Introduction
The lymph node (LN) status largely dictates
prognosis and adjuvant therapy strategy for colorectal cancer
patients. The NCCN guidelines recommend that a minimum of 12 LNs
should be dissected to accurately stage colorectal cancer (1). Even though larger LNs are more prone to
having metastatic deposits, the presence of metastases could also
be identified in small measuring nodes (1 mm, 6.5%; 2 mm, 12.4%;
15.3%, 3 mm) (2). These small LNs
are hard to be found and may lead to downstage of tumors. Some
patients who might benefit from adjuvant therapy were misclassified
as node-negative due to incomplete sampling of LNs (3). Therefore, researchers suggested that
more nodes should be examined to increase the likelihood of proper
staging (3-6).
The number of LNs retrieved from colorectal cancer specimens are
influenced by multiple factors, including age of the patient, sex,
tumor grade, tumor site, neoadjuvant radiotherapy, operating
surgeon and examining pathologist (7-9).
One of the most important reasons is that very small LNs are
difficult to find, especially amid large amounts of
pericolic/perirectal fat.
Various methods mainly concerning fat clearance have
been recommended to increase LN harvest (10-14).
However, these traditional fat clearance techniques are noxious,
time-consuming, costly and troublesome. It cannot be widely used in
clinical practice, only for cases in which few LNs are initially
identified.
Sodium hypochlorite is the most commonly used
irrigating solution in endodontics because of its antimicrobial
effect and tissue dissolution capacity. The antimicrobial activity
is related to bacterial essential enzymatic sites promoting
irreversible inactivation and the chloramination reaction. The
dissolution action can be observed in the saponification reaction
when sodium hypochlorite degrades lipids and fatty acids resulting
in the formation soap and glycerol (15). In this study, we intend to
investigate the use of sodium hypochlorite to clear
pericolic/perirectal fat and improve harvest of LNs.
Materials and methods
Dissolving time of LNs and fat tissue
in different concentrations of sodium hypochlorite
The mesentery is now recognised as an organ composed
by the tissues of vessels, lymphatic, nerve and adipose (16). Since sodium hypochlorite dissolved
organic tissue unselectively, we firstly investigated the
dissolving time of different tissues from mesocolon or mesorectum.
LNs and fat tissue were obtained from fresh colorectal surgical
specimens. LNs diameter (length x width) measuring about 3x2 and
10x5 mm were separately chosen. Because the exposed surface area
had a great impact on the dissolving capability of sodium
hypochlorite, pericolic/perirectal fat were cut to produce samples
of similar size and shape.
Sodium hypochlorite in concentrations of 1, 3 and
5.25% were commercially purchased and respectively tested at room
temperature. Specimens from each group were individually immersed
in plastic specimen bags filled with 50 ml of the test solution,
then placed without mechanical agitation. The time of complete
dissolution of LNs and fat were recorded. These procedures were
repeated 5 times.
Properties of LNs after sodium
hypochlorite treatment
Alterations of the chemical composition of the
dentin have been reported after exposure to sodium hypochlorite
(17). Here, we also focused on
whether sodium hypochlorite would change the properties of LNs. LNs
measuring about 5x5 mm with possible metastases was immersed in 1%
sodium hypochlorite for 30 min and then was fixed in 10% formalin
overnight. Afterwards, 4 µm sections were cut from the
paraffin-embedded blocks for hematoxylin and eosin (H&E)
examination.
Case selection and specimen
treatment
Between January 2018 and June 2018, 65 colorectal
cancer patients who underwent either open or laparoscopic radical
surgery at the Affiliated Cancer Hospital and Institute of
Guangzhou Medical University were included in this study. All cases
were without distant metastases and had not received preoperative
chemoradiotherapy. Patients with recurrent tumors were excluded.
The surgical procedure was conducted according to the standard of
total or complete mesocolic excision.
LNs of these colorectal resection specimens were
firstly harvested by traditional manual gross dissection method.
After standard manual gross dissection, the bulk of the mesentery
was dissected from the bowel wall and tumor. The mesentery
immediately related to the tumour was left in situ since
this was to be examined for gross evidence of circumferential
resection margin. The tumor/bowel was fixed in 10% buffered neutral
formalin solution for 24 h before embedded, whereas the entire
remaining mesenteric tissue was separately immersed in
approximately three times its volume of 1% sodium hypochlorite for
30 min. After the fat was ‘washed’ by sodium hypochlorite, manual
dissection method was again applied for the visible LN harvest. In
order to reduce opportunity for operator bias in the dissection
process, only one experienced staff, S.C., performed dissections
both before and after immersion in sodium hypochlorite. The number
and size of LNs were recorded for each case. All of the LNs were
fixed in 10% buffered neutral formalin as mentioned above. After
paraffin embedding, 4 µm thin sections were cut, stained with
H&E and then scanned for metastases.
In addition, 68 patients who were treated for
colorectal cancer from July 2017 to December 2017 were identified
from our collected database. All of these patients were neither
with distant metastases nor given chemoradiotherapy before radical
operation. Patients with recurrent tumors were also excluded. The
manual dissection method without fat clearance was applied to the
surgically removed specimens for LN harvest. For these selected
patients, the number but not the size of LNs had been recorded.
The present study protocol was approved by The
Affiliated Cancer Hospital and Institute of Guangzhou Medical
University Ethical Committee. All selected patients provided
written statements of their informed consent and the Ethical
approval was granted.
Statistical analysis
Statistical analysis was conducted using the
statistical software SPSS 17.0 (SPSS, Inc.). Measurement data were
compared by the independent-samples t-test or the one-way ANOVA
followed by Student-Newman-Keuls post hoc test. The associations
between categorical data were performed using the χ2
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Sodium hypochlorite dissolved fat
tissue significantly faster than LNs
The complete dissolving time of small fat tissue
(3x2 mm) in 1, 3 and 5.25% sodium hypochlorite was 13.6±1.1,
6.8±0.83 and 5.6±0.54 min respectively, while it was 120.6±5.6,
48.0±1.8 and 29.0±3.8 min respectively for small LNs (Table I). Moreover, the complete dissolving
time of bigger fat tissue (10x5 mm) in 1, 3 and 5.25% sodium
hypochlorite was 27.0±4.0, 18.6±1.1 and 14.8±0.83 min, while it was
474.0±19.4, 118.0±8.3 and 97.0±6.7 min for bigger LNs (Table II). These results indicated that
sodium hypochlorite dissolved fat tissue significantly faster than
LNs (P<0.001).
 | Table IDissolving time of lymph nodes and fat
tissue in different concentrations of sodium hypochlorite
(min). |
Table I
Dissolving time of lymph nodes and fat
tissue in different concentrations of sodium hypochlorite
(min).
| Different
concentrations of sodium hypochlorite | Lymph node, mean ± SD
(n=5) | Fat tissue, mean ± SD
(n=5) | P-valuea |
|---|
| 3x2 mm | | | |
|
1% Sodium
hypochlorite | 120.6±5.6 | 13.6±1.1 | <0.001 |
|
3% Sodium
hypochlorite | 48.0±1.8 | 6.8±0.83 | <0.001 |
|
5.25% Sodium
hypochlorite | 29.0±3.8 | 5.6±0.54 | <0.001 |
|
P-valueb | <0.001 | <0.001 | |
| 10x5 mm | | | |
|
1% Sodium
hypochlorite | 474.0±19.4 | 27.0±4.0 | <0.001 |
|
3% Sodium
hypochlorite | 118.0±8.3 | 18.6±1.1 | <0.001 |
|
5.25% Sodium
hypochlorite | 97.0±6.7 | 14.8±0.83 | <0.001 |
|
P-valueb | <0.001 | <0.001 | |
 | Table IIClinicopathological features of
patients included. |
Table II
Clinicopathological features of
patients included.
| Variables | Sodium hypochlorite
group (n=65) | Manual method group
(n=68) | P-value |
|---|
| Sex | | | 0.929 |
|
Male | 32 (49.2%) | 34 (50.0%) | |
|
Female | 33 (50.8%) | 34 (50.0%) | |
| Age, years | 56.6±11.3 | 56.2±11.8 | 0.568 |
| BMI | 21.2±4.7 | 20.7±4.6 | 0.851 |
| Tumor size, cm | 4.3±1.8 | 4.1±1.6 | 0.414 |
| Location | | | 0.832 |
|
Right
colon | 16 (24.6%) | 20 (29.4%) | |
|
Transverse
colon | 3 (4.6%) | 4 (5.9%) | |
|
Left
colon | 6 (9.2%) | 9 (13.2%) | |
|
Sigmoid
colon | 20 (30.8%) | 17 (25.0%) | |
|
Rectum | 20 (30.8%) | 18 (26.5%) | |
| Histologic
type | | | 0.651 |
|
Well
differentiated adenocarcinoma | 27 (41.5%) | 23 (33.8%) | |
|
Moderately
differentiated adenocarcinoma | 24 (36.9%) | 29 (42.6%) | |
|
Poorly
differentiated adenocarcinoma | 14 (21.5%) | 16 (23.5%) | |
| T stage | | | 0.818 |
|
T1 | 5 (7.7%) | 5 (7.4%) | |
|
T2 | 13 (20.0%) | 16 (23.5%) | |
|
T3 | 23 (35.4%) | 19 (27.9%) | |
|
T4 | 24 (36.9%) | 28 (41.2%) | |
| N stage | | | 0.166 |
|
N0 | 14 (21.5%) | 16 (23.5%) | |
|
N1 | 24 (36.9%) | 34 (50.0%) | |
|
N2 | 27 (41.5%) | 18 (26.5%) | |
| Total lymph node
harvest | 28.2±12.1 | 16.5±8.7 | 0.010 |
| Positive lymph node
harvest | 3.0±2.3 | 2.3±2.1 | 0.181 |
Though high concentration sodium hypochlorite
dissolved fat tissue and LNs more quickly than low concentration
sodium hypochlorite, the low concentration sodium hypochlorite had
the max dissolving time difference between fat tissue and LNs
(P<0.001, Table I). So 1% sodium
hypochlorite was chosen for further study in order to avoid rapid
destruction of LNs.
Sodium hypochlorite would not change
the properties of LNs
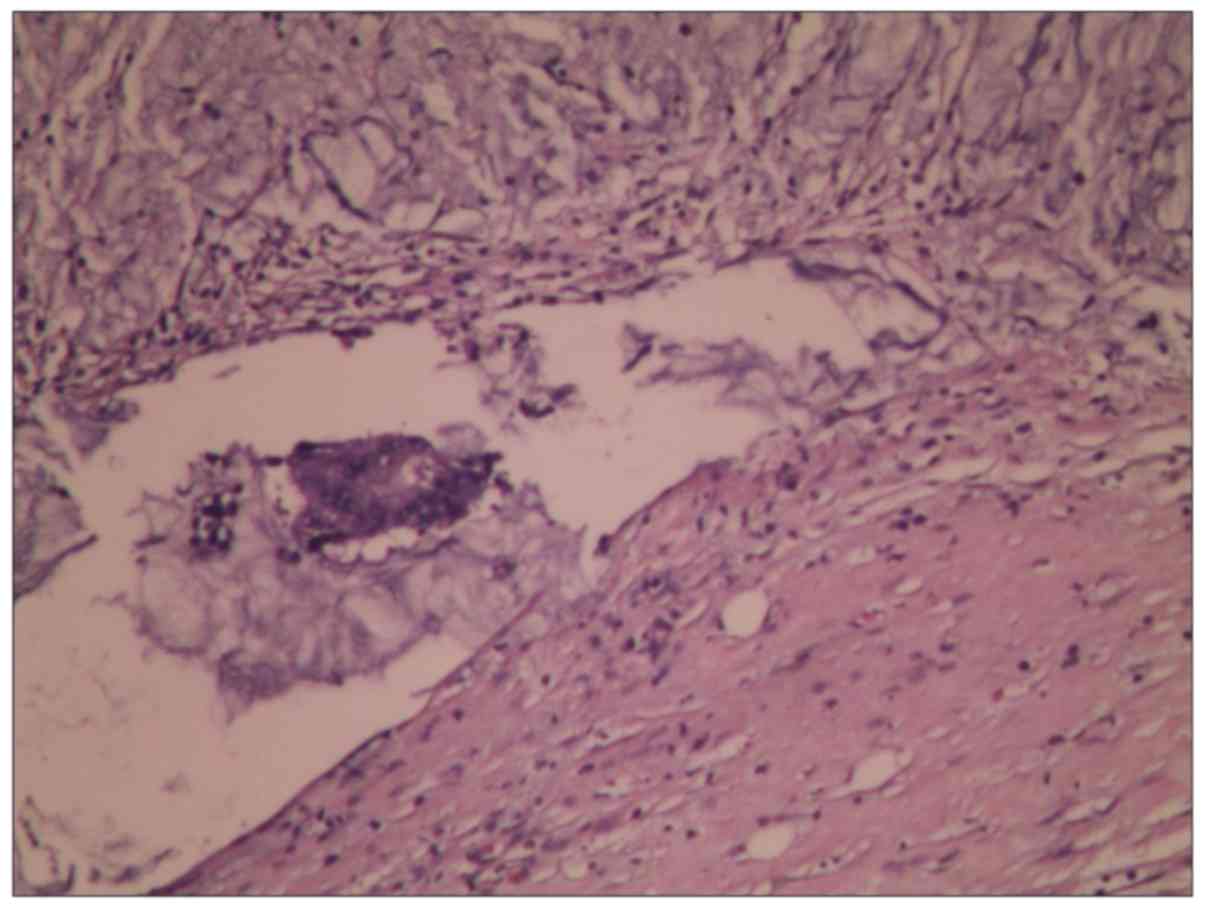
H&E staining examination showed that LNs
immersed in 1% sodium hypochlorite would not change their
biological characteristics in a short period of time (Fig. 1).
Sodium hypochlorite significantly
improved LN harvest
Clinicopathological details in relation to the two
different dissection methods were summarized in Table II. There were no significant
differences in sex, age, body mass index (BMI), tumour size or
tumour location between the two groups (P>0.05). Moreover, no
significant association was found when histologic types, T and N
stage were taken into account (P>0.05). The very small LNs
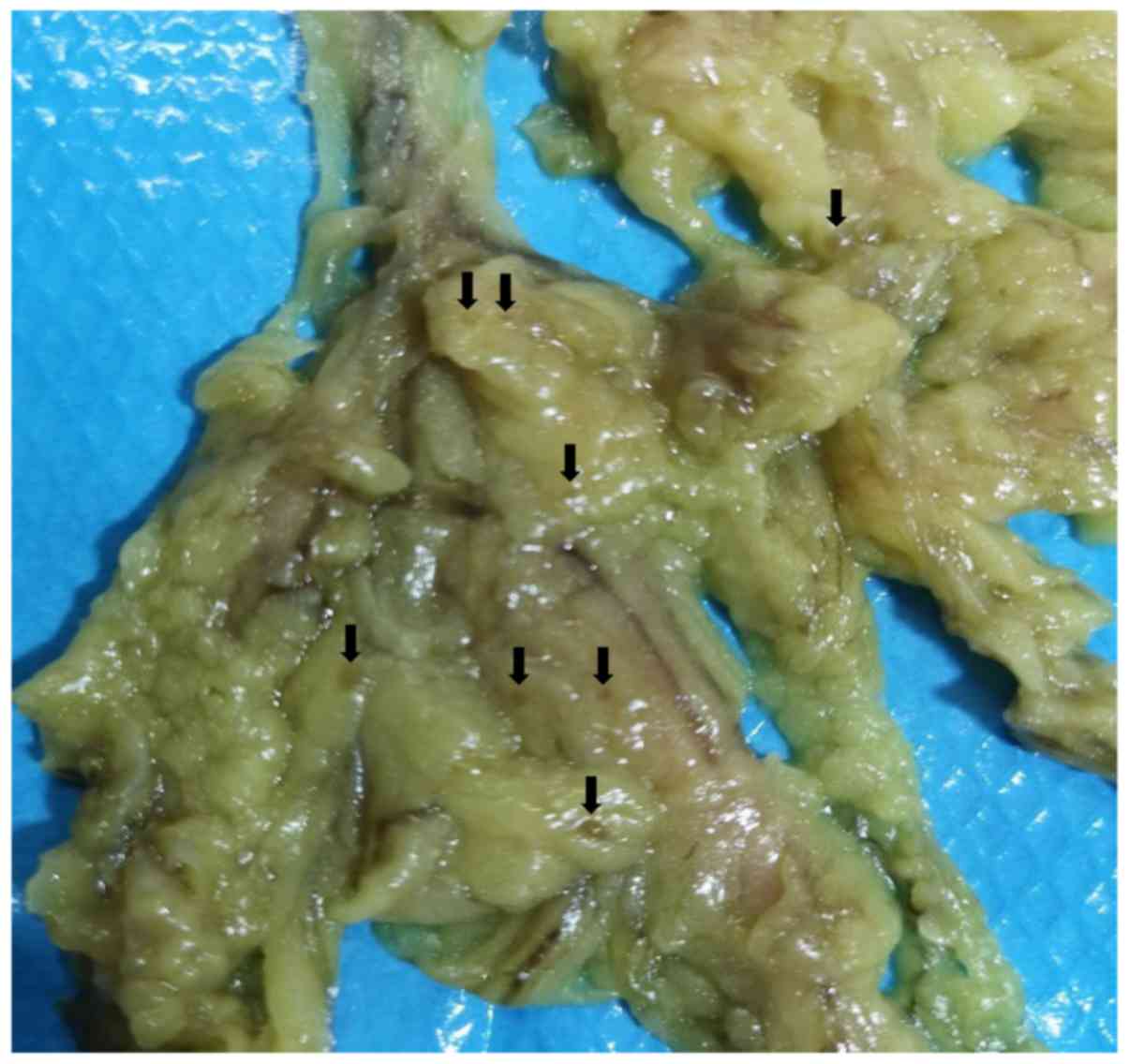
became easily visible after sodium hypochlorite treatment (Fig. 2). When the total number of LNs was
compared, the sodium hypochlorite group increased more LN harvest
than manual method group (28.2±12.1 vs. 16.5±8.7, P=0.010).
However, the number of positive LNs did not increase in the sodium
hypochlorite group when compared with manual method group
(P=0.181).
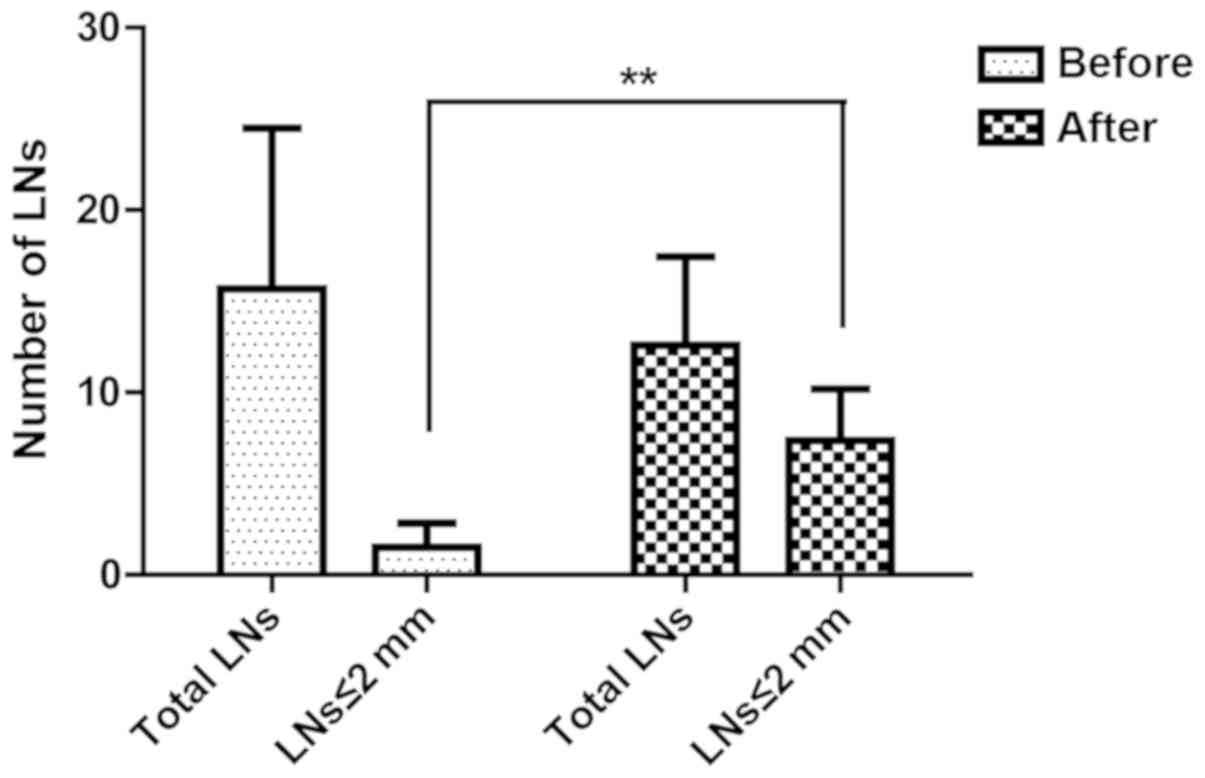
Within the sodium hypochlorite group, a total of
1,020 LNs (15.6±8.7 per case) were revealed in initial inspection.
After treated in sodium hypochlorite for 30 min, 818 additional LNs
(12.5±4.8 per case) were identified, which account for 44.5% of all
the LNs revealed in this group (Fig.
3). LNs ≤2 mm were 9.6% (98/1,020) and 58.4% (478/818)
separately before and after sodium hypochlorite treatment. Use of
sodium hypochlorite method could obviously increase LN harvest,
especially those smaller than 2 mm (Fig.
3).
After treated with sodium hypochlorite, 16
additional metastatic LNs were found in 10 patients. Seven of the
10 patients were upstaged, including 2 initially N0 cases (Table III).
 | Table IIIChanges in the staging of 10 patients
after sodium hypochlorite treatment. |
Table III
Changes in the staging of 10 patients
after sodium hypochlorite treatment.
| Case | Number of LNs (+)
before sodium hypochlorite treatment | Initial N
stage | Number of extra LNs
(+) after sodium hypochlorite treatment | Final N stage |
|---|
| 1 | 3 | N1b | 1 | N2a |
| 2 | 3 | N1b | 3 | N2a |
| 3 | 4 | N2a | 1 | N2a |
| 4 | 0 | N0 | 1 | N1a |
| 5 | 0 | N0 | 2 | N1b |
| 6 | 6 | N2a | 2 | N2b |
| 7 | 6 | N2a | 1 | N2b |
| 8 | 5 | N2a | 2 | N2b |
| 9 | 4 | N2a | 2 | N2a |
| 10 | 5 | N2a | 1 | N2a |
Discussion
LN size may not be a reliable indicator for LN
metastasis. Studies had showed that most nodal metastases in
colorectal cancer were found in small LNs (5 mm or less in
diameter) which were often missed during routine dissection of
specimens (2,18-21).
Brown et al even reported that 75% of all positive nodes
were under 2 mm in size (6).
Furthermore, the total number of LNs investigated was considered an
independent risk factor no matter metastases were present or not
(22). Thus, the detection of the
largest possible number of LNs and a complete pathologic assessment
of nodal status are essential for accurate staging, therapeutic
decisions, and prognosis of patients (3,23).
Manual LN dissection is the current standard method
at most institutions and it depends on visual isolation and
palpation of the mesenteric tissue. It is certainly that smaller
LNs could be found using manual techniques by experienced personnel
who take appropriate time. Sampling the entire pericolic fat is
theoretically the most accurate method for total lymph node
examination. However, very small lymph nodes are rarely found and
it requires time, money and is not practical in routine surgical
pathology (11,24,25). So,
many researchers suggested that additional techniques combined with
manual dissection could help to increase small LN harvest more
easily. Fat clearance method has been recently recommended for the
detection of small or inconspicuous LNs. However, this technique
requires the use of sequential immersions of the mesenteric tissue
in baths of alcohol, xylene, followed by methyl salicylate. These
solutions are noxious and the fat has to be dissected in a
ventilated cabinet using transillumination to locate the LNs. In
addition, the clearance process takes dozens of hours or even weeks
and causes an unacceptable reporting delay (10,26).
Furthermore, serial sectioning of the cleared mesenteric tissue at
1-2 mm intervals is necessary to reveal small LNs. Nodes may be
probably cut into 2 halves during multilevel step sectioning
process, resulting in error of LN count. The disadvantages of fat
clearance precludes its use in routine pathological assessment,
only for cases in which an unacceptably low number of LNs are
retrieved (27).
Other techniques such as LN revealing solution
(11,28), sentinel node procedures with
methylene blue injection (29), and
fat-dissociation method using enzymes (25) are applied to increase the yield of
LNs. However, these methods are not used routinely largely because
they are hazardous, time-consuming and expensive and do not provide
rapid pathology report.
Sodium hypochlorite is still the most commonly used
irrigation solution for endodontic procedures because of their
characteristics such as wide-spectrum antimicrobial activity and
organic tissue dissolution capacity (17). The tissue-dissolving capability of
sodium hypochlorite relies on its concentration, volume, contact
time of the solution, the surface area of the exposed tissue, pH,
temperature, and mechanical agitation (30,31).
Here, we showed that sodium hypochlorite could easily dissolve fat
tissue when compared with LNs even in a low concentration at room
temperature without agitation.
High concentrations may be more toxic and dissolve
the whole mesenteric tissue with no dissolving time difference,
while the low concentration sodium hypochlorite has the maximum
dissolving time difference between fat tissue and LNs. In addition,
H&E examination indicated that low concentration sodium
hypochlorite avoided changes in tissue composition of LNs. This
study found that the use of sodium hypochlorite at lower
concentration, such as 1%, demonstrated to be effective in
promoting a suitable dissolution of mesenteric fat and preventing a
pronounced damage to LNs.
We had showed that 1% sodium hypochlorite had the
maximum dissolving time difference between fat tissue and LNs, and
the complete dissolving time of bigger fat tissue (10x5 mm) in 1%
sodium hypochlorite was about 30 min (27.0±4.0 min, Table I) without change in the properties of
LNs. So, we considered that 30 min would be the appropriate
processing time. After 1% sodium hypochlorite immersion for about
30 min, the perienteric adipose tissue was cleared and the LNs were
easily visible. The sodium hypochlorite group detected more LNs in
total than manual dissection method group. More LNs harvest means
more accurate pathological staging. Though the number of positive
LNs did not significantly increase after sodium hypochlorite
treatment, this method is still meaningful because it is not a
question about ‘significant statistical difference’ but a question
about ‘have or have no’. Even a single additional LN metastasis
identified will be sufficient to upstage the malignancy from pN0 to
pN1 when adjuvant therapy is required.
Furthermore, 818 additional LNs were revealed after
sodium hypochlorite treatment, which account for 44.5% of all the
LNs found in sodium hypochlorite group. In line with other
assistant techniques (6,32), sodium hypochlorite solution was
greatly helpful in detecting small LNs and most of these additional
LNs (58.4%) were smaller than 2 mm. Besides, 16 additional
metastatic LNs were found in 10 cases after sodium hypochlorite
treatment and the stage of the disease was upgraded in 7 of the 10
cases. More importantly, additional metastatic LNs had been found
in 2 initially pN0 cases, resulting in upstaging from TNM stage II
to stage III, implying that postoperative adjuvant chemotherapy had
to be given.
However, there were still some limits in our
research. The sample number of this study was small. Also, because
it was a retrospective study, the size of lymph nodes for the
control group had not been recorded before and the data cannot be
gathered. In order to reduce opportunity for operator bias in the
dissection process, only one experienced person performed
dissections both before and after immersion in sodium
hypochlorite.
For the first time, this study indicates that sodium
hypochlorite at low concentration can be used to reveal LNs in
colorectal carcinoma specimens. As we know, it may be the most
simple, rapid (30 min), cost-saving (US $1.0), nontoxic and
effective method to improve LN harvest so far. We suggest that
sodium hypochlorite after manual dissection should be used
routinely regardless of whether the number of LNs is less than 12
or not.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NY and HL conceived and designed the present study,
and gave final approval. JL and SC contributed to the data analysis
and interpretation. SC was responsible for provision of the study
material and wrote the manuscript. All of the authors revised the
manuscript critically. All authors read and approved the final
draft of the manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by The
Affiliated Cancer Hospital and Institute of Guangzhou Medical
University Ethical Committee. All selected patients provided
written statements of their informed consent and the Εthical
approval was granted.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benson AB III, Venook AP, Cederquist L,
Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC,
Fichera A, et al: Colon cancer, version 1.2017, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw.
15:370–398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Murphy J, O'Sullivan GC and Fitzgibbon J:
The effect of node size on the detection of nodal metastases in
Dukes C rectal carcinoma. Gut. 44(A84)1999.
|
|
3
|
Tepper JE, O'Connell MJ, Niedzwiecki D,
Hollis D, Compton C, Benson AB III, Cummings B, Gunderson L,
Macdonald JS and Mayer RJ: Impact of number of nodes retrieved on
outcome in patients with rectal cancer. J Clin Oncol. 19:157–163.
2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Goldstein NS, Sanford W, Coffey M and
Layfield LJ: Lymph node recovery from colorectal resection
specimens removed for adenocarcinoma. Trends over time and a
recommendation for a minimum number of lymph nodes to be recovered.
Am J Clin Pathol. 106:209–216. 1996.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim YW, Kim NK, Min BS, Lee KY, Sohn SK
and Cho CH: The influence of the number of retrieved lymph nodes on
staging and survival in patients with stage II and III rectal
cancer undergoing tumor-specific mesorectal excision. Ann Surg.
249:965–972. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brown HG, Luckasevic TM, Medich DS,
Celebrezze JP and Jones SM: Efficacy of manual dissection of lymph
nodes in colon cancer resections. Mod Pathol. 17:402–406.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sarli L, Bader G, Iusco D, Salvemini C,
Mauro DD, Mazzeo A, Regina G and Roncoroni L: Number of lymph nodes
examined and prognosis of TNM stage II colorectal cancer. Eur J
Cancer. 41:272–279. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Valsecchi ME, Leighton J Jr and Tester W:
Modifiable factors that influence colon cancer lymph node sampling
and examination. Clin Colorectal Cancer. 9:162–167. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Baxter NN, Morris AM, Rothenberger DA and
Tepper JE: Impact of preoperative radiation for rectal cancer on
subsequent lymph node evaluation: A population-based analysis. Int
J Radiat Oncol Biol Phys. 61:426–431. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Scott KW and Grace RH: Detection of lymph
node metastases in colorectal carcinoma before and after fat
clearance. Br J Surg. 76:1165–1167. 1989.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Koren R, Siegal A, Klein B, Halpern M,
Kyzer S, Veltman V and Gal R: Lymph node-revealing solution: Simple
new method for detecting minute lymph nodes in colon carcinoma. Dis
Colon Rectum. 40:407–410. 1997.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang H, Safar B, Wexner SD, Denoya P and
Berho M: The clinical significance of fat clearance lymph node
harvest for invasive rectal adenocarcinoma following neoadjuvant
therapy. Dis Colon Rectum. 52:1767–1773. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Newell KJ, Sawka BW, Rudrick BF and Driman
DK: GEWF solution. Arch Pathol Lab Med. 125:642–645.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Prabhudesai AG, Dalton R, Kumar D and
Finlayson CJ: Mechanised one-day fat clearance method to increase
the lymph node yield in rectal cancer specimens. Br J Biomed Sci.
62:120–123. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Estrela C, Estrela CR, Barbin EL, Spano
JC, Marchesan MA and Pecora JD: Mechanism of action of sodium
hypochlorite. Braz Dent J. 13:113–117. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Coffey JC and O'Leary DP: The mesentery:
Structure, function, and role in disease. Lancet Gastroenterol
Hepatol. 1:238–247. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tartari T, Bachmann L, Maliza AG, Andrade
FB, Duarte MA and Bramante CM: Tissue dissolution and modifications
in dentin composition by different sodium hypochlorite
concentrations. J Appl Oral Sci. 24:291–298. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Herrera-Ornelas L, Justiniano J, Castillo
N, Petrelli NJ, Stulc JP and Mittelman A: Metastases in small lymph
nodes from colon cancer. Arch Surg. 122:1253–1256. 1987.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rodriguez-Bigas MA, Maamoun S, Weber TK,
Penetrante RB, Blumenson LE and Petrelli NJ: Clinical significance
of colorectal cancer: Metastases in lymph nodes <5 mm in size.
Ann Surg Oncol. 3:124–130. 1996.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Haboubi NY, Abdalla SA, Amini S, Clark P,
Dougal M, Dube A and Schofield P: The novel combination of fat
clearance and immunohistochemistry improves prediction of the
outcome of patients with colorectal carcinomas: A preliminary
study. Int J Colorectal Dis. 13:99–102. 1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Monig SP, Baldus SE, Zirbes TK, Schröder
W, Lindemann DG, Dienes HP and Hölscher AH: Lymph node size and
metastatic infiltration in colon cancer. Ann Surg Oncol. 6:579–581.
1999.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Le Voyer TE, Sigurdson ER, Hanlon AL,
Mayer RJ, Macdonald JS, Catalano PJ and Haller DG: Colon cancer
survival is associated with increasing number of lymph nodes
analyzed: A secondary survey of intergroup trial INT-0089. J Clin
Oncol. 21:2912–2919. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Washington MK: Colorectal carcinoma:
Selected issues in pathologic examination and staging and
determination of prognostic factors. Arch Pathol Lab Med.
132:1600–1607. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim YM, Suh JH, Cha HJ, Jang SJ, Kim MJ,
Yoon S, Kim B, Chang H, Kwon Y, Hong EK and Ro JY: Additional lymph
node examination from entire submission of residual mesenteric
tissue in colorectal cancer specimens may not add clinical and
pathologic relevance. Hum Pathol. 38:762–767. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fujino S, Miyoshi N, Ohue M, Noura S,
Tomita Y, Yano M and Sakon M: New enhanced and effective method for
staging cancer to detect lymph nodes after fat-dissociation. Oncol
Rep. 32:922–926. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Haboubi NY, Clark P, Kaftan SM and
Schofield PF: The importance of combining xylene clearance and
immunohistochemistry in the accurate staging of colorectal
carcinoma. J R Soc Med. 85:386–388. 1992.PubMed/NCBI
|
|
27
|
Compton CC, Fielding LP, Burgart LJ,
Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV,
Nagle RB, et al: Prognostic factors in colorectal cancer. College
of American pathologists consensus statement 1999. Arch Pathol Lab
Med. 124:979–994. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vogel C, Kirtil T, Oellig F and Stolte M:
Lymph node preparation in resected colorectal carcinoma specimens
employing the acetone clearing method. Pathol Res Pract. 204:11–15.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Markl B, Kerwel TG, Wagner T, Anthuber M
and Arnholdt HM: Methylene blue injection into the rectal artery as
a simple method to improve lymph node harvest in rectal cancer. Mod
Pathol. 20:797–801. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Moorer WR and Wesselink PR: Factors
promoting the tissue dissolving capability of sodium hypochlorite.
Int Endod J. 15:187–196. 1982.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Christensen CE, McNeal SF and Eleazer P:
Effect of lowering the pH of sodium hypochlorite on dissolving
tissue in vitro. J Endod. 34:449–452. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ma XL, Ye JX, Su J, Qi FF, Meng QY and Shi
XY: A modified GEWF solution is cost-saving and effective for lymph
node retrieval in resected colorectal carcinoma specimens. Pathol
Res Pract. 210:543–547. 2014.PubMed/NCBI View Article : Google Scholar
|