Introduction
Extraskeletal osteosarcoma is a rare sarcoma of the
non-skeletal tissue that produces neoplastic osteoid or bone
(1). The tumor typically appears as
a solid mass with variable mineralization and seldom has a cystic
lesion.
Roller et al, reported the features of 19
patients with extraskeletal osteosarcoma radiographically and
pathologically (2). In their report,
mineralization on computed tomogram (CT) was seen in only 26% (5 of
19) of cases, and central necrosis on magnetic resonance imaging
(MRI) was observed in 47% (9 of 16) of cases. They confirmed
osteoid formation in the histology of all specimen. However, they
did not mention whether or not central necrosis was observed
pathologically in the excised specimen of the tumors.
Extraskeletal osteosarcoma is classified into six
subtypes, just like intraosseous osteosarcoma, in the 2013 WHO
classification (1). Osteosarcoma
with a cystic lesion can be classified as the telangiectatic-type,
although this type typically manifests as tumors predominantly
composed of cystic spaces filled with blood (3). Telangiectatic-type intraosseous
osteosarcoma is occasionally seen, and telangiectatic-type
extraskeletal osteosarcoma that meets the strict diagnostic
criteria is rarely reported (4-6).
However, no previous reports have described the gross and
pathological characteristics of extraskeletal osteosarcoma with
non-hemorrhagic cystic change.
When a soft tissue tumor consisting of a solid
lesion with calcification and a cystic lesion are observed, both
benign (myositis ossificans and hemangioma) and malignant entities
(dedifferentiated liposarcoma, synovial sarcoma, malignant
peripheral nerve sheath tumor and undifferentiated pleomorphic
sarcoma) are included in the differential diagnosis (2). It is very important to make an accurate
pathological diagnosis in order to facilitate appropriate treatment
with a chemotherapy regimen suited for the histological type. The
clinical course and imaging findings are also useful for making a
diagnosis, especially in cases of rare tumors.
We herein report three rare cases of extraskeletal
osteosarcoma with cystic change. The Ethical Institutional Review
Board of Kanazawa University Hospital approved the present study,
and written informed consent was obtained from all patients.
Case report
Case 1
The patient was an 81-year-old man who presented
with a 2-year history of a gradually enlarging mass in his left
thigh. A large, ill-defined, non-movable, non-tender, firm mass was
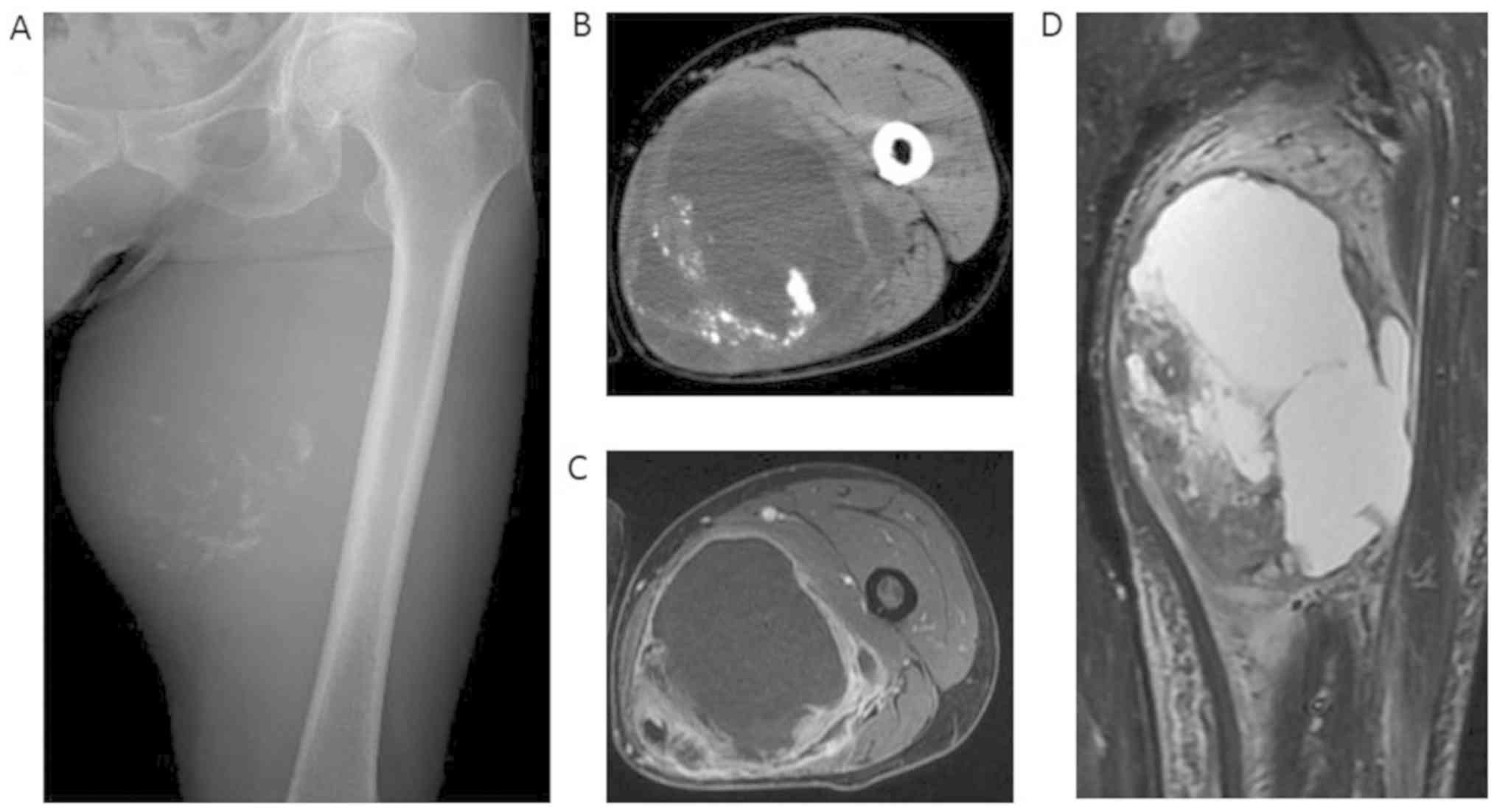
noted on the upper medial portion of the left thigh. Plain film
showed a large soft tissue mass with numerous small calcifications,
located more or less centrally (Fig.
1A). CT showed a large deep-seated mass in the adductor magnus
muscle (Fig. 1B). On MRI, the cystic
lesion appeared hypointense on T1-weighted images and hyperintense
on T2-fat suppression images, and the solid lesion showed a
heterogeneous intensity on T2-fat suppression images (Fig. 1D). An axial view of dynamic MRI
showed enhancement of the tumor periphery and the solid lesion
(Fig. 1C). Scintigraphy with
thallium-201 showed the increased uptake of the whole tumor, which
was more pronounced in the solid component than in the cystic
lesion. No distant metastases were observed on a close examination
of the whole body.
A needle biopsy revealed undifferentiated
pleomorphic sarcoma. We considered the possibility of extraskeletal
osteosarcoma, but a biopsy showed no sign of malignant osteoid
formation.
Surgical treatment was selected because the patient
was already of advanced age and might not tolerate chemotherapy.
The tumor was widely excised with the adductor magnus,
semimembranosus and semitendinosus, preserving the femoral vessels,
nerve and sciatic nerve. The surgical margins were negative for
tumor involvement. The patient received no other adjuvant
treatment. About three months after surgery, lower leg lymphoedema
appeared. He was observed conservatively at an outpatient clinic
but did not improve significantly. At one and half a year after
surgery, recurrence was detected on imaging and confirmed by a
needle biopsy. He underwent reoperation and adjuvant chemotherapy
with the Adriamycin regimen. However, he was dead from his disease
at three years after the primary surgery.
Case 2
A 78-year-old man presented with a soft tissue mass
on the left upper posterior thigh. The mass in the adductor muscles
gradually grew for a few years and was ill-defined, painless, firm,
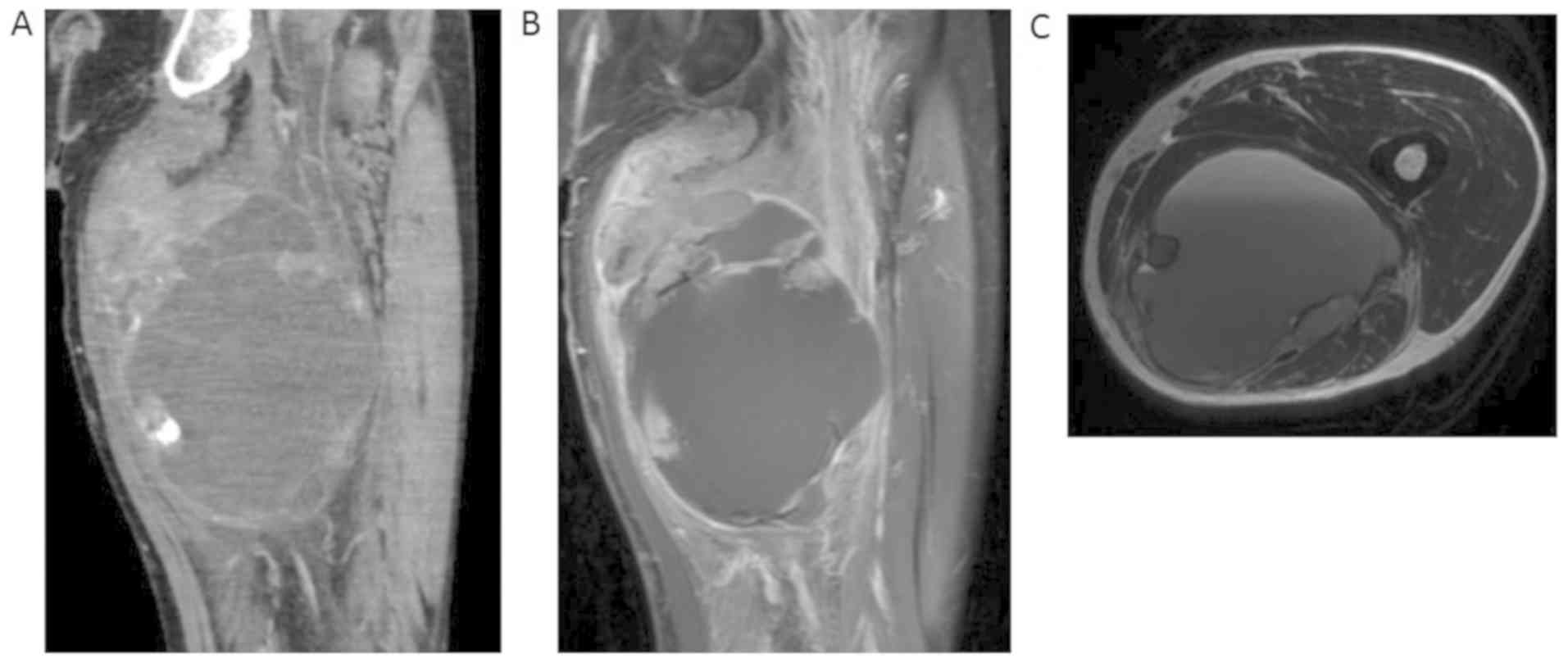
and non-movable. Radiologic findings showed both a solid and cystic
lesion; the solid component included calcification on X-ray and CT,
and the cystic component showed fluid-fluid levels on MRI (Fig. 2A, B
and C). No distant metastases were
observed on a close examination of the whole body. The tumor was
pathologically diagnosed as undifferentiated pleomorphic sarcoma by
a needle biopsy.
As the patient was already of advanced age and
potentially unable to tolerate chemotherapy, wide excision was
planned for local control. The tumor was widely excised with the
adductor magnus, semimembranosus, semitendinosus and biceps
femoris, preserving the sciatic nerve. A diagnosis of extraskeletal
osteosarcoma was made. No invasion to the lymphatic ducts was
observed in the excised specimen. The surgical margins were
negative for tumor involvement. There was no hemorrhaging in the
cystic spaces of the tumor, and only a yellowish-brown fluid with
little blood flowed from the tumor when the resected specimen was
cut (Fig. 3B).
There was no other adjuvant treatment; however,
solitary lymph node metastasis was detected on MRI five months
after excision of the primary tumor. The patient received oral
pazopanib after the excision of the involved lymph node. However,
lung metastases developed 10 months after the first surgery.
Cyclophosphamide was administered as palliative chemotherapy. The
patient died approximately one year after the detection of
pulmonary metastasis.
Case 3
The patient was a 33-year-old woman with a soft
tissue tumor in the distal part of the left posterior thigh. The
patient had noticed a mass that had been gradually growing in size
for approximately six months. She felt a well-circumscribed,
non-tender firm mass between the biceps femoris muscle and
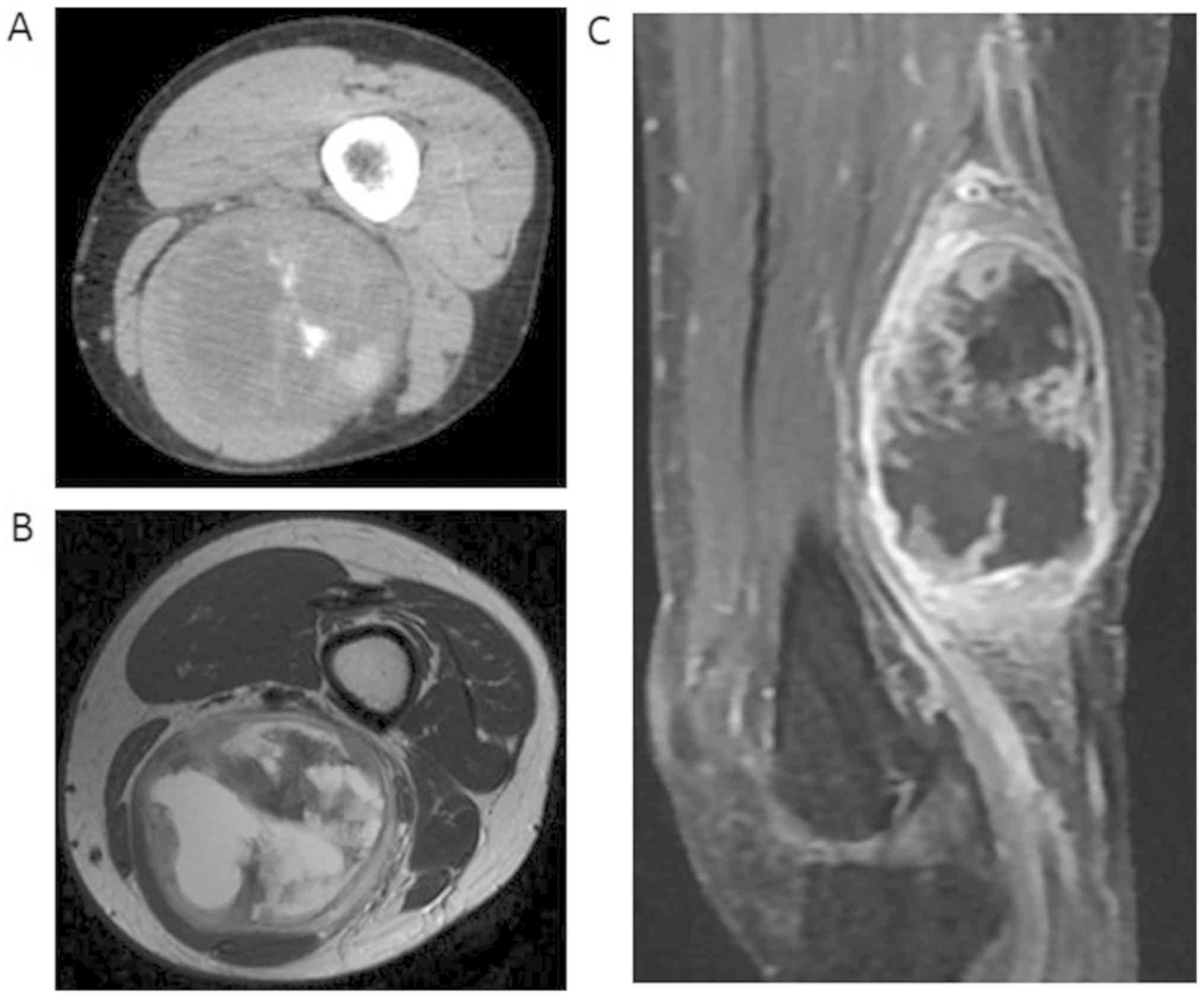
semitendinosus muscle. CT showed a solid component with foci of
calcification (Fig. 4A). On MRI, the
tumor consisted of both a solid lesion and a cystic lesion. The
solid lesion appeared hypointense on T1-weighted images and
hypointense to hyperintense on T2-weighted images. The cystic
lesion appeared hypointense on T1-weighted images and hyperintense
on T2-weighted images (Fig. 4B). The
periphery of the tumor and solid lesion was highlighted on enhanced
MRI (Fig. 4C). No distant metastases
were observed on a close examination of the whole body. The tumor
was diagnosed as extraskeletal osteosarcoma by a needle biopsy.
She received a neo-adjuvant chemotherapy regimen for
osteosarcoma (adriamycin and cisplatin) followed by surgery. The
tumor showed complete remission after 5 cycles of chemotherapy and
was widely excised with the semimembranosus and semitendinosus,
preserving the sciatic nerve. The surgical margins were negative
for tumor involvement. After neoadjuvant chemotherapy had been
completed, a decreased renal function was observed, so the adjuvant
chemotherapy regimen was changed. She was given three cycles of
adjuvant chemotherapy with ifosfamide and etoposide and discharged.
She is being regularly observed at an outpatient clinic. At
present, over 10 years from the time of the diagnosis, she remains
alive and free of disease.
Pathological examination procedures and
findings
Immunohistochemical staining
For fixation of operatively extracted specimen, 10%
formaldehyde (Muto Pure Chemicals Co., Ltd.) was used at room
temperature. After immersing in the formaldehyde for 24 h,
paraffin-embedded specimen were made by Tissue-Tek VIP®
6AI (Sakura Finetek Japan Co., Ltd.). Four-micrometer-thick
sections cut from the representative block of each tumor were
deparaffinized. The preparations were autoclaved in citrate buffer
(pH 6.0), and endogenous peroxidase activity was blocked with 3%
hydrogen peroxide. The following primary antibodies were used:
Anti-alpha-smooth muscle actin mouse monoclonal (M0851, dilution
1:100; DAKO A/S, Glostrup), anti-desmin mouse monoclonal (M0760,
dilution 1:100; DAKO A/S, Glostrup), anti-S-100 rabbit polyclonal
(Z0311, dilution 1:5,000; DAKO A/S, Glostrup), anti-cytokeratin
mouse monoclonal (IS053, no dilution; DAKO A/S, Glostrup),
anti-epithelial membrane antigen (EMA) mouse monoclonal (IS629, no
dilution; DAKO A/S, Glostrup), anti-D2-40 (Podoplanin) mouse
monoclonal (M3619, dilution 1: 50; DAKO A/S, Glostrup) and
anti-Ki-67 rabbit monoclonal (RM-9106-S, dilution 1: 100; Thermo
Fisher Scientific Anatomical Pathology). Slides were incubated for
1 h at room temperature with the primary antibody, and subsequently
labelled by use of the secondary antibody (Histofine®
Simple Stain MAX PO (MULTI), Nichirei Biosciences Inc.). The
sections were examined with a confocal microscope (Olympus).
Fluorescence in situ
hybridization
The probes used for the fluorescence in situ
hybridization (FISH) analyses were as follows; Vysis LSI MDM2
Spectrum Orange Probe, Vysis CEP (D12Z3) (alpha-satellite) Spectrum
Green Probe (Abbott Molecular Inc.), and KreatechTM CDK4
(12q13)/SE12FISHprobe (Leica Biosystems). The tissue sections were
counterstained in phosphate-buffered saline containing
4',6-diamidine-2'- phenylindole dihydrochloride (DAPI II
Counterstain; Abbott Molecular Inc.), p-phenylenediamine, and
glycerol (Abbott Molecular Inc.), then examined with a fluorescence
microscope (Olympus) equipped with a Triple Bandpass FilterTM set
(Abbott Molecular Inc.) for detecting DAPI II, Spectrum Orange, and
Spectrum Green.
Pathological findings
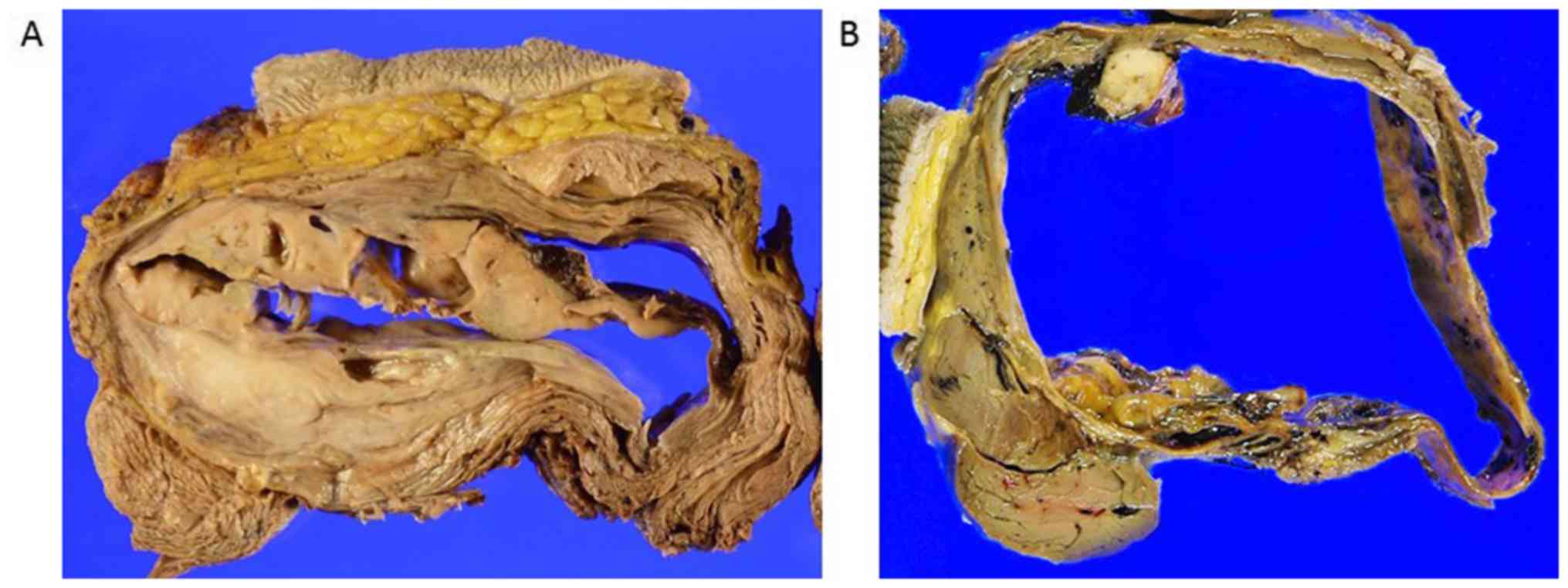
Grossly, the tumors of the three cases were fairly
defined and predominantly consisted of several large cystic spaces
with non-hemorrhagic fluid content (Fig.
3A) or a small amount of hemorrhaging (Fig. 3B). A grayish, tan-white, fleshy solid
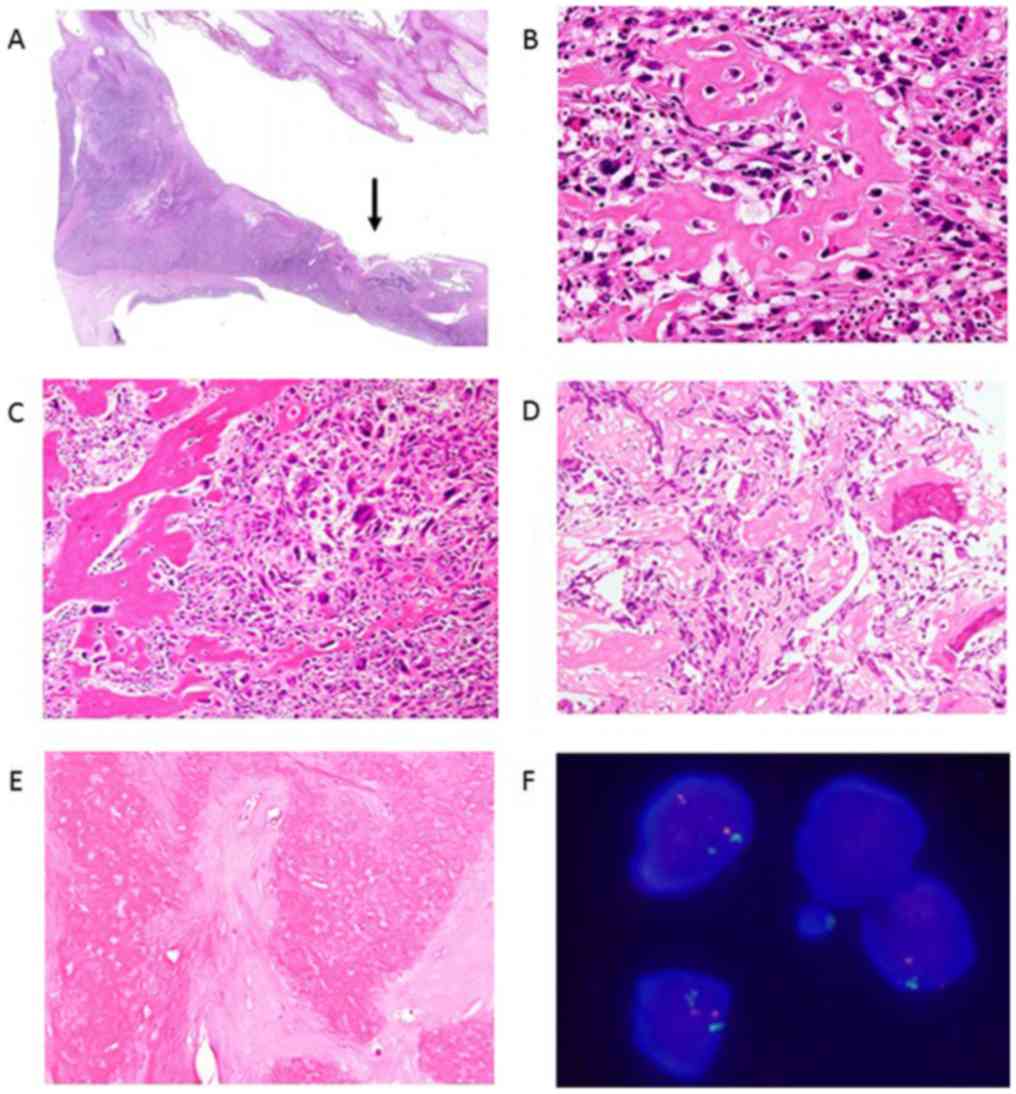
component was noted on the periphery of the cysts (Fig. 3A and B). Microscopically, the osteoid was located
in the septa of the cyst wall (Fig.
5A). On a low-power view, the tumor was composed of haphazardly
arranged, highly anaplastic sarcomatous cells. On a higher-power
view, spindle cells with enlarged, hyperchromatic and pleomorphic
nuclei were observed. There were numerous typical and atypical
mitoses. Scattered tumor giant cells were also seen. Focal areas of
osteoid production by sarcomatous tumor cells were seen (Fig. 5B, C
and D).
Case 3 was diagnosed with extraskeletal osteosarcoma
based on the histological analysis of a biopsy specimen (Fig. 5D). Neoadjuvant chemotherapy was very
effective, and necrosis was observed in more than 95% of the area
of the resected specimen (Fig.
5E).
Immunohistochemistry revealed focal positivity for
alpha-smooth muscle actin but negativity for desmin, S100 protein,
cytokeratin, and EMA. There were no lymphatic ducts with positivity
for D2-40 in the area of tumor involvement. The Ki-67 proliferation
index was approximately 30%. The MDM2 gene was not amplified on
FISH in any of the three cases (Fig.
5F). The cdk4 gene amplification was not observed on FISH in
any of the three cases.
Discussion
Extraskeletal osteosarcoma is a rare soft tissue
sarcoma that accounts for approximately 1-2% of all soft tissue
sarcomas and 2-5% of all osteosarcomas (1). Various etiologies of extraskeletal
osteosarcoma, such as previous trauma or radiotherapy, have been
reported but the actual cause of this tumor is unknown (1,7,8). Approximately 10% of cases show
extensive hemorrhagic change (1);
however, no reports have described a tumor with a non-hemorrhagic
fluid lesion, as was observed in our cases.
Roller et al, reported the radiographical and
pathological features of 19 cases of extraskeletal osteosarcoma
(2). They noted mineralization on CT
in only 26% (5 of 19) of cases, while central necrosis on MRI was
observed in 47% (9 of 19). Regarding central necrosis, they did not
clearly confirm the pathological characteristics of the specimen.
Cystic lesions were observed in all of our cases on MRI, but
central necrosis was not observed in the pathological examination
of the specimens, in which only non-hemorrhagic fluid was
found.
Cases of high-grade extraskeletal osteosarcoma are
reported to be larger in size and to have more necrosis than
low-grade cases (2,9-11).
In all of our cases, the maximum diameter was >17 cm, and a
histological examination revealed high-grade malignancy (Table I). On MRI, the cystic lesion findings
were typically consistent with necrosis. However, there was no
clear necrotic tissue. Only yellowish-brown fluid with little or no
blood flowed from the tumor when the resected specimen was cut. A
histological examination of the tumor did not show central
necrosis. These findings suggest that the necrotic lesion or
bleeding in the tumor may have gradually changed to fluid content
over a long time-course. Indeed, more than half a year had passed
between the patients first noticing the mass and the diagnosis,
which might have been a sufficient duration to allow a change to
non-hemorrhagic fluid. Case 2 showed fluid-fluid levels on MRI
before surgery, but no hemorrhaging was noted in the resected
tumor, and only a small amount of blood flowed with yellowish-brown
fluid on cutting the specimen. This might indicate the course of
changing to non-hemorrhagic fluid.
 | Table ISummary of three cases of cystic
extraskeletal osteosarcoma. |
Table I
Summary of three cases of cystic
extraskeletal osteosarcoma.
| | | | | | | | | MRI | Pathology | | | | |
|---|
| Case | Age/sex | Site | Depth | Tumor size (mm) | Cyst size (mm) | Cyst area (%)
(Cyst/tumor) | X-ray/CT
mineralization | Solid lesion | Cyst | Fluid-fluid
level | Osteoid | MDM2 | cdk4 | Neoadjuvant ChX | Op. | Follow-up periods
(months) | Outcome |
|---|
| 1 | 81/M | Thigh | Deep | 229x145 | 168x118 | 60 | ++ | + | + | - | + | - | - | - | + | 36 | DOD |
| 2 | 78/M | Thigh | Deep | 176x111 | 132x109 | 74 | ++ | + | + | + | + | - | - | - | + | 23 | DOD |
| 3 | 33/F | Thigh | Deep | 122x65 | 86x51 | 55 | + | + | + | - | + | - | - | + | + | 121 | CDF |
The tumors in our cases were located at the adductor
muscles of the thigh in two patients and at the intermuscular
region of the hamstrings in one patient, where lymphatic channels
from the lower legs are abundantly gathered. Lymph node involvement
is likely associated with extraskeletal osteosarcoma and
intratumoral lymphorrhea due to lymphatic channel invasion were
assumed to have been the cause of the cystic lesion (12). However, there were no tumor-involved
areas positive for D2-40, a specific marker of lymphatic ducts, in
any surgical specimens.
At present, extraskeletal osteosarcoma is classified
into six subtypes, just like intraosseous osteosarcoma, in the 2013
WHO classification (1). Intraosseous
osteosarcoma with cystic change is typically diagnosed as
telangiectatic osteosarcoma, but the cyst of intraosseous
osteosarcoma is necessarily filled with blood (2). The criteria for the diagnosis of
telangiectatic osteosarcoma are as follows: The absence of
sclerosis on plain film imaging; a purely lytic lesion; and a
predominant composition of cystic spaces filled with blood.
Microscopically, the tumor comprises a sarcomatous component lining
the septa and the presence inconspicuous osteoid production. In our
cases, the tumors had marked cystic degeneration with little or no
blood. An abundant mineralization pattern was also seen on
radiography, and abundant osteoid production was pathologically
observed in the specimen. With such features, the lesion did not
meet the criteria for telangiectatic-type extraskeletal
osteosarcoma and was appropriately diagnosed as extraskeletal
osteosarcoma with cystic change. We have never encountered a case
of intraosseous osteosarcoma with a non-hemorrhagic cyst. However,
rare cases of cystic extraskeletal osteosarcoma do exist, as seen
in the present cases. A different entity from intraosseous
osteosarcoma may therefore exist among extraskeletal osteosarcoma
cases.
The clinical prognosis of patients with
extraskeletal osteosarcoma is poor because these patients typically
show high-grade malignancy (1).
Surgical resection is the standard treatment; however,
perioperative chemotherapy may improve the survival (13-15).
Although extraskeletal osteosarcoma is classified as a soft tissue
sarcoma, chemotherapy regimens for osteosarcoma are more effective
than those for soft tissue sarcoma (16,17).
Thus, an accurate pretreatment diagnosis of extraskeletal
osteosarcoma is very important for acquiring a good survival.
Biopsy specimens are essential for differentiating the entity from
benign tumors or other soft tissue sarcomas. The increased
vascularity or arteriovenous malformation that is often seen in
children, adolescents and young adults with a hemangioma (18,19); or
the zonal pattern of peripheral ossification that is typically seen
with myositis ossificans were not observed in our cases (20). MDM2 amplification is useful for
ruling out dedifferentiated liposarcoma with osteogenic
differentiation; recently, however, MDM2 amplification has been
reported in extraskeletal osteosarcomas, and caution is required
when MDM2 amplification is observed (21-23).
Synovial sarcoma sometimes shows similar imaging findings, but the
histological morphology differs from the pleomorphism of
extraskeletal osteosarcoma. Malignant peripheral nerve sheath
tumors are a type of neurogenic malignant tumor and sometimes show
heterotopic differentiation; however, immunohistochemical staining
is positive for neurogenic markers, such as S-100 protein, in most
cases. Case 3 was diagnosed as extraskeletal osteosarcoma based on
the histological analysis of a biopsy specimen, and neoadjuvant
chemotherapy for osteosarcoma was administered. Necrosis was
observed in >95% of the area of the operation specimen. A good
response to chemotherapy is a prognostic factor (24), and Case 3 has achieved a long
survival (>10 years) with a disease-free condition.
Extraskeletal osteosarcoma can show diverse
radiological findings; however, more than half a year had passed
since the patients had first become aware of their tumors, which
may have resulted in relatively specific imaging characteristics.
It is important to consider extraskeletal osteosarcoma as a
differential diagnosis of soft tissue tumors with calcification and
a large cystic lesion, especially in cases with a long clinical
course before consulting a doctor. Larger-scale studies will be
required in order to clarify the clinical implications of this
category of extraskeletal osteosarcoma.
Acknowledgements
The authors would like to thank Higuchi Takashi, Abe
Kensaku, and Asano Youhei (all, Department of Orthopaedic Surgery,
Graduate School of Medical Sciences, Kanazawa University, Kanazawa,
Japan) for assistance in the data collection for the current
study.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HT, NY, KH and AT performed the surgery and managed
the patients postoperatively. TN made a diagnosis of extraskeletal
osteosarcoma with cystic change pathologically. HT, NY, SMi, KI and
TN contributed to the concept and design of the study and to the
acquisition, analysis or interpretation of working data. YT, HY and
SMo assisted in data collection. YA analyzed all the patient's data
and was involved in drafting the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethical Institutional
Review Board of the Kanazawa University Hospital [approval no.
2019-61(3094)], and written informed consent was obtained from all
study participants.
Patient consent for publication
The consent for publication of the manuscript and
the related images from the patients and/or their relatives was
obtained by the Kanazawa University Hospital.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fletcher CDM, Bridge JA, Hogendoorn P and
Mertens F (eds): Extraskeletal osteosarcoma. In: WHO Classification
of Tumours of Soft Tissue and Bone. 4th edition. IARC Press, Lyon.
pp161–162. 2013.
|
|
2
|
Roller LA, Chebib I, Bredella MA and Chang
CY: Clinical, radiological, and pathological features of
extraskeletal osteosarcoma. Skeletal Radiol. 47:1213–1220.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matsuno T, Unni KK, McLeod RA and Dahlin
DC: Telangiectatic osteogenic sarcoma. Cancer. 38:2538–2547.
1976.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mirra JM, Fain JS, Ward WG, Eckardt JJ,
Eilber F and Rosen G: Extraskeletal telangiectatic osteosarcoma.
Cancer. 71:3014–3019. 1993.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dubec JJ, Munk PL, O'Connell JX, Lee MJ,
Janzen D, Connell D, Masri B and Logan PM: Soft tissue osteosarcoma
with telangiectatic features: MR imaging findings in two cases.
Skeletal Radiol. 26:732–736. 1997.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lee KH, Joo JK, Kim DY, Lee JS, Choi C and
Lee JH: Mesentric extraskeletal osteosarcoma with telangiectatic
features: A case report. BMC Cancer. 7(82)2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Healy C, Kahn LB and Kenan S: Subcutaneous
extraskeletal osteosarcoma of the forearm: A case report and review
of the literature. Skeletal Radiol. 45:1307–1311. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Savant D, Kenan S, Kenan S and Kahn L:
Extraskeletal osteosarcoma arising in myositis ossificans: A case
report and review of the literature. Skeletal Radiol. 46:1155–1161.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bane BL, Evans HL, Ro JY, Carrasco CH,
Grignon DJ, Benjamin RS and Ayala AG: Extraskeletal osteosarcoma: A
clinicopathologic review of 26 cases. Cancer. 65:2762–2770.
1990.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Thampi S, Matthay KK, Boscardin WJ,
Goldsby R and DuBois SG: Clinical features and outcomes differ
between skeletal and extraskeletal osteosarcoma. Sarcoma.
2014(902620)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schaefer IM, Cote GM and Hornick JL:
Contemporary sarcoma diagnosis, genetics and genomics. J Clin
Oncol. 36:101–110. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thampi S, Matthay KK, Goldsby R and DuBois
SG: Adverse impact of regional lymph node involvement in
osteosarcoma. Eur J Cancer. 49:3471–3476. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee JS, Fetsch JF, Wasdhal DA, Lee BP,
Pritchard DJ and Nascimento AG: A review of 40 patients with
extraskeletal osteosarcoma. Cancer. 76:2253–2259. 1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ahmad SA, Patel SR, Ballo MT, Baker TP,
Yasko AW, Wang X, Feig BW, Hunt KK, Lin PP, Weber KL, et al:
Extraosseous osteosarcoma: Response to treatment and long term
outcome. J Clin Oncol. 20:521–527. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Choi LE, Healey JH, Kuk D and Brennan MF:
Analysis of outcomes in extraskeletal osteosarcoma: A review of
fifty-three cases. J Bone Joint Surg Am. 96(e2)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Longhi A, Bielack SS, Grimer R, Whelan J,
Windhager R, Leithner A, Gronchi A, Biau D, Jutte P, Krieg AH, et
al: Extraskeletal osteosarcoma: A European musculoskeletal oncology
society study on 266 patients. Eur J Cancer. 74:9–16.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Paludo J, Fritchie K, Haddox CL, Rose PS,
Arndt CAS, Marks RS, Galanis E, Okuno SH and Robinson SI:
Extraskeletal Osteosarcoma: Outcomes and the Role of Chemotherapy.
Am J Clin Oncol. 41:832–837. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wildgruber M, Sadick M, Müller-Wille R and
Wohlgemuth WA: Vascular tumors in infants and adolescents. Insights
Imaging. 10(30)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
DeHart A and Richter G: Hemangioma: Recent
advances. F1000Res 8: F1000 Faculty Rev-1926, 2019.
|
|
20
|
Walczak BE, Johnson CN and Howe BM:
Myositis Ossificans. J Am Acad Orthop Surg. 23:612–622.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sabatier R, Bouvier C, de Pinieux G,
Sarran A, Brenot-Rossi I, Pedeutour F, Chetaille B, Viens P,
Weiller PJ and Bertucci F: Low-grade extraskeletal osteosarcoma of
the chest wall: Case report and review of literature. BMC Cancer.
10(645)2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yamashita K, Kohashi K, Yamada Y, Nishida
Y, Urakawa H, Oda Y and Toyokuni S: Primary extraskeletal
osteosarcoma: A clinicopathological study of 18 cases focusing on
MDM2 amplification status. Hum Pathol. 63:63–69. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Makise N, Sekimizu M, Kubo T, Wakai S,
Watanabe SI, Kato T, Kinoshita T, Hiraoka N, Fukayama M, Kawai A,
et al: Extraskeletal osteosarcoma: MDM2 and H3K27me3 analysis of 19
cases suggest disease heterogeneity. Histopathology. 73:147–156.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Miwa S, Takeuchi A, Ikeda H, Shirai T,
Yamamoto N, Nishida H, Hayashi K, Tanzawa Y, Kimura H, Igarashi K
and Tsuchiya H: Prognostic value of histological response to
chemotherapy in osteosarcoma patients receiving tumor-bearing
frozen autograft. PLoS One. 8(e71362)2013.PubMed/NCBI View Article : Google Scholar
|