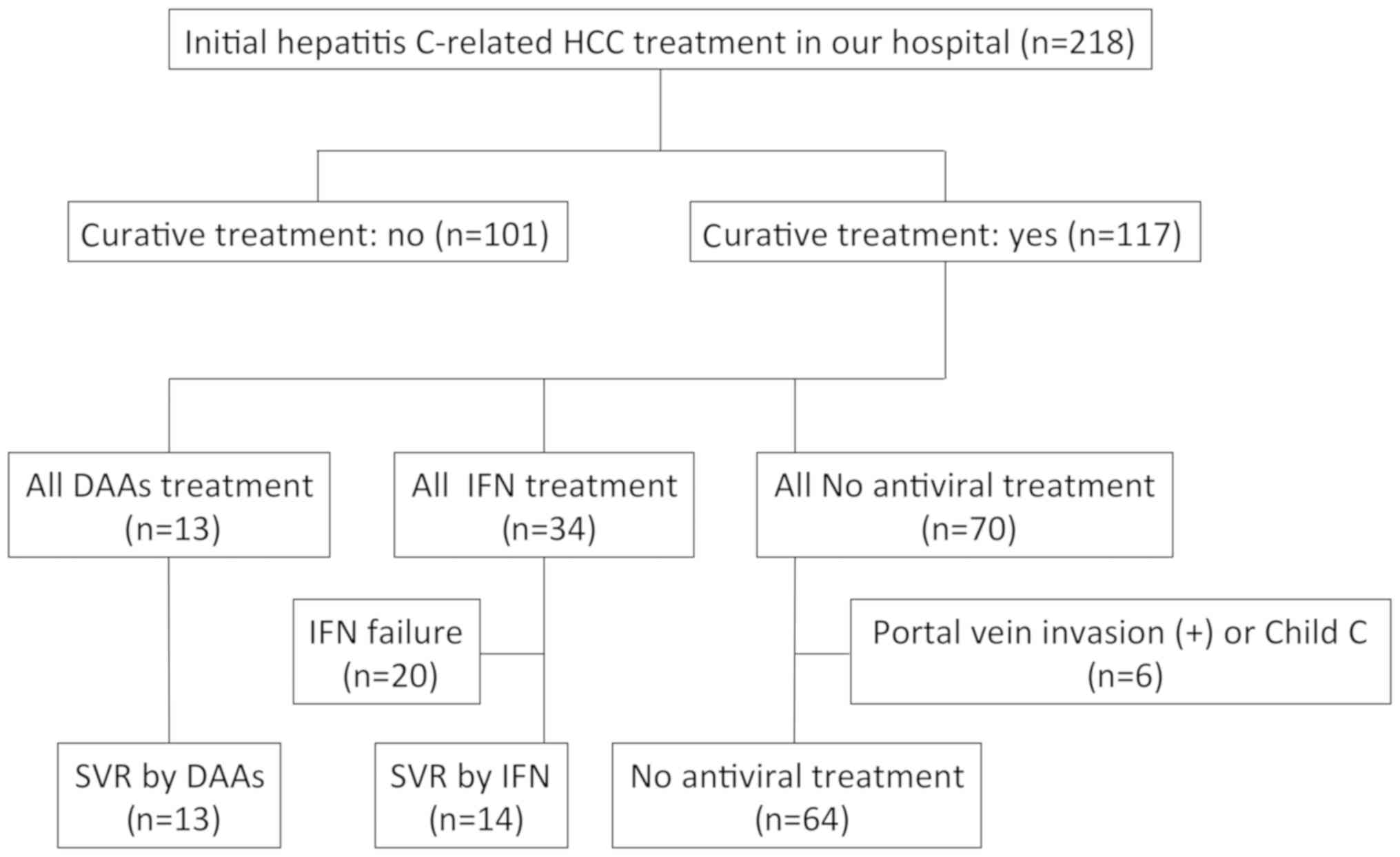
This retrospective study included 218 patients with
HCV-related initial HCC who were treated in Gifu University
Hospital (Gifu, Japan) between May 2006 and December 2017. Of these
patients, 117 patients who received curative treatment, including
surgical resection or radiofrequency ablation, were evaluated.
HCC nodules were detected using imaging modalities,
including dynamic computed tomography (CT), dynamic magnetic
resonance imaging (MRI), and abdominal arteriography. HCC was
diagnosed based on a typical hypervascular tumor stain on
angiography and typical dynamic study findings of enhanced staining
in the early phase and attenuation in the delayed phase. All
patients were followed on an outpatient basis and underwent dynamic
CT, MRI, or ultrasound scans every 3 months after the initial
treatment. Recurrent HCC was diagnosed when the typical findings of
HCC were observed in segments different from where the initial
lesions arose. Recurrence-free survival (RFS) time was defined as
the interval from the date of the initial treatment to the date of
the recurrence or December 2017 for recurrence-free survivors.
All study participants provided verbal informed
consent, which was considered sufficient as this study followed an
observational research design that did not require new human
specimens and instead relied only on preexisting samples. The study
design, including this consent procedure, was approved by the
Ethics Committee of the Gifu University School of Medicine.
Statistical analysis
Baseline characteristics among the groups were
compared using one-way analysis of variance for continuous
variables or the χ2 test for categorical variables.
Univariate analysis was performed using the Cox proportional
hazards model to identify the factors that affected RFS. RFS was
estimated using the Kaplan-Meier method, and differences between
curves were evaluated using the log-rank test. The Holm method was
used as a post hoc test to counteract the problem of multiple
comparisons (18). Statistical
significance was defined as P<0.05. All statistical analyses
were performed using R version 3.3.2 (The R Project for Statistical
Computing, Vienna, Austria; http://www.R-project.org/).
Results
Baseline characteristics of the
enrolled patients
Among the 117 patients who received curative
treatment for initial HCC, 13 patients received DAA therapy and all
of them achieved SVR (DAA group; asunaprevir/daclatasvir [n=6],
sofosbuvir/ledipasvir [n=4], and ombitasvir/paritaprevir/ritonavir
[n=3]). IFN-based therapy was administered to 34 patients and,
among them, 14 achieved SVR (IFN group; pegylated-IFN alone [n=7],
pegylated-IFN/ribavirin [n=5], and pegylated-IFN/ribavirin plus
simeprevir [n=2]). In order to examine whether HCV eradication by
DAA or IFN therapies can suppress the recurrence of HCC after
curative treatment, IFN failure cases (n=20) were excluded in the
present study. Antiviral therapies were started as soon as possible
after initial HCC was completely cured. Among 70 patients who had
not been treated with any antiviral therapy, 6 patients with portal
vein invasion or Child-Pugh class C were excluded because such
patients are known to have high risk for HCC recurrence and poor
prognosis, and the remaining 64 patients were set as the control
group (non-treatment group). No patient had such high risk
conditions in the DAA or IFN groups. The patient flow in this study
is shown in Fig. 1.
The baseline characteristics and laboratory data of
the DAA, IFN, and non-treatment groups are shown in Table I. When compared to the DAA (74.1
years; P=0.007) and non-treatment (73.2 years; P=0.002) groups, the
patients in the IFN group (65.5 years) were significantly younger.
The serum concentration of alanine aminotransferase (ALT) was
significantly lower both in the DAA (40.4 IU/L; P=0.003) and
non-treatment (44.7 IU/L; P<0.001) groups than in the IFN group
(79.5 IU/L). No significant differences were observed in
HCC-related factors, including tumor size, tumor numbers, portal
vein invasion, stage, and initial treatment among the three
groups.
 | Table IBaseline demographic and clinical
characteristics of the DAA, IFN and no treatment groups. |
Table I
Baseline demographic and clinical
characteristics of the DAA, IFN and no treatment groups.
| Variables | DAA (n=13) | IFN (n=14) | No treatment
(n=64) | P-value |
|---|
| Sex
(male/female) | 9/4 | 12/2 | 44/20 | 0.513 |
| Age (years) | 74.1±3.8 | 65.5±6.8 | 73.2±8.0 | 0.002a |
| BMI
(kg/m2) | 21.9±3.4 | 22.0±2.9 | 22.4±2.8 | 0.759 |
| Child-Pugh score
(5/6/7/8/9) | 7/4/1/1/0 | 8/4/2/0/0 | 33/17/7/6/1 | 0.985 |
| ALB (g/dl) | 3.6±0.5 | 3.7±0.4 | 3.5±0.5 | 0.437 |
| ALT (IU/l) | 40.4±22.3 | 79.5±49.8 | 44.7±26.9 | 0.001b |
| T-Bil (mg/dl) | 0.9±0.4 | 0.9±0.3 | 1.1±0.6 | 0.513 |
| PLT
(x104/µl) | 13.5±6.0 | 12.0±6.0 | 11.2±4.7 | 0.313 |
| PT (%) | 83.3±17.0 | 84.5±11.2 | 85.7±14.9 | 0.856 |
| AFP (ng/dl) | 500±913 | 202±670 | 174±617 | 0.363 |
| PIVKA-II
(mAU/ml) | 469±1256 | 2,170±7,952 | 816±2648 | 0.428 |
| Tumor size (cm) | 2.0±1.1 | 2.0±1.2 | 2.4±1.4 | 0.541 |
| Tumor number
(1/≥2) | 11/2 | 11/3 | 49/15 | 0.947 |
| Stage (I/II/III) | 7/6/0 | 6/7/1 | 26/31/7 | 0.873 |
| Initial treatment
(resection/RFA) | 5/8 | 6/8 | 17/47 | 0.590 |
Comparison of RFS among the DAA, IFN
and non-treatment groups
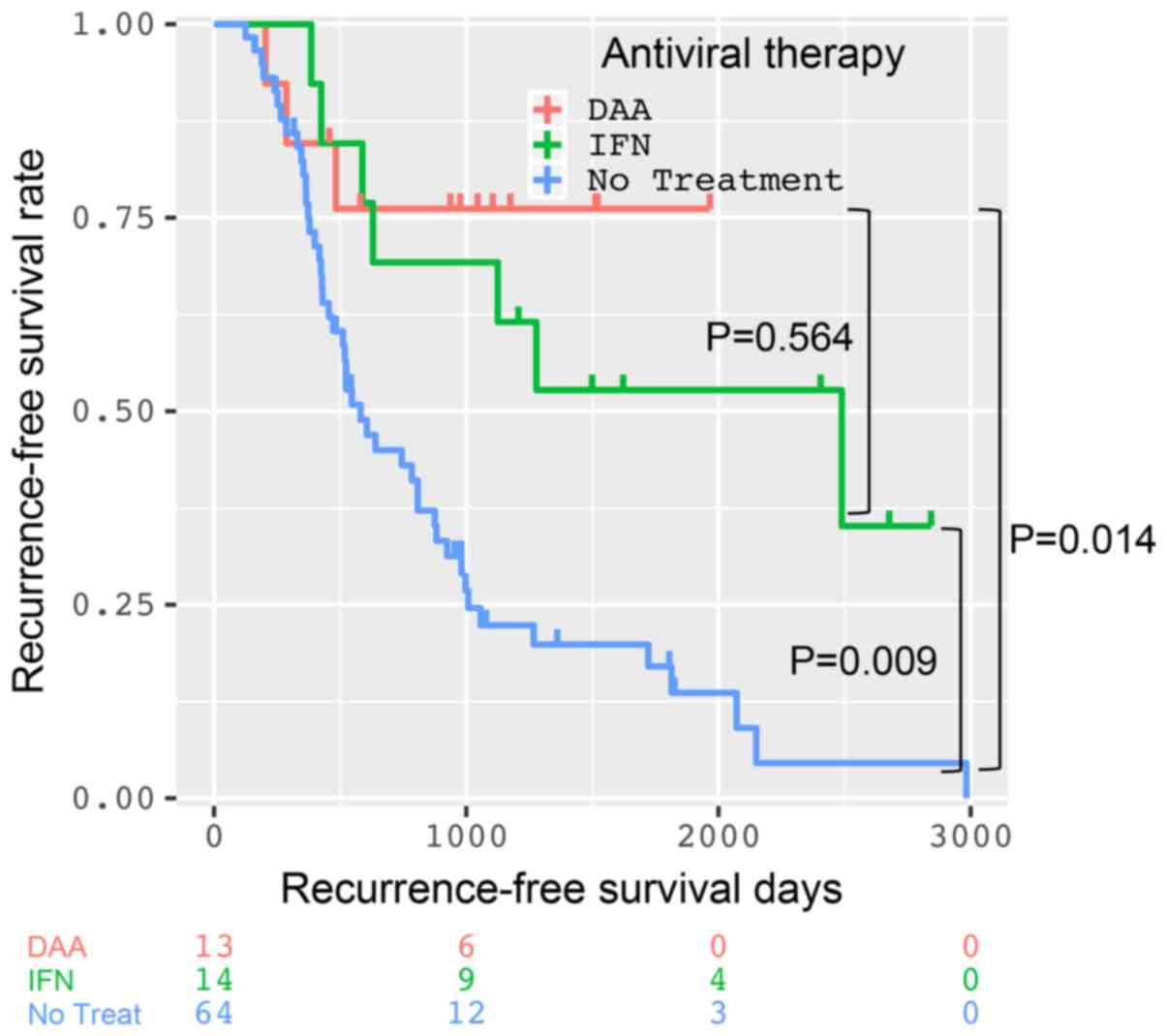
The 1-, 2-, and 3-year RFS rates of the DAA, IFN,
and non-treatment groups were 84.6, 76.2 and 76.2%; 100, 69.2 and
69.2%; and 76.8, 45.0 and 22.4%, respectively (Fig. 2). The shortest intervals for RFS of
the DAA, IFN, and non-treatment groups were 204, 245 and 125 days,
respectively. In comparison to the non-treatment group, RFS was
significantly improved in the DAA (P=0.014) and IFN groups
(P=0.009). However, there was no significant difference in RFS
between the DAA and IFN groups (P=0.564).
Possible risk factors for HCC
recurrence
Table II shows the
possible risk factors for HCC recurrence by Cox proportional
hazards model, and IFN therapy (HR 0.327, 95% CI 0.145-0.742,
P=0.07) and DAA therapy (HR 0.222, 95% CI 0.069-0.758, P=0.011)
were independent factors, which could reduce the risk of HCC
recurrence.
 | Table IIAnalysis of possible risk factors for
HCC recurrence using a Cox proportional hazards model. |
Table II
Analysis of possible risk factors for
HCC recurrence using a Cox proportional hazards model.
| | Univariate
analysis |
|---|
| Variables | HR (95% CI) | P-value |
|---|
| Sex (male vs.
female) | 1.084
(0.612-1.920) | 0.782 |
| Age (years) | 0.986
(0.948-1.025) | 0.475 |
| BMI
(kg/m2) | 1.002
(0.914-1.099) | 0.960 |
| Child-Pugh grade (A
vs. B) | 0.970
(0.488-1.927) | 0.931 |
| ALB (g/dl) | 0.769
(0.436-1.357) | 0.364 |
| ALT (IU/l) | 0.995
(0.987-1.004) | 0.265 |
| T-Bil (mg/dl) | 1.398
(0.759-2.578) | 0.283 |
| PLT
(x104/µl) | 0.962
(0.908-1.019) | 0.184 |
| PT (%) | 0.999
(0.980-1.019) | 0.925 |
| AFP (ng/dl) | 0.999
(0.999-1.000) | 0.424 |
| Tumor size
(cm) | 1.124
(0.882-1.431) | 0.345 |
| Tumor number (1 vs.
≥2) | 1.111
(0.663-1.861) | 0.689 |
| Cancer stage | 1.016
(0.652-1.584) | 0.944 |
| Initial treatment
(RFA vs. resection) | 1.286
(0.711-2.33) | 0.406 |
| Antiviral
therapy |
|
IFN vs. no
treatment | 0.327
(0.145-0.742) | 0.007 |
|
DAA vs.
IFN | 1.470
(0.373-5.788) | 0.581 |
|
DAA vs. no
treatment | 0.222
(0.069-0.758) | 0.011 |
Discussion
The results of the present study revealed that the
patients in whom HCV infection was successfully eradicated by DAAs
after curative treatment of initial HCC had a similar recurrence
rate as those who underwent IFN-based therapy. Furthermore,
compared with the non-treatment group, successful treatment with
DAAs reduced the risk of HCC recurrence after curative treatment by
about one-fifth, which was almost the same as that reported
previously for IFN-based therapy (10). These results strongly encourage us to
introduce DAA treatment among patients treated with curative
treatment for primary HCV-related HCC.
Recently, DAAs have become the dominant therapy for
HCV infection in place of conventional IFN-based therapy because of
its higher tolerability and SVR rate (11). In fact, all the patients in the DAA
group did not develop serious complications during DAA treatment
and achieved SVR. On the other hand, it is reported that patients
with HCC had significantly higher DAA failure (19). The presence of active HCC was the
primary predictor of DAA failure even after controlling for other
variables (19). Therefore, we did
not introduce DAA treatment to HCV positive patients with active
HCC and this might lead to the achievement of extremely high SVR
rate in this study. In order to achieve SVR, it is important to
confirm that there is no active HCC before introducing DAA
treatment.
Importantly, it is also expected that treatment with
DAAs may exert an anti-liver tumorigenesis effect, which was proven
by IFN-based therapy in patients with cirrhosis (10). Several studies have revealed the
association between successful DAA treatment and the reduction of
the risk of HCC (8,9,14). HCV
eradication can improve hepatic inflammation and fibrosis, both of
which are associated with the progression of precancerous lesions
into malignant cell clones (20).
Therefore, HCV eradication from the liver exerts a protective
effect against HCC development, regardless of the type of antiviral
treatment. Importantly, not the type of antiviral treatment (IFN or
DAA) but treatment response (SVR or non-SVR) was the only
independent predictive factor of HCC recurrence after curative
treatment (21).
On the other hand, some studies have indicated that
DAAs could instead promote the development of HCC (15-17).
To explain these contrasting results, the following hypotheses can
be considered: DAAs cause a deregulation of the immune system
through an abrupt reduction in the HCV load, leaving immune cells
less active against tumor cells already present at the beginning of
antiviral treatment (22,23). Moreover, it can persist even after
successful DAA treatment, while exogenous IFN used as part of HCV
treatment plays a protective role in HCC by switching on
inflammatory cells with pro-apoptotic and anti-tumoral activities
(24,25).
However, it should be recognized that patients
treated with DAAs have a significantly higher risk of HCC
occurrence or recurrence than those with IFN-based therapy because
of their background factors. Namely, DAA-treated patients have a
significantly higher rate of known risk factors for HCC, including
cirrhosis, older age, and a higher baseline alpha-fetoprotein (AFP)
level (14). Age and serum AFP
levels were higher and liver fibrosis was more advanced in patients
who achieved SVR with DAA therapy than those in patients who
achieved SVR with IFN-based therapy (26). The higher prevalence of underlying
HCC risks among DAA-treated individuals could be because patients
treated with earlier DAA regimens had previously failed treatment
with IFN-based therapy (14).
Indeed, patients were significantly older in the DAA group in the
present study. Furthermore, two patients had recurrent HCC within a
year in the DAA group, while none did in the IFN group.
Another emphasized point of the present study is
that even though HCV could be eradicated successfully by either
DAA- or IFN-based therapy, the risk of recurrence almost never
changes within a year. Because DAAs might not be able to inactivate
tumor cells already present at the beginning of antiviral
treatment, close follow-up for surveying HCC development is
required. All of the guidelines from the American Associated for
the Study of Liver Diseases, the European Association for the Study
of the Liver, and the Japan Society of Hepatology recommended
surveillance every 3-4 months using image examination with or
without tumor maker measurements after curative treatment of HCC
(27-29).
The appropriate surveillance interval also should be determined on
the basis of risk factors of HCC recurrence such as male sex, older
age, presence of cirrhosis, alcohol consumption, higher AFP levels,
and advanced stage of primary HCC (1,27-33).
The main limitation of our study is the small sample
size and relatively short follow-up time. Our study is also limited
by its study design as a retrospective-cohort study. Further
prospective multicenter studies with larger sample sizes and longer
follow-up periods are warranted to further verify the anti-liver
tumorigenesis effect of DAAs. However, although there are such
limitations, the result of the present study indicating the
anti-liver tumorigenesis effect of DAAs is extremely important
because there is a concern that DAAs might promote HCC (15-17).
In conclusion, SVR by DAAs showed a suppressive
effect on HCV-related HCC recurrence after curative treatment,
which was almost equal to that obtained by IFN-based therapy.
However, the risk of HCC recurrence almost never changes within a
year and, therefore, strict surveillance should be performed at
least within a year after curative treatment for primary HCC even
if successful DAA treatment is achieved.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KI, KT, TH, AS, MShir and MShim designed the study.
KI analyzed the data and drafted the manuscript. KT supervised the
treatment of the participants. KT, TH, AS and MShir contributed to
the selection of the participants and collected the data. KT, TH,
AS and MShir revised the manuscript, and MShim mainly reviewed and
amended the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All study participants provided verbal informed
consent, which was considered sufficient as the present study
followed an observational research design that did not require new
human specimens and instead relied only on preexisting samples. The
authors declare that publication of clinical datasets does not
compromise anonymity or confidentiality or breach local data
protection laws, for the dataset to be considered for publication.
The study design, including this consent procedure, was approved by
the Ethics Committee of the Gifu University School of Medicine
(Gifu, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Serag HB: Hepatocellular carcinoma: An
epidemiologic view. J Clin Gastroenterol. 35 (5 Suppl 2):S72–S78.
2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Perz JF, Armstrong GL, Farrington LA,
Hutin YJ and Bell BP: The contributions of hepatitis B virus and
hepatitis C virus infections to cirrhosis and primary liver cancer
worldwide. J Hepatol. 45:529–538. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang BE, Ma WM, Sulaiman A, Noer S,
Sumoharjo S, Sumarsidi D, Tandon BN, Nakao K, Mishiro S, Miyakawa
Y, et al: Demographic, clinical, and virological characteristics of
hepatocellular carcinoma in Asia: Survey of 414 patients from four
countries. J Med Virol. 67:394–400. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Poon RT: Prevention of recurrence after
resection of hepatocellular carcinoma: A daunting challenge.
Hepatology. 54:757–759. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shiina S, Tateishi R, Arano T, Uchino K,
Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, et al:
Radiofrequency ablation for hepatocellular carcinoma: 10-year
outcome and prognostic factors. Am J Gastroenterol. 107:569–577;
quiz 578. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Foster GR, Irving WL, Cheung MC, Walker
AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson
WT, et al: Impact of direct acting antiviral therapy in patients
with chronic hepatitis C and decompensated cirrhosis. J Hepatol.
64:1224–1231. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ikeda K, Kawamura Y, Kobayashi M, Kominami
Y, Fujiyama S, Sezaki H, Hosaka T, Akuta N, Saitoh S, Suzuki F, et
al: Direct-acting antivirals decreased tumor recurrence after
initial treatment of hepatitis C virus-related hepatocellular
carcinoma. Dig Dis Sci. 62:2932–2942. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kobayashi M, Suzuki F, Fujiyama S,
Kawamura Y, Sezaki H, Hosaka T, Akuta N, Suzuki Y, Saitoh S, Arase
Y, et al: Sustained virologic response by direct antiviral agents
reduces the incidence of hepatocellular carcinoma in patients with
HCV infection. J Med Virol. 89:476–483. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Morgan RL, Baack B, Smith BD, Yartel A,
Pitasi M and Falck-Ytter Y: Eradication of hepatitis C virus
infection and the development of hepatocellular carcinoma: A
meta-analysis of observational studies. Ann Intern Med.
158:329–337. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zoulim F, Liang TJ, Gerbes AL, Aghemo A,
Deuffic-Burban S, Dusheiko G, Fried MW, Pol S, Rockstroh JK,
Terrault NA and Wiktor S: Hepatitis C virus treatment in the real
world: Optimising treatment and access to therapies. Gut.
64:1824–1833. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Younossi ZM, Stepanova M, Afdhal N,
Kowdley KV, Zeuzem S, Henry L, Hunt SL and Marcellin P: Improvement
of health-related quality of life and work productivity in chronic
hepatitis C patients with early and advanced fibrosis treated with
ledipasvir and sofosbuvir. J Hepatol. 63:337–345. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Deterding K, Höner Zu Siederdissen C, Port
K, Solbach P, Sollik L, Kirschner J, Mix C, Cornberg J, Worzala D,
Mix H, et al: Improvement of liver function parameters in advanced
HCV-associated liver cirrhosis by IFN-free antiviral therapies.
Aliment Pharmacol Ther. 42:889–901. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li DK, Ren Y, Fierer DS, Rutledge S,
Shaikh OS, Lo Re V III, Simon T, Abou-Samra AB, Chung RT and Butt
AA: The short-term incidence of hepatocellular carcinoma is not
increased after hepatitis C treatment with direct-acting
antivirals: An ERCHIVES study. Hepatology. 67:2244–2253.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Reig M, Mariño Z, Perelló C, Iñarrairaegui
M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, et al:
Unexpected high rate of early tumor recurrence in patients with
HCV-related HCC undergoing interferon-free therapy. J Hepatol.
65:719–726. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Conti F, Buonfiglioli F, Scuteri A, Crespi
C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi
G, et al: Early occurrence and recurrence of hepatocellular
carcinoma in HCV-related cirrhosis treated with direct-acting
antivirals. J Hepatol. 65:727–733. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cardoso H, Vale AM, Rodrigues S, Gonçalves
R, Albuquerque A, Pereira P, Lopes S, Silva M, Andrade P, Morais R,
et al: High incidence of hepatocellular carcinoma following
successful interferon-free antiviral therapy for hepatitis C
associated cirrhosis. J Hepatol. 65:1070–1071. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aickin M and Gensler H: Adjusting for
multiple testing when reporting research results: The Bonferroni
vs. Holm methods. Am J Public Health. 86:726–728. 1996.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Prenner SB, VanWagner LB, Flamm SL, Salem
R, Lewandowski RJ and Kulik L: Hepatocellular carcinoma decreases
the chance of successful hepatitis C virus therapy with
direct-acting antivirals. J Hepatol. 66:1173–1181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tampaki M, Savvanis S and Koskinas J:
Impact of direct-acting antiviral agents on the development of
hepatocellular carcinoma: Evidence and pathophysiological issues.
Ann Gastroenterol. 31:670–679. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nagaoki Y, Imamura M, Nishida Y, Daijo K,
Teraoka Y, Honda F, Nakamura Y, Morio K, Fujino H, Nakahara T, et
al: The impact of interferon-free direct-acting antivirals on
clinical outcome after curative treatment for hepatitis C
virus-associated hepatocellular carcinoma: Comparison with
interferon-based therapy. J Med Virol. 91:650–658. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Innes H, McDonald S, Hayes P, Dillon JF,
Allen S, Goldberg D, Mills PR, Barclay ST, Wilks D, Valerio H, et
al: Mortality in hepatitis C patients who achieve a sustained viral
response compared to the general population. J Hepatol. 66:19–27.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wirth TC and Manns MP: The impact of the
revolution in hepatitis C treatment on hepatocellular carcinoma.
Ann Oncol. 27:1467–1474. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hengst J, Strunz B, Deterding K, Ljunggren
HG, Leeansyah E, Manns MP, Cornberg M, Sandberg JK, Wedemeyer H and
Björkström NK: Nonreversible MAIT cell-dysfunction in chronic
hepatitis C virus infection despite successful interferon-free
therapy. Eur J Immunol. 46:2204–2210. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Goossens N and Hoshida Y: Hepatitis C
virus-induced hepatocellular carcinoma. Clin Mol Hepatol.
21:105–114. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Toyoda H, Tada T, Takaguchi K, Senoh T,
Shimada N, Hiraoka A, Michitaka K, Ishikawa T and Kumada T:
Differences in background characteristics of patients with chronic
hepatitis C who achieved sustained virologic response with
interferon-free versus interferon-based therapy and the risk of
developing hepatocellular carcinoma after eradication of hepatitis
C virus in Japan. J Viral Hepat. 24:472–476. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kokudo N, Hasegawa K, Akahane M, Igaki H,
Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, et al:
Evidence-based clinical practice guidelines for hepatocellular
carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC
Guidelines). Hepatol Res. 45:2015 doi: 10.1111/hepr.12464.
PubMed/NCBI View Article : Google Scholar
|
|
28
|
European Association For The Study Of The
Liver; European Organisation For Research And Treatment Of Cancer:
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol 56: 908-943, 2012.
|
|
29
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Watanabe N, Takai K, Imai K, Shimizu M,
Naiki T, Nagaki M and Moriwaki H: Increased levels of serum leptin
are a risk factor for the recurrence of stage I/II hepatocellular
carcinoma after curative treatment. J Clin Biochem Nutr.
49:153–158. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Imai K, Takai K, Nishigaki Y, Shimizu S,
Naiki T, Hayashi H, Uematsu T, Sugihara J, Tomita E, Shimizu M, et
al: Insulin resistance raises the risk for recurrence of stage I
hepatocellular carcinoma after curative radiofrequency ablation in
hepatitis C virus-positive patients: A prospective, case series
study. Hepatol Res. 40:376–382. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Koike Y, Shiratori Y, Sato S, Obi S,
Teratani T, Imamura M, Hamamura K, Imai Y, Yoshida H, Shiina S and
Omata M: Risk factors for recurring hepatocellular carcinoma differ
according to infected hepatitis virus-an analysis of 236
consecutive patients with a single lesion. Hepatology.
32:1216–1223. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nagashima I, Hamada C, Naruse K, Osada T,
Nagao T, Kawano N and Muto T: Surgical resection for small
hepatocellular carcinoma. Surgery. 119:40–45. 1996.PubMed/NCBI View Article : Google Scholar
|