Introduction
According to the World Health Organization, the
number of newly diagnosed colorectal cancer (CRC) cases will
increase by 77% and the number of deaths by 80% by 2030 (1,2). The
expected increase in CRC will mainly occur in less developed
countries,due to the development of a lifestyle closed to Western
countries (3-5).
The neoplastic transformation time of CRC is ~10-15
years (6-9),
and the 5 year survival following treatment 50-60% (10,11).
Reliable diagnostic, prognostic and predictive biomarkers are
required (12). At present, the most
accepted tests for CRC are fecal occult blood test, colonoscopy and
sigmoidoscopy (13). Tumor node
metastasis (TNM) staging and carcinoembryonic antigen level are
also used. However, most of these tests have been reported to be
too insensitive for accurate individual prognosis (14,15).
Tumor biomarkers with a high sensitivity and specificity are
required. In this meta-analysis, the use of serum thymidine kinase
1 (STK1p) concentration for prognosis and treatment monitoring in
CRC patients is discussed.
STK1p has been proven useful for predicting
recurrence and survival in many types of human cancer (16,17). TK1
is a kinase enzyme that converts deoxythymidine to deoxythymidine
monophosphate and is involved in the synthesis of DNA, and thus
related to cell growth rate (proliferation). The new-generation
STK1p concentration assay shows an area under the curve (AUC) value
of 0.96(18), and is a more reliable
assay than the serum TK activity (19,20) and
serum TK1 sandwich ELISA (21)
assays. Low STK1p values are associated with a better prognosis
(16,18,22-27).
The STK1p marker is an independent prognostic factor for survival
in CRC patients (n=504) (28). STK1p
can distinguish malignant patients from benign tumor patients and
healthy individuals, as well as predict the prognosis of survival
and relapse. STK1p can also monitor the effect of the treatment,
and is a useful biomarker for predicting the development of
malignancies and discovering early-stage tumors (18,26,28).
A number of studies have focused on the use of STK1p
in colorectal benign and malignant tumor patients, but most of them
included a limited number of cases, thus reducing the reliability
of the conclusions. Therefore, 20 colorectal studies were collected
for the present meta-analysis, in order to obtain a sufficient
number of cases. The results showed that STK1p values are
significantly higher in CRC patients, as compared to heathy
individuals or patients with benign tumors. STK1p was also used to
monitor the effect of the surgery.
Materials and methods
Literature search
A systematic literature search was conducted through
the PubMed, Embase, CENTRAL, CNKI, Wanfang, VIP and SinoMed
databases until August 31, 2019, using the following strategy
keywords: (‘thymidine kinase 1’ or ‘TK1’) and (‘colorectal’ or
‘colon’ or ‘rectal’ or ‘colorectum’ or ‘rectum’) and (‘cancer’ or
‘tumor’ or ‘carcinoma’ or ‘malignancy’). A more detailed
description of the search strategy used is described in the
beginning of the Results section. The literature search was
restricted to human studies. There were no language
restrictions.
The meta-analysis followed the PRISMA guidelines.
The quality of the meta-analysis as a study was investigated using
the Newcastle-Ottawa Scale Document Quality Assessment Scale (NOS).
Only studies using the STK1p assay developed by us, tested in many
clinical studies and statistically proved to be reliable were
selected (summary in refs. 27,28), in
order to guarantee the high quality of the STK1p results. Since no
specific review protocol suitable for our meta-analysis was found,
our own protocol was used.
All analyses were based on previously published
studies, and therefore no ethical approval and patient consent were
required.
Inclusion and exclusion criteria
Inclusion criteria
i) STK1p as an endpoint; ii) STK1p was measured by
the kit developed by SSTK Ltd; iii) patients with adenomatous
polyps were identified by clinical endoscopy and pathological
diagnosis. Patients were identified by pathological diagnosis,
classified as stage I-III and grade 1-3, and confirmed to have no
residual tumor following surgery; iv) healthy individuals were used
as the control group. Healthy people were defined as disease-free
checked by different imaging, blood tests and other pathological
methods.
Exclusion criteria
i) Insufficient data; ii) TK1 immunohistochemistry
and TK1 activity; iii) Invalid research data, which included
physiological stress responses such as immunological reaction,
inflammation and activation of metabolic adenosine mediators.
Oxidative stress was considered a surgical stress response,
together with myocardial injury, sepsis, pulmonary edema, and
kidney and liver failure, which could increase mortality; iv)
Healthy people were excluded when containing diseases associated
with tumors proliferation, such as precancerous (moderate/severe
types of hyperplasia of breast, prostate, gastrointestinal, cervix,
liver cirrhosis, refractory anemia). Also excluded were people with
risk-diseases associated with tumors progression such as liver
disease, moderate/severe fatty liver, high risk for hepatitis B,
abnormal liver function, obesity and benign tumors (such as renal,
thyroid); and any of the following conditions: severe cardiac
disease; using any medication that could affect the STK1 levels
such as exogenous hormone therapy; pregnancy; or acute illness such
as inflammation/virus infection within 4 weeks.
Literature screening and data
extraction Primary screening
The title and abstract of literatures were carefully
reviewed, and 10% of the excluded papers were randomly selected to
check the concordance rate.
Secondary screening
After checking the abstracts, the full text of the
papers was re-evaluated, and it was decided whether these papers
should be included to the study or not, according to the criteria.
Authors 1, 2 and 3 screened papers independently and discussed to
reach an agreement; when met with a disagreement, the papers were
rechecked by authors 5 and 6.
The following data were extracted from each study:
First author's name, publication year, title of publication,
published journal, study population, number of samples, design type
and results.
Statistical analysis
RevMan 5.1 statistical software provided by Networks
of Cochrane Review Groups and Stata 12.0 data analysis and
statistical software were used (29). A heterogeneity test was performed at
the beginning, and depending on the results, a fixed or random
effects model was used. A fixed effects model with an I2
of <50% or random effects model with an I2 of >50%
was used to calculate the weighted mean difference and 95%
confidence interval. In addition, Funnel plot and Egger's linear
regression test was used to assess literature bias. For the
comparison of STK1p concentration among the different groups of
controls and patients, one-way analysis of variance followed by a
post hoc least significant difference test was performed. SPSS
version 19 was utilized for statistical analysis (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Literature search and study
characteristics
The healthy control group included individuals with
no evidence of tumors. Data from patients with colorectal
neoplastic (adenomatous) polyps were used in the meta-analysis.
Adenomatous polyps are benign tumors that originate from the
mucus-secreting colonic epithelial cells (9). Tumors from CRC patients pathologically
identified as clinical I-III degree and grade G1-G3 were defined as
malignant. We had no information on whether other types of tumors
besides CRC were identified in the benign or malignant tumor
groups.
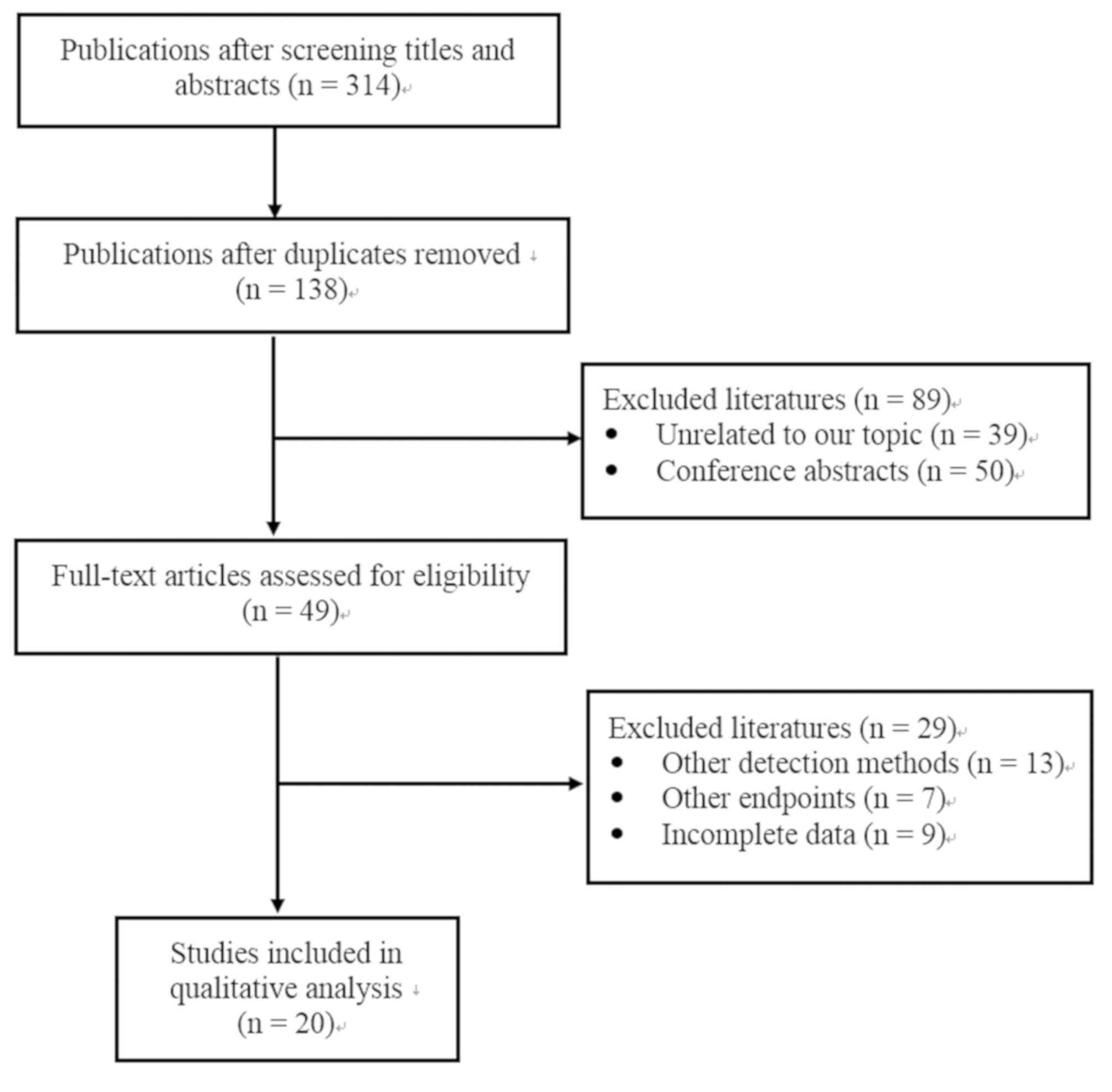
As shown in Fig. 1, a
total of 314 publications were initially identified through a
search of the Pubmed, Embase, CENTRAL, CNKI, Wanfang, VIP and
SinoMed databases. A total 176 publications were removed due to
duplications and 138 were kept. Next, 89 publications were excluded
from the remaining articles following a screening of the titles and
abstracts, due to being conference abstracts or focusing on
unrelated topics. Therefore, 49 full-text articles were assessed
for eligibility. Among them, 13 were excluded, since another STK1
method was used instead of the SSTK dot blot ECL assay, 7 because
they used different endpoints, and 9 due to incomplete data.
Finally, 20 studies were included in this meta-analysis (30-49).
The number of manuscripts, patients involved and the
STK1p mean values in the control, benign and malignant tumor groups
are shown in Table I. Detailed
information on the number of cases, age distribution, type of CRC,
clinical stages grades and just before start of the surgery and one
month after surgery reported in the individual publications are
presented in Table II.
 | Table INo of publications in the group of
controls, benign and malignant persons, no of samples and STK1p
mean values in the various groups included in the present
study. |
Table I
No of publications in the group of
controls, benign and malignant persons, no of samples and STK1p
mean values in the various groups included in the present
study.
| Variables | Control | Benign | Malignant |
|---|
| Publications
(n) | 19 | 10 | 20 |
| Samples (n) | 1,701 | 774 | 1,836 |
| STK1p (mean ±
SD) | 0.88±0.50 | 1.30±0.84 | 3.14±2.55 |
| P-value |
0.00078a |
<0.0001b |
<0.0001c |
 | Table IISummary of the clinical data included
in each selected publication. |
Table II
Summary of the clinical data included
in each selected publication.
| | | | | | | | | Clinical stage
(n) | Pathological
grading (n) | CRC type | Surgery |
|---|
| Author/(Ref),
year | Location | Control (n) | Benign (n) | CRC (n) | M (n) | F (n) | Age | I | II | III | Low diff. | Medium/high
diff. | Colon | Rectum | Before | After |
|---|
| An et al
(36), 2016 | Southwest | 33 | | 45 | 45 | | 31-76 | | | 45 | | | 24 | 21 | 13 | 13 |
| Huo et al
(41), 2015 | East China | 32 | 35 | 76 | 51 | 25 | 36-83 | | | 76 | | | 76 | | | |
| Li (46), 2012 | Southwest | 120 | 100 | 120 | 78 | 42 | 31-80 | | | 120 | | | 60 | 60 | 120 | 120 |
| Li and Wang
(49), 2009 | East China | 48 | 45 | 108 | 79 | 29 | 31-78 | | | 108 | | | | 108 | | |
| Liu et al
(40), 2015 | South China | 600 | 137 | 65 | 65 | | 22-67 | | | 65 | | | 65 | | | |
| Lu et al
(44), 2014 | South China | 40 | 61 | 77 | 43 | 34 | 38-88 | | | 77 | | | 38 | 39 | | |
| Shen et al
(47), 2011 | East China | 60 | 50 | 43 | 28 | 15 | 30-79 | | | 43 | | | 21 | 22 | 43 | 43 |
| Tian et al
(48), 2010 | East China | 33 | | 66 | 45 | 21 | 25-72 | | | 66 | | | 66 | | 66 | 66 |
| Xia et al
(39), 2015 | East China | 41 | | 61 | 33 | 28 | 23-85 | | | 61 | 43 | 18 | 61 | | 16 | 16 |
| Qi et al
(45), 2013 | East China | 45 | | 104 | 104 | | 32-81 | | | 104 | | | 52 | 52 | | |
| Zeng and Zhang
(38), 2015 | East China | | 103 | 133 | 77 | 56 | 32-84 | | | 133 | | | 133 | | | |
| Zhang et al
(42), 2015 | Huazhong | 40 | 36 | 150 | 88 | 62 | 30-78 | | | 150 | | | 150 | | 150 | 150 |
| Zhang et al
(43), 2014 | East China | 161 | | 64 | 64 | | 35-84 | | | 64 | | | 35 | 29 | | |
| Zhu et al
(35), 2017 | Huazhong | 52 | 162 | 82 | 49 | 33 | 36-68 | 17 | | 65 | 34 | 48 | | 82 | | |
| Zhu et al
(37), 2015 | East China | 67 | | 33 | 33 | | 16-85 | 7 | 8 | 18 | | | | 33 | 33 | 33 |
| Fan et al
(30), 2019 | Northwest | 60 | 45 | 50 | 23 | 27 | 60-70 | | | 50 | 31 | 19 | 50 | | | |
| Jiang et al
(32), 2018 | East China | 70 | | 71 | 50 | 21 | 32-82 | 9 | 26 | 36 | 18 | 53 | 22 | 49 | | |
| Weng (31), 2018 | South China | 64 | | 64 | 36 | 28 | 42-73 | 15 | 29 | 20 | | | | 64 | | |
| Sun et al
(33), 2018 | Northeast | 60 | | 80 | 50 | 30 | 24-80 | | | 80 | 54 | 26 | 80 | | | |
| Ning et al
(34), 2018 | South China | 75 | | 344 | 344 | | | 132 | | 212 | | | 177 | 167 | | |
| Total number | | 1,701 | 774 | 1,836 | 1,385 | 451 | | 180 | 63 | 1,593 | 180 | 164 | 1,110 | 726 | 441 | 441 |
| Age
distribution | | 16-85 | 35-84 | | | | 23-85 | | | | | | | | 30-85 | 30-85 |
A total of 1,836 CRC patients were included in the
20 publications, including 774 benign tumor patients and 1,701
healthy individuals. The majority of CRC patients were male with an
age-distribution of 16-85 years and colon cancer clinical stage of
III and grade low/medium/high. The expression of STK1p increased
significantly in the following manner: Controls < benign <
malign (P<0.001). The quality of the meta-analysis as a study
was checked by the NOS and was found to be high, with 19
publications meeting 8 of the requirements of NOS, and 1
publication meeting 6. The results are presented in Table III.
 | Table IIILiterature quality evaluation by
Newcastle-Ottawa Scale Document Quality Assessment Scale (NOS). |
Table III
Literature quality evaluation by
Newcastle-Ottawa Scale Document Quality Assessment Scale (NOS).
| Author/(Ref),
year | Is the definition
adequate? | Representativeness
of cases | Section selection
of controls | Definition of
controls | Comparability of
cases and controls on the basis of design and analysis | Ascertainment of
exposure | Exposure same
method of ascertainment for cases and controls | Non-response
rate |
|---|
| An et al
(36), 2016 | * | * | * | * | ** | * | * | * |
| Huo et al
(41), 2015 | * | * | * | * | ** | * | * | * |
| Li (46), 2012 | * | * | * | * | ** | * | * | * |
| Li and Wang
(49), 2009 | * | * | * | * | ** | * | * | * |
| Liu et al
(40), 2015 | * | * | * | * | ** | * | * | * |
| Lu et al
(44), 2014 | * | * | * | * | ** | * | * | * |
| Shen et al
(47), 2011 | * | * | * | * | ** | * | * | * |
| Tian et al
(48), 2010 | * | * | * | * | ** | * | * | * |
| Xia et al
(39), 2015 | * | * | * | * | ** | * | * | * |
| Zeng and Zhang
(38), 2015 | * | * | | | ** | * | * | * |
| Zhang et al
(42), 2015 | * | * | * | * | ** | * | * | * |
| Zhang et al
(43), 2014 | * | * | * | * | * | * | * | * |
| Zhu et al
(35), 2017 | * | * | * | * | ** | * | * | * |
| Zhu et al
(37), 2015 | * | * | * | * | ** | * | * | * |
| Qi et al
(45), 2013 | * | * | * | * | ** | * | * | * |
| Fan et al
(30), 2019 | * | * | * | * | ** | * | * | * |
| Jiang et al
(32), 2018 | * | * | * | * | ** | * | * | * |
| Weng (31), 2018 | * | * | * | * | ** | * | * | * |
| Sun et al
(33), 2018 | * | * | * | * | ** | * | * | * |
| Ning et al
(34), 2018 | * | * | * | * | ** | * | * | * |
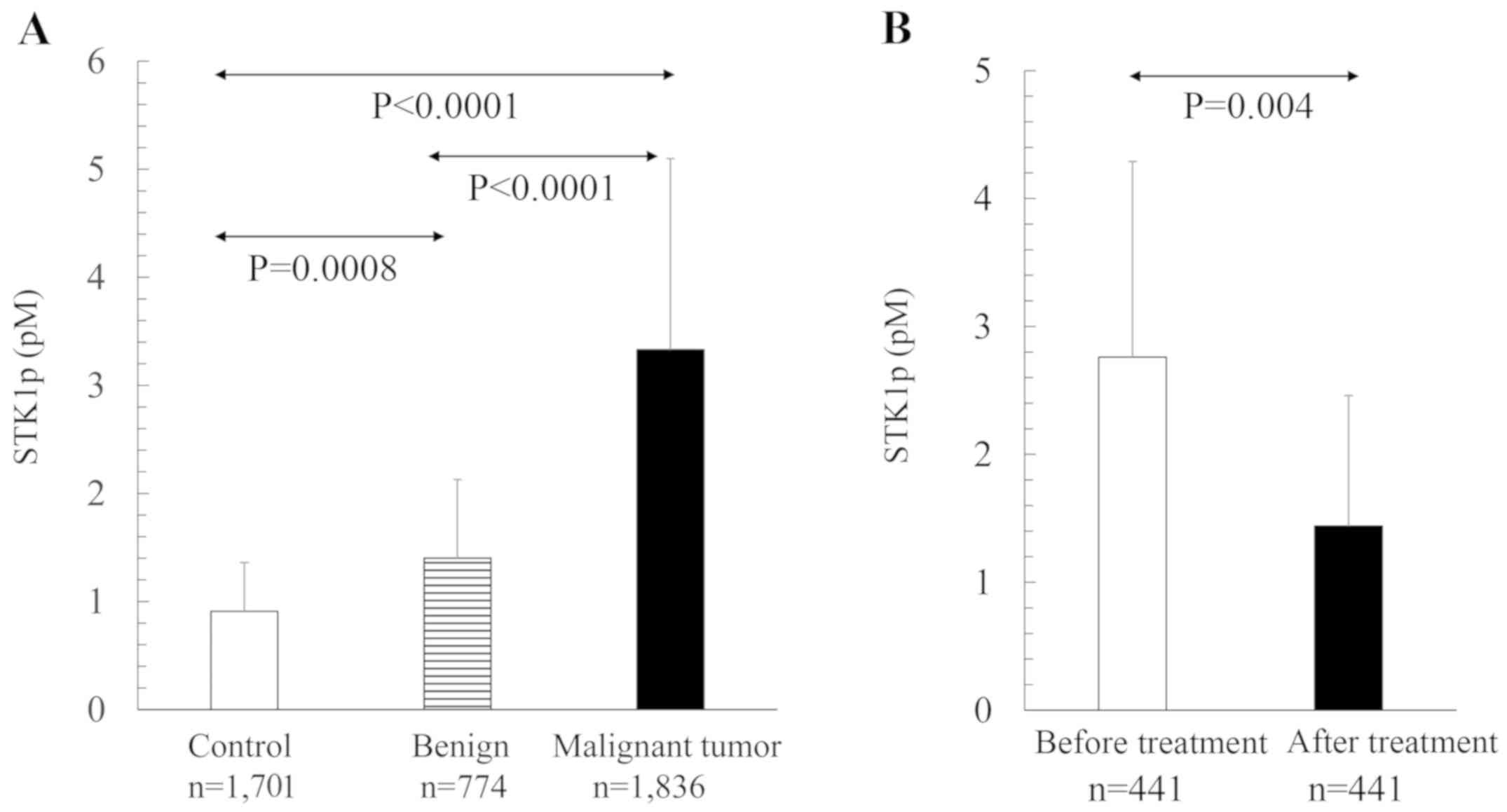
STK1p values in different
populations
The STK1p values were significantly different
between healthy individuals and benign tumor patients, healthy
individuals and CRC patients, and benign tumor and CRC patients
(Table I, Fig. 2A). The number of publications
involved among the healthy controls were 19, the benign 10 and the
malignant 20, corresponding to 1,701, 774 and 1,836 persons,
respectively. The STK1p values decreased significantly (40%) 1
month after surgery (Fig. 2B).
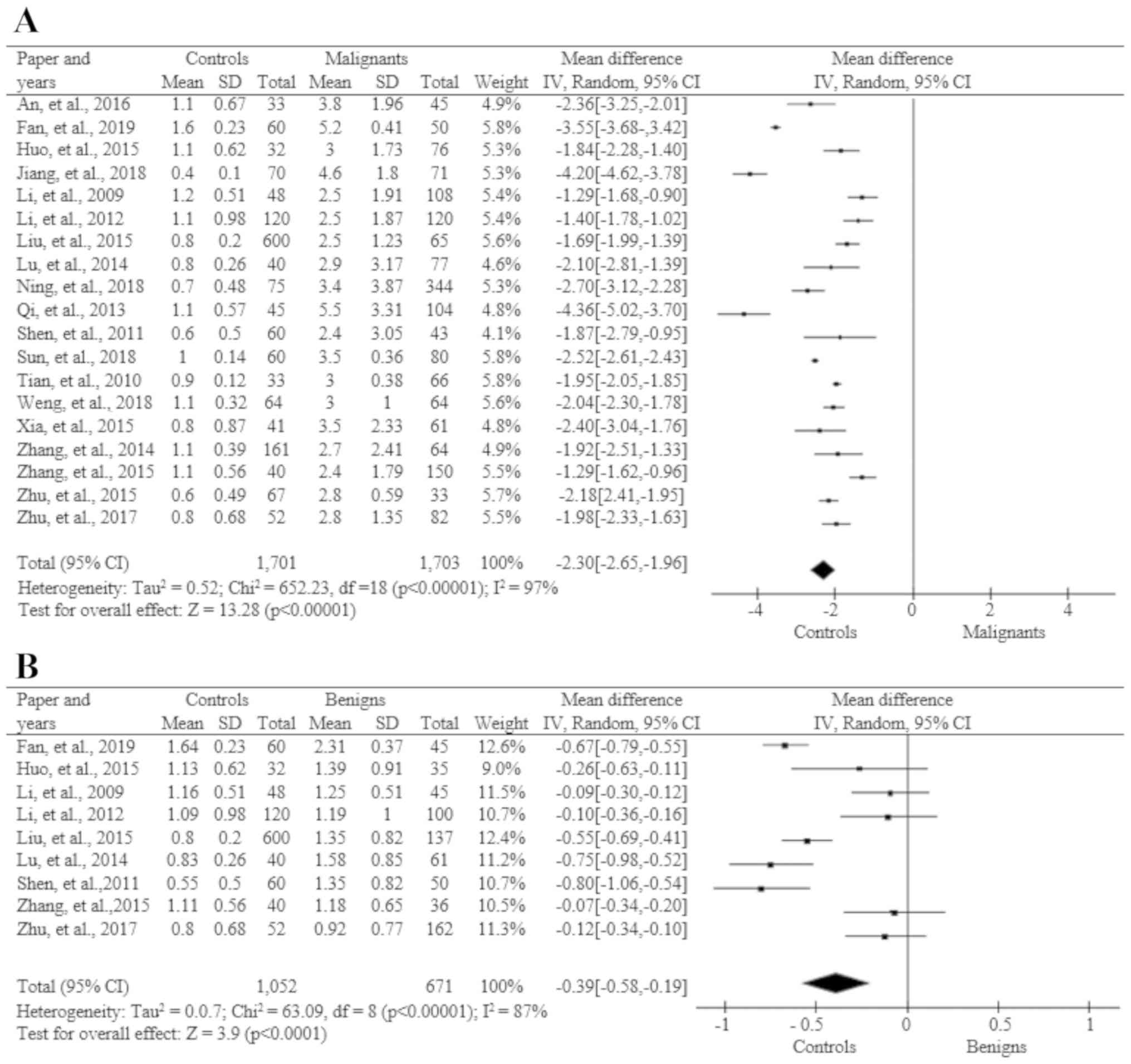
Meta-analysis statistical
calculation
Significant values were calculated using statistical
programs for meta-analysis (see Material and methods sections). The
results for the different test groups are shown in Figs. 3 and 4. The statistical values were
calculated between healthy individuals and benign tumor patients
(Fig. 3B), healthy individuals and
CRC malignant tumor patients (Fig.
3A), benign and malignant tumor patients (Fig. 3C), and before and after surgery
(Fig. 3D).
STK1p of healthy individuals compared
to benign tumor patients
In this statistical calculation 1,052 healthy
individuals and 671 benign tumor patients from 9 studies were used.
Based on a random effects model, the heterogeneity test showed that
the STK1p value in healthy individuals was significantly lower than
that in benign tumor patients (Fig.
3B).
STK1p of healthy individuals compared
to CRC patients
Of the 20 publications received, 19 were used in
this comparison, including 1,701 healthy individuals and 1,703
patients with CRC (Fig. 3A). A
heterogeneity test based on the random effects model showed that
the STK1p value in CRC patients was statistically higher than that
in healthy individuals.
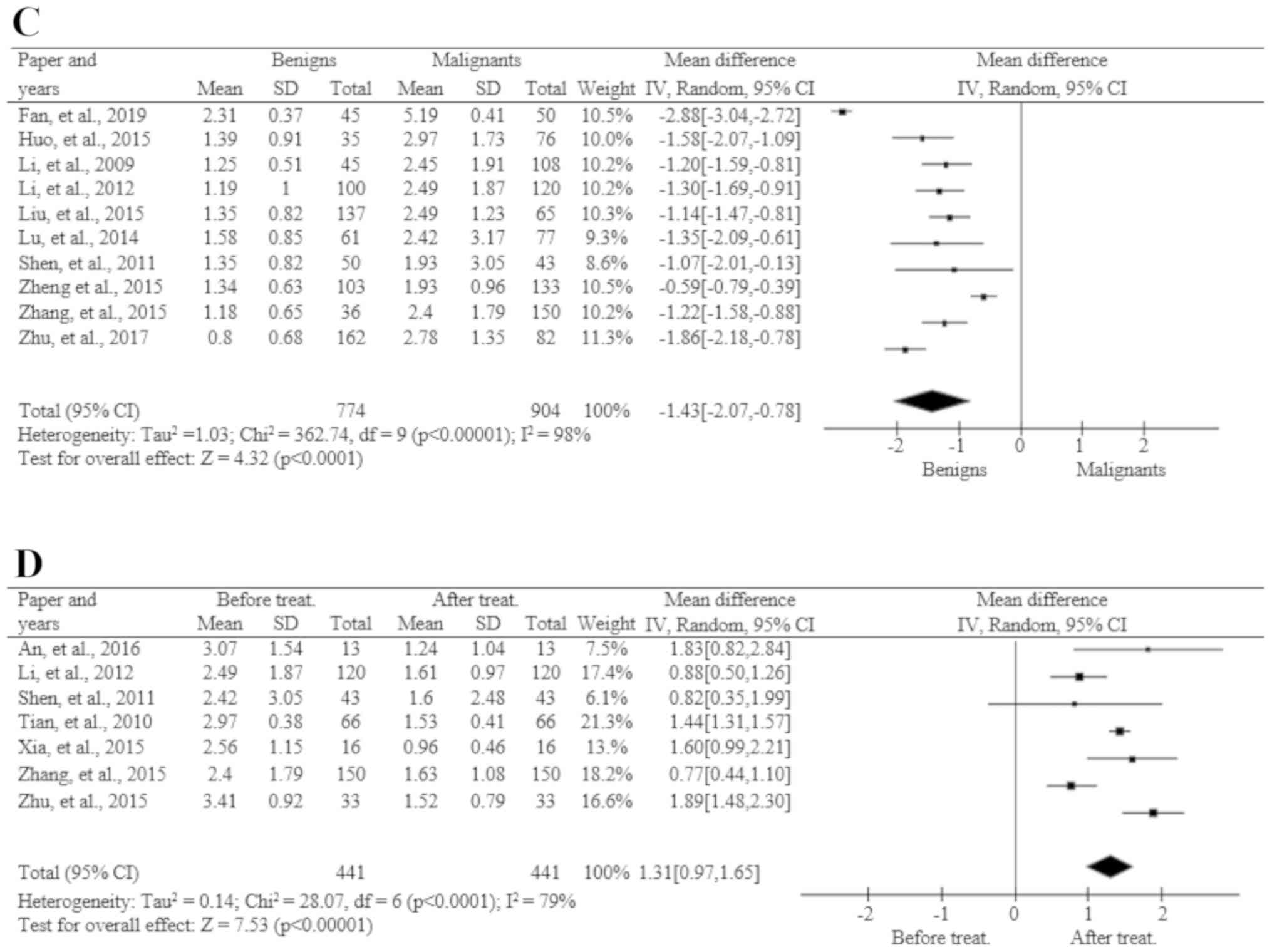
STK1p in benign tumor patients
compared to CRC patients
Out of the 20 publications, 10 included data
regarding STK1p in benign tumors and CRC (Fig. 3C). There was a total of 774 cases in
the benign tumor group and 904 in the CRC group. A heterogeneity
test based on the random effects model showed that the STK1p values
were significantly higher in CRC patients, as compared to benign
tumor patients.
STK1p level before and after
surgery
All 20 publications included data regarding the
STK1p level before and 1 month after surgery. A heterogeneity test,
based on the random effects model, showed a significant decrease by
40% 1 month after surgery (Fig.
3D).
Sensitivity analysis
Sensitivity analysis was conducted to evaluate the
effect of excluding any individual study. By excluding 1 article at
a time in turn, the summary results of the remaining literatures
did not substantially change (Fig.
4A).
Publication bias
Begg's Funnel-plot (Fig.
4B) and Egger' test (Table IV)
were used to examine the potential publication bias. There was no
evidence of bias between the healthy individuals and the benign
tumor group, healthy individuals and the malignant group, the
benign and malignant tumor groups, or the before and after
treatment groups. The Egger's test showed that the possibility of
potential bias from each comparison analysis was very low (all
P>0.05; Table IV). Based on
these results, no significant publication bias was identified in
this meta-analysis.
 | Table IVEgger's linear regression test for
the assessment of publication bias. |
Table IV
Egger's linear regression test for
the assessment of publication bias.
| Standard
effect | Coefficient | Standard error | T value | p>|t| | 95% CI |
|---|
| Control vs.
benign | | | | | |
|
Slope | -0.8601885 | 0.2631424 | -3.27 | 0.014 | -1.482421 to
-0.2379556 |
|
Bias | 4.134483 | 2.616981 | 1.58 | 0.158 | -2.053694 to
10.32266 |
| Control vs.
malignant | | | | | |
|
Slope | -2.533914 | 0.249529 | -10.15 | 0.000 | -3.060375 to
-2.007454 |
|
Bias | 1.303459 | 2.242794 | 0.58 | 0.569 | -3.428422 to
6.03534 |
| Benign vs.
malignant | | | | | |
|
Slope | -2.50849 | 0.6528756 | -3.84 | 0.005 | -4.014024 to
-1.002956 |
|
Bias | 5.555459 | 4.287575 | 1.30 | 0.231 | -4.331706 to
15.44262 |
| Before vs. after
treatment | | | | | |
|
Slope | 1.414582 | 0.2186961 | 6.47 | 0.001 | 0.8524054 to
1.976758 |
|
Bias | -0.6030739 | 1.460007 | -0.34 | 0.744 | -4.256143 to
3.249995 |
Discussion
Meta-analysis is a statistical analysis that
combines the results of multiple studies (50-52).
In the present study, a meta-analysis was performed according to
the aforementioned inclusion and exclusion criteria. No bias was
found. We also found that STK1p was able to distinguish between
healthy individuals and benign tumor patients, as well as between
healthy/benign tumor and malignant tumor patients. STK1p also
monitored the results of the surgery. Thus, STK1p is a reliable
biomarker for the prognosis of benign tumor and CRC patients, and a
useful follow-up tool for surgery.
The STK1p was determined by an assay with a high
sensitivity (0.80) and specificity (0.99), and an AUC value of
0.96. This assay is the most sensitive assay for TK1 in the serum
on the market today (16). The mean
value of STK1p in the benign tumor patients was 1.64-fold higher
than that in healthy individuals, but significantly lower than that
in CRC patients (1.60-fold). Although the mean STK1p value in
benign tumor patients was higher than that in healthy individuals,
there was a deviation, with both low and high individual STK1p
values. The benign tumor patients with higher STK1p values (>2.0
pM) may be in a higher risk of developing malignancies than benign
tumor patients with low STK1p values (<2.0 pM). Based on a
health screening study (n=35,365) (18), where patients with a STK1p value of
>2.0 pM were found to have a 3-5 times higher risk to develop a
malignancy, we concluded that the risk for developing colorectal
malignancies from a benign colorectal tumor should be higher in
benign tumor patients with an STK1p value of >2.0 pM. On the
other hand, benign tumor patients with STK1p values of <2.0 pM
should have a low risk of developing CRC. STK1p was also found to
have a prognostic potential in a randomised clinical trial that
included patients with CRC (n=504) (28). Patients were followed up for 3-8
years. STK1p was compared with pathological stage and grade, lymph
node metastasis, gender and age in relation to survival. A
significantly worse survival was found among patients with high
STK1p values (>0.9 pM), as compared to patients with low STK1p
values (≤0.9 pM) (P<0.0001). Cox regression analysis
demonstrated that STK1p, clinical stage and lymph node metastasis
were independent prognostic factors, but Dukes' stage (P=0.633),
sex (P=0.976) and age (P=0.520) were not.
In the present meta-analysis, the cut-off STK1p
value was set to 0.9 pM, based on receiver operating characteristic
statistical analysis. It is important to understand that the
cut-off value of STK1p may differ depending on the type of tumor
and how the measurement of STK1p was performed (healthy screening,
clinical trials, etc.).
CRC is a heterogeneous disease, with most cases
originating from polyp precursors. Different clinical stages and
pathological grades of CRC can lead to heterogeneity.
CRC, a complex disease, is caused by both genetic
and environmental factors. Certain studies have shown that
inherited genetic factors account for ~35% of the disease etiology
(53,54). It has been suggested that there may
be two distinct categories of cancer: Right- and left-sided colon
cancers that arise proximally or distally to the splenic flexure,
respectively (55). A meta-analysis
on vitamin E concentration in the serum suggested that serum
vitamin E concentration was lower in patients with CRC than in
healthy controls. Reduced serum vitamin E levels may therefore be a
risk factor for CRC. However, prospective cohort studies are still
required to assess the risk of serum vitamin E on CRC in the future
(56). Although the original Dukes
staging system has been modified several times, the extent of
cancer invasion through the bowel wall and that of regional lymph
node invasion is still the mainstay of TNM staging for CRC. A
17,641 patient-cohort study demonstrated that right- and left-sided
colon carcinoma (CC) are significantly different in terms of
epidemiological, clinical and histological parameters. Right-sided
CC has been found to have a worse prognosis. These discrepancies
may be due to genetic differences that determine distinct
carcinogenesis and biological behaviour (57). There was no significant difference in
recurrence rates between right- and left-sided CC and rectum
carcinoma (RC), but the right-sided CC had a worse prognosis than
left-sided CC and RC, possibly due to more advanced staging and
fewer curative resections (58).
Another study demonstrated that the patients with stage I
right-sided CC had a significantly better 5 year disease free
survival rate than those with left-sided CC; however, no
significant difference was observed in the distribution of the
first patients with recurrence (59). A retrospective design and
single-institution study showed that patients with right-sided
colon cancers presented with a significantly increased risk of
locoregional recurrence. Right-sided location, female sex, T4
disease, lymph node metastasis, and perineural invasion are
independent risk factors for the locoregional recurrence of colon
cancer (60). Qin et al
(61) found that right- and
left-sided colon cancer had significantly different
clinicopathological characteristics. Right-sided colon cancer had a
higher incidence of recurrence than left-sided colon cancer.
Patients with stage III right-sided colon cancer had a worse
prognosis than those with stage III left-sided colon cancer. In
addition, CRC results may vary from region to region, resulting in
heterogeneity, depending on patients' genetic characteristics and
living conditions.
With regard to the heterogeneity in the STK1
results, in a preliminary CRC study on 492 patients (Prof. Desong
Wan, Sun Yet-Sen University Cancer Centre, China) no significant
difference (P≈0.628-0.645) was found in the STK1 values between
right-(n=91) and left-(n=124) side colon cancer, and rectum
(n=277). The STK1p value of right colon, left colon and rectum was
1.8±1.8, 1.9±1.8 and 1.9±1.8 pM, respectively. STK1p is therefore
useful for assessing the proliferation rate in CRC serum
samples.
Monitoring the response to treatment is important.
Previous studies have shown that STK1p can be used not only to
predict prognosis, relapse and survival, but also to monitor tumor
treatment (28). In this
meta-analysis, the half-life time of STK1p following surgery was
found to be ~1 month, which is the half-life time identified
following surgery in patients with lung cancer (26) and gastric carcinoma (62). In the case of gastric carcinoma, the
STK1p values were significantly reduced to 52.7% 35 days after open
surgery (P=0.0106). On the contrary, in the patients with distant
metastases, the STK1p value increased to 173% at 35 days
post-operatively. There was no significantly difference in TK
activity. Similar results were also found in breast cancer
(20). However, in the case of
minimally invasive surgery, the half-life time of STK1p in patients
with bladder carcinoma was only 1 week (63).
It is recommend that STK1p is combined with other
tools to evaluate the treatment effect on patients with CRC. This
will help individual treatment planning. The following parameters
in combination with STK1p should be considered when designing a CRC
study: i) CRC is a heterogeneous disease (63), the majority of which is developed
from polyp precursors. Therefore, a complete study should use tools
useful for the early detection, diagnosis, prognosis and management
of CRC development from benign tumors; ii) Since CC and RC are two
different types of malignancy (64),
they should be evaluated separately; the same goes for right- and
left-sided CC and RC; iii) While monitoring the effect of the
treatment, the STK1p levels may change depending on clinical
stage/grade and tumor type on an individual bases; iv) Since CRC
results may differ between living area, depending on the genetic
properties and living conditions of the patients, studies should
include data from different health centres and oncology
hospitals.
In summary, STK1p could potentially be used for the
early detection of benign lesions to prevent their future
development into colorectal malignancies, as well as for individual
clinical dynamic monitoring of the results of surgery in patients
with CRC. The combination of STK1p with colorectal imaging tools
after treatment can provide a precise evaluation of the results of
the therapy. Together with the use of colorectal-related
biomarkers, tumor stage and grade for predicting the risk of
relapse, STK1p can help doctors develope more accurate,
individualized and rational treatment plans for patients.
Acknowledgements
The authors would like to thank Professor Desong Wan
from the Cancer Centre of Sun Yet-Sen University (Guangdong, China)
for providing STK1p serum samples of CRC.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LD, HM, AH and SX searched the literature,
independently extracted the data, produced the figures and tables,
and discussed the articles until an agreement was reached. JZ, EH
and SS carefully re-analyzed all results. LD, HM and AH wrote the
first version of the manuscript. EH and SS revised the introduction
and discussion sections of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
JZ is the owner of SSTK Ltd., which produces the
STK1p kit. Authors declare they have no competing interests.
References
|
1
|
Boyle P and Levin B: World Cancer Report
2008. International Agency for Research on Cancer; Distributed by
WHO Press, c2008, International Agency for Research on Cancer.
France, Publisher: Lyon: Geneva, 2008.
|
|
2
|
Stewart BW and Wild CP: World Cancer
Report 2014. International Agency for Research on Cancer; World
Health Organization. France, Publisher Lyon, 2014.
|
|
3
|
Stewart BW, Bray F, Forman D, Ohgaki H,
Straif K, Ullrich A and Wild CP: Cancer prevention as part of
precision medicine: ‘Plenty to be done’. Carcinogenesis. 37:2–9.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huxley RR, Ansary-Moghaddam A, Clifton P,
Czernichow S, Parr CL and Woodward M: The impact of dietary and
lifestyle risk factors on risk of colorectal cancer: A quantitative
overview of the epidemiological evidence. Int J Cancer.
125:171–180. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jasperson KW, Tuohy TM, Neklason DW and
Burt RW: Hereditary and familial colon cancer. Gastroenterology.
138:2044–2058. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cappellani A, Zanghì A, D Vita M,
Cavallaro A, Piccolo G, Veroux P, Lo Menzo E, Cavallaro V, de Paoli
P, Veroux M and Berretta M: Strong correlation between diet and
development of colorectal cancer. Front Biosci. 18:190–198.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Abu-Remaileh M, Bender S, Raddatz G,
Ansari I, Cohen D, Gutekunst J, Musch T, Linhart H, Breiling A,
Pikarsky E, et al: Chronic inflammation induces a novel epigenetic
program that is conserved in intestinal adenomas and in colorectal
cancer. Cancer Res. 75:2120–2130. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shussman N and Wexner SD: Colorectal
polyps and polyposis syndromes. Gastroenterol Rep. 2:1–15.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I: EUROCARE-4 Working
Group. Recent cancer survival in Europe: A 2000-02 period analysis
of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ciccolallo L, Capocaccia R, Coleman MP,
Berrino F, Coebergh JW, Damhuis RA, Faivre J, Martinez-Garcia C,
Møller H, Ponz de Leon M, et al: Survival differences between
European and US patients with colorectal cancer: Role of stage at
diagnosis and surgery. Gut. 54:268–273. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767.
1990.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Binefa G, Rodríguez-Moranta F, Teule A and
Medina-Hayas M: Colorectal cancer: From prevention to personalized
medicine. World J Gastroenterol. 20:6786–6808. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ribero D, Viganò L, Amisano M and
Capussotti L: Prognostic factors after resection of colorectal
liver metastases: From morphology to biology. Future Oncol.
9:45–57. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gasparri F, Wang N, Skog S, Galvani A and
Eriksson S: Thymidine kinase 1 expression defines an activated G1
state of the cell cycle as revealed with site-specific antibodies
and arrayscan assays. Eur J Cell Biol. 88:779–785. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou J, He E and Skog S: The proliferation
marker thymidine kinase 1 in clinical use. Mol Clin Oncol. 1:18–28.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cao X, Zhou J and Chen Z: Standardized
centile curves and reference intervals of serum thymidine kinase 1
levels in a normal Chinese population using the LMS method. Genet
Test Mol Biomarkers. 20:445–450. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen ZH, Huang SQ, Wang Y, Yang AZ, Wen J,
Xu XH, Chen Y, Chen QB, Wang YH, He E, et al: Serological thymidine
kinase 1 is a biomarker for early detection of tumours-a health
screening study on 35,365 people, using a sensitive
chemiluminescent dot blot assay. Sensors (Basel). 11:11064–11080.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
He Q, Zhang P, Zou L, Li H, Wang X, Zhou
S, Fornander T and Skog S: Concentration of thymidine kinase 1 in
serum (S-TK1) is a more sensitive proliferation marker in human
solid tumors than its activity. Oncol Rep. 14:1013–1019.
2005.PubMed/NCBI
|
|
20
|
He Q, Fornander T, Johansson H, Johansson
U, Hu GZ, Rutqvist LE and Skog S: Thymidine kinase 1 in serum
predicts increased risk of distant or loco-regional recurrence
following surgery in patients with early breast cancer. Anticancer
Res. 26:4753–4759. 2006.PubMed/NCBI
|
|
21
|
Kumar JK, Aronsson AC, Pilko G, Zupan M,
Kumer K, Fabjan T, Osredkar J and Eriksson S: A clinical evaluation
of the TK 210 ELISA in sera from breast cancer patients
demonstrates high sensitivity and specificity in all stages of
disease. Tumour Biol. 37:11937–11945. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu Y, Ling Y, Qi Q, Tang Y, Xu J, Tong Z,
Sheng G, Yang Q and Pan Y: Changes in serum thymidine kinase 1
levels during chemotherapy correlate with objective response in
patients with advanced gastric cancer. Exp Ther Med. 2:1177–1181.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen F, Tang L, Xia T, He E, Hu G, Zhang
M, Li Y, Zhou J, Eriksson S and Skog S: Serum thymidine kinase 1
levels predictcancer-free survival following neoadjuvant, surgical
and adjuvant treatment of patients with locally advanced breast
cancer. Mol Clin Oncol. 1:894–902. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Aufderklamm S, Hennenlotter J, Todenhoefer
T, Gakis G, Schilling D, Vogel U, Kuehs U, Dlugosch J, Knapp J,
Merseburger A, et al: XPA-210: A new proliferation marker
determines locally advanced prostate cancer and is a predictor of
biochemical recurrence. World J Urol. 30:547–552. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang Y, Jiang X, Dong S, Shen J, Yu H,
Zhou J, Li J, Ma H, He E and Skog S: Serum TK1 is a more reliable
marker than CEA and AFP for cancer screening survey in a study of
56,286 people. Cancer Biomark. 16:529–536. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lou X, Zhou J, Ma H, Xu S, He E, Skog S
and Wang H: The half-life of serum thymidine kinase 1 concentration
is an important tool for monitoring surgical response in patients
with lung cancer: A meta-analysis. Genet Test Mol Biomarkers.
21:471–478. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yurish SY: Serum-biomarker thymidine
kinase 1 for early discovery of tumour process -160,086
participants using a sensitive immune-ECL-dot-blot detection
system. Advances in Sensors: Reviews. 6:529–540. 2018.http://www.sensorsportal.com.
|
|
28
|
Skog S, He E and Haghdoost S: Prevention
and early detection of human tumour. LAP lambert academic
publishing, Schaltungsdienst Lange O.H.G., Berlin. p74, p183-257.
http://www.get-morebook.com.
|
|
29
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fan XJ, Shi ZT and Sun W: Significance of
TuM2-PK, TK1, CEA, CA19-9 and CA72-4 in the diagnosis of colon
cancer patients. Chin J Gerontol. 39:51–53. 2019.
|
|
31
|
Weng YL: Diagnostic value of serum
TuM2-PK, TK1 and HER-2 expression levels in rectal cancer. Henan
Med Res. 1:1795–1797. 2018.
|
|
32
|
Jiang XL, Bo YX and Ye Y: Correlation
between TK1 and tumor markers in diagnosis and pathological
features of digestive tract tumors. J Anhui Med Univ. 53:110–114.
2018.
|
|
33
|
Sun HW, Mei M, Liu YY and Liu XZ: Clinical
value of serum thymidine kinase 1 in the evaluation of chemotherapy
for stage IV colon cancer. Continuing Medical Education. 8:81–83.
2018.
|
|
34
|
Ning S, Wei W, Li J, Hou B, Zhong J, Xie
Y, Liu H, Mo X, Chen J and Zhang L: Clinical significance and
diagnostic capacity of serum TK1, CEA, CA 19-9 and CA 72-4 levels
in gastric and colorectal cancer patients. J Cancer. 3:494–501.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhu LP, Li XL, Liu H, Ping YF and Zhang
YN: Expression and clinical significance of thymidine kinase 1 in
rectal cancer. J Xiangnan Univ (Medical Sciences). 19:9–12.
2017.
|
|
36
|
An N, Zhang M, Lan HT and Li HM:
Significance of detecting serum thymidine kinase 1 in metastatic
large intestine carcinoma before and after chemotherapy. Med J West
China. 28:1257–1260. 2016.
|
|
37
|
Zhu YZ, Ma T, Zhang CJ and Sun GP: The
expression and clinical significance of serum thymidine kinase 1 in
cancer patients. Acta Univ Med Anhui. 7:1012–1015. 2015.
|
|
38
|
Zeng QH and Zhang SY: Diagnostic value of
combined detection of CEA, CA199 and TK1 for colorectal cancer. Med
Equipment. 10:2–4. 2015.
|
|
39
|
Xia Y, Liu Y, Qi Q, Zhu M, Zhang Y and
Ling Y: Value of serum thymidine kinase 1 in the chemotherapy
efficacy evaluation for patients with stage IV colon cancer. J
Basic Clin Oncol. 28:6–8. 2015.
|
|
40
|
Liu X, Feng Z, Lai Z, Jiang Y, Xu M and
Zhou X: An analysis on expression difference of serum TK1 between
patients with gastric carcinoma, lung cancer, colon cancer and
healthy person and its clinical significance. J Mathematical Med.
58:913–915. 2015.
|
|
41
|
Huo Y, Yu X and Kong AP: The application
value of TK1 in colon cancer patients. Int J Lab Med. 36:3151–3152.
2015.
|
|
42
|
Zhang Y, Wang J, Xie J, Yang D, Han G and
Zhang Y: The assay and clinical significance of serum thymidine
kinase 1 in patients with colorectal carcinoma. Eur Surg.
47:248–253. 2015.
|
|
43
|
Zhang ZJ, Zheng YP, Lin YH and Mei XQ:
Clinical detecting application of TK1 in the diagnosis of common
malignant tumors. Int J Lab Med. 35:2636–2637. 2014.
|
|
44
|
Lu ZQ, Xie YM, Cheng GH and Li JH:
Clinical diagnostic value of combined detection of serum TK1, CAl99
and CA724 in colorectal cancer. China Practical Medicine. 9:5–7.
2014.
|
|
45
|
Qi QF, Zhu M, Bao YQ and Liu YP: Detection
of serum TK1 in patients with colorectal cancer after operation to
evaluate the effect of value. World Latest Med Information.
13:60–61. 2013.
|
|
46
|
Li QF: Clinical application of serum
thymidine kinase 1 used in colorectal carcinoma patients.
Laboratory Med. 27:1080–1081. 2012.
|
|
47
|
Shen J, Chen W, Xu RH, Zhang JF and Jiang
H: Evaluation of the significance of serum thymidine kinase 1
detection in the diagnosis and curative effect of gastrointestinal
malignant tumors. Exp Lab Med. 29:531–533. 2011.
|
|
48
|
Tian J, Jia Y and Liu H: Serum TK1, EGF,
HGF and CRP and IL-6 levels in patiens with colon cancer using
laparoscopic and open surgery. Shangdong Med. 50:42–43. 2010.
|
|
49
|
Li X and Wang F: Significance of serum
thymidine kinase determination in patients with carcinoma of
rectum. Jiangsu Medical J. 35:1436–1437. 2009.
|
|
50
|
Streiner DL: I have the answer, now what's
the question?: Why meta-analyses do not provide definitive
solutions. Can J Psychiat. 50:829–831. 2005.
|
|
51
|
Chatterji M: Grades of evidence:
Variability in quality of findings in effectiveness studies of
complex field interventions. Am J Eval. 28:239–255. 2007.
|
|
52
|
Baas M, De Dreu CK and Nijstad BA: A
meta-analysis of 25 years of mood-creativity research: Hedonic
tone, activation, or regulatory focus? Psychol Bull. 134:779–806.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
53
|
Zou L, Zhang PG, Zou S, Li Y and He Q: The
half-life of cytosolic thymidine kinase in serum by ECL dot bolt
potential marker for monitoring the response to surgery of patients
with gastric cancer. Int J Biol Markers. 17:135–140.
2002.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhang J, Jia Q, Zou S, Zhang P, Zhang X,
Skog S, Luo P, Zhang W and He Q: Thymidine kinase 1: A
proliferation marker for determining prognosis and monitoring the
surgical outcome of primary bladder carcinoma patients. Oncol Rep.
15:455–461. 2006.PubMed/NCBI
|
|
55
|
Ogino S, Chan AT, Fuchs CS and Giovannucci
E: Molecular pathological epidemiology of colorectal neoplasia: An
emerging transdisciplinary and interdisciplinary field. Gut.
60:397–411. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Dong Y, Liu Y, Shu Y, Chen X, Hu J, Zheng
R, Ma D, Yang C and Guan X: Link between risk of colorectal cancer
and serum vitamin E levels: A meta-analysis of case-control
studies. Medicine (Baltimore). 96(e7470)2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Benedix F, Kube R, Meyer F, Schmidt U,
Gastinger I and Lippert H: Colon/Rectum Carcinomas (Primary Tumor)
Study Group. Comparison of 17,641 patients with right- and
left-sided colon cancer: Differences in epidemiology, perioperative
course, histology, and survival. Dis Colon Rectum. 53:57–64.
2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lichtenstein P, Holm NV, Verkasalo PK,
Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A and Hemminki
K: Environmental and heritable factors in the causation of
cancer-analyses of cohorts of twins from Sweden, Denmark, and
Finland. N Engl J Med. 343:78–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Hemminki K and Chen B: Familial risk for
colorectal cancers are mainly due to heritable causes. Cancer
Epidemiol Biomarkers Prev. 13:1253–1256. 2004.PubMed/NCBI
|
|
60
|
Moritani K, Hasegawa H, Okabayashi K,
Ishii Y, Endo T and Kitagawa Y: Difference in the recurrence rate
between right- and left-sided colon cancer: A 17 year experience at
a single institution. Surg Today. 44:1685–1691. 2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Qin Q, Yang L, Sun YK, Ying JM, Song Y,
Zhang W, Wang JW and Zhou AP: Comparison of 627 patients with
right- and left-sided colon cancer in China: Differences in
clinicopathology, recurrence, and survival. Chronic Dis Transl Med.
13:51–59. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zou L, Zhang PG, Zou S, Li Y..He Q: The
half-life of cytosolic thymidine kinase in serum by ECL dot blot
potential marker for monitoring the response to surgery of patients
with gastric cancer. International Journal of Biological Markers.
17:135–140. 2002.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhang J, Jia Q, Zou S, Zhang P, Zhang X,
Skog S, Luo P, Zhang W and He Q: Thymidine kinase 1: a
proliferation marker for determining prognosis and monitoring the
surgical outcome of primary bladder carcinoma patients. Oncology
Reports. 15:455–461. 2006.PubMed/NCBI
|
|
64
|
Paschke S, Jafarov S, Staib L, Kreuser ED,
Maulbecker-Armstrong C, Roitman M, Holm T, Harris CC, Link KH and
Kornmann M: Are colon and rectal cancer two different tumor
entities? A proposal to abandon the term colorectal cancer. Int J
Mol Sci. 19(E2577)2018.PubMed/NCBI View Article : Google Scholar
|