Introduction
Radiotherapy (RT) is a well-established treatment
modality for patients with early laryngeal carcinoma; however,
laser therapy and partial laryngectomy may also be used to
definitively treat early laryngeal carcinomas (1-3).
The goals of treatment are cancer cure, preservation of the vocal
cords with acceptable voice quality, and minimal treatment-related
mortality. Definitive RT may achieve all these goals in the
majority of patients with early laryngeal carcinoma, and salvage
laryngectomy may be effective in cases of relapse. The local
control rate for patients with early laryngeal carcinoma who
undergo salvage laryngectomy for recurrence after initial RT is
90-100% (4-9).
Laryngeal carcinoma is classified into glottic,
supraglottic and subglottic types according to the place of origin,
with glottic carcinomas being the most common (70%). The majority
of glottic carcinomas are at an early stage and account for ~70% of
all cases. The most commonly used dose-fractionation schedule for
T1 glottic carcinoma is 66 Gy/33 fractions. The local control rate
for T1N0 glottic carcinoma treated with conventional fractionation
is 80-90% (8,10-12).
Thus, RT alone results in an adequate local control rate for T1
glottic lesions, with a low incidence rate of severe complications.
However, some patients may experience local failure. The local
control rate for T1 glottic carcinoma may be improved by
identifying the risk factors for local failure. Therefore, the aim
of the present study was to retrospectively investigate the risk
factors of local failure in patients with T1 glottic carcinoma
irradiated with a prescription dose of 66 Gy.
Patients and methods
Patients
Between July 2006 and December 2017, 69 consecutive
patients with early (T1) glottic squamous cell carcinoma were
treated with definitive RT. All patients provided written informed
consent, and the study was approved by the Ethics Review Board of
the Tokyo Medical University Hospital (Tokyo, Japan). Among the 69
patients, 64 who underwent irradiation with a dose-fractionation
schedule of 66 Gy/33 fractions were selected for the retrospective
analysis. The characteristics of the 64 patients are listed in
Table I. Tumor stage was defined
according to the 2016 TNM classification (13) (8th edition, International Union
Against Cancer). Of the 64 patients, 57 were men and 7 were women.
The median patient age was 72 years (range, 47-86 years). A total
of 98% of the patients had an Eastern Cooperative Oncology Group
performance status score of 0 or 1. The primary tumor stage was T1a
in 43 and T1b in 21 patients. None of the patients had clinical
neck or distant metastasis. Among the 64 patients, 55 experienced
hoarseness. In addition, 15 patients (23%) had double cancers, 4
(6%) had triple cancers, and 2 (3%) had quadruple cancers,
including the glottic tumor.
 | Table IPatient and tumor characteristics
(n=64). |
Table I
Patient and tumor characteristics
(n=64).
| Characteristics | No. (%) |
|---|
| Sex | |
|
Male | 57(89) |
|
Female | 7(11) |
| Age, years [median
(range)] | 72 (47-86) |
| Performance status
score | |
|
0 | 60 |
|
1 | 3 |
|
2 | 0 |
|
3 | 1 |
| Stage of primary
tumors | |
|
T1a | 43(67) |
|
T1b | 21(33) |
| Smoking during/after
treatment | |
|
Yes | 28(44) |
|
No | 36(56) |
| Anterior commissure
involvement by tumor | |
|
Yes | 23(36) |
|
No | 41(64) |
| Histological
grade | |
|
Well-differentiated | 45(70) |
|
Moderately/poorly
differentiated | 19(30) |
| Pretreatment
hemoglobin level, g/dl | |
|
≤14 | 25(39) |
|
>14 | 39(61) |
RT
Three-dimensional RT was planned and performed using
a shell with the patient placed in the supine position. For
treatment planning, all patients underwent cervical computed
tomography (CT) with a 2.5 mm slice thickness. Treatment planning
was performed using the Eclipse™ (Varian Medical Systems) treatment
planning system. The standard RT technique involved parallel
opposing lateral fields using photons of 4-MV X-rays for all
patients over 5 days per week. The volume of the glottic larynx was
defined as the vocal cord and was contoured by a single radiation
oncologist. The gross tumor volume was defined based on endoscopy
findings. However, it was not delineated in the present study,
owing to non-visualization on CT and magnetic resonance imaging.
The clinical target volume (CTV) encompassed the glottis,
subglottis and part of the supraglottis; cranially and anteriorly,
the CTV extended to the thyroid notch at the level of the vocal
process of the arytenoid cartilage, and caudally and posteriorly it
extended to the middle of the cricoid cartilage. A 5 mm isotropic
expansion of the CTV provided the planning target volume (PTV). A
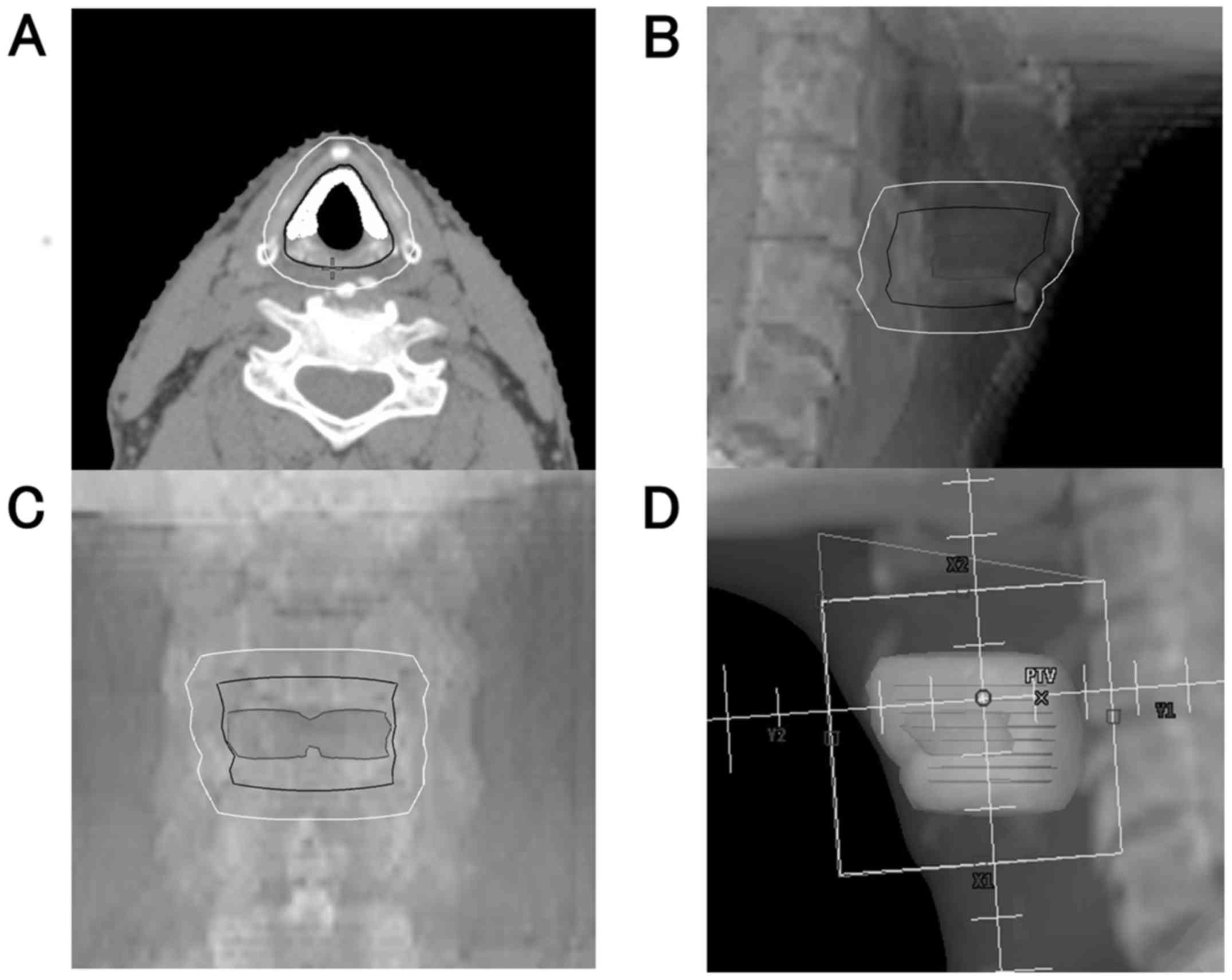
typical contouring of the target volume and beam's eye view are
shown in Fig. 1. Irradiation was
delivered via local portals (mostly 5-6x5-6 cm) covering only the
primary lesion. The cervical lymph nodes were not electively
treated. The dose and fractionation for all patients was 66 Gy/33
fractions delivered over 6.6 weeks.
Evaluation of local response and
adverse effects
The local response was evaluated by laryngoscopy at
1 month after completion of RT. In the absence of clinical
symptoms, regular follow-up visits were performed at 2-3-month
intervals for the first 2 years, and every 4-6 months thereafter.
At each follow-up visit, the evaluation included laryngoscopy,
medical history taking, physical examination, CT, and tumor marker
assessment. The data pertaining to adverse effects were collected
retrospectively from patient files. Local failure was considered to
occur when local recurrence developed after an initial complete
response. The Common Terminology Criteria for Adverse Events
(14), version 3.0 (CTCAE v3.0) were
used for evaluating the acute and late effects of RT.
Risk factors for local failure
The following factors were investigated to determine
the clinical risk factors for local failure: Sex, age, performance
status, T stage, overall treatment time (OTT), anterior commissure
involvement (ACI), smoking status during/after treatment,
histological tumor grade, and pretreatment hemoglobin levels. The
pretreatment hemoglobin level was measured within 1 month prior to
the initiation of RT. The maximum, mean and minimum doses and the
homogeneity index (HI) for the glottic larynx, CTV and PTV were
evaluated as dosimetric risk factors for local failure. The HI was
calculated as the maximum dose divided by the minimum dose to the
target volume (15).
Statistical analysis
The endpoint was local control, calculated from the
first date of RT. The associations between local failure and the
clinical factors were calculated using the Fisher's exact
probability test. The associations between local failure and
dosimetric factors were analyzed using the Mann-Whitney U test. The
local control rate was plotted using the Kaplan-Meier method, with
statistical significance assessed by the log-rank test. Univariate
logistic regression analyses were performed to evaluate the data
using SPSS 20.0 (IBM Corp.). Differences with P-values <0.05
were considered statistically significant.
Results
Local control and overall
survival
The median follow-up duration was 51 months (range,
4-132 months). All patients with local failure of the primary
lesion treatment who were successfully salvaged by surgery were
considered to have had local failure with RT. The overall survival
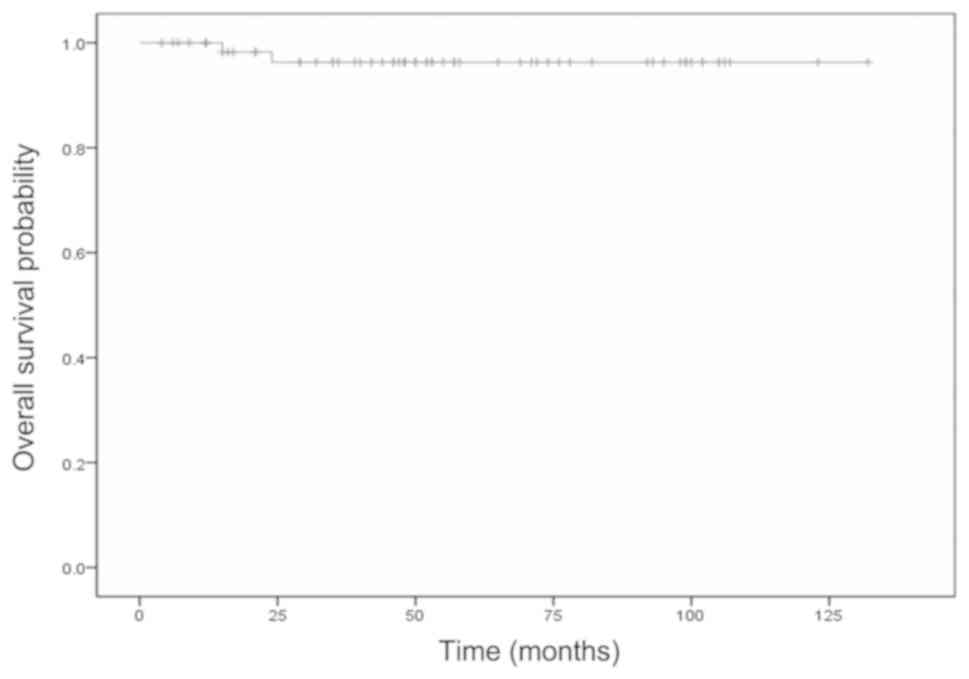
and local control curves are shown in Figs. 2 and 3. The 5-year overall survival rate was 96%,
and 2 (3.1%) of the 64 cases died from gastric cancer and
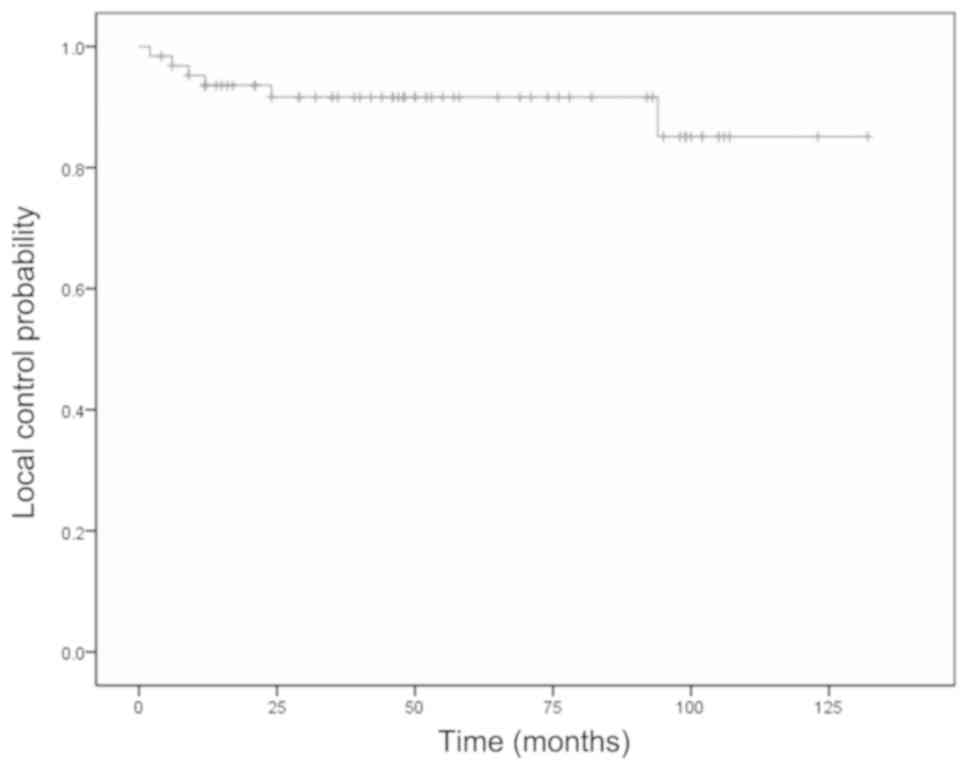
pneumonia. The 5-year local control rate was 92%, and local failure
was observed in 6 (9.5%) of the 64 cases; local failure alone
occurred in 5 patients, whereas local failure and neck metastasis
occurred in 1 patient. The median time for local failure was 12
months (range, 2-94 months) after the start of RT.
The associations between the clinical factors and
local failure are summarized in Table
II. No factor exhibited a significant association. Multivariate
analysis was not performed owing to the limited data. The
associations between the dosimetric factors and local failure in
all the patients are shown in Table
III. On univariate analysis, the minimum dose to the glottic
larynx, calculated using Mann-Whitney U test, was the only factor
significantly associated with the occurrence of local failure
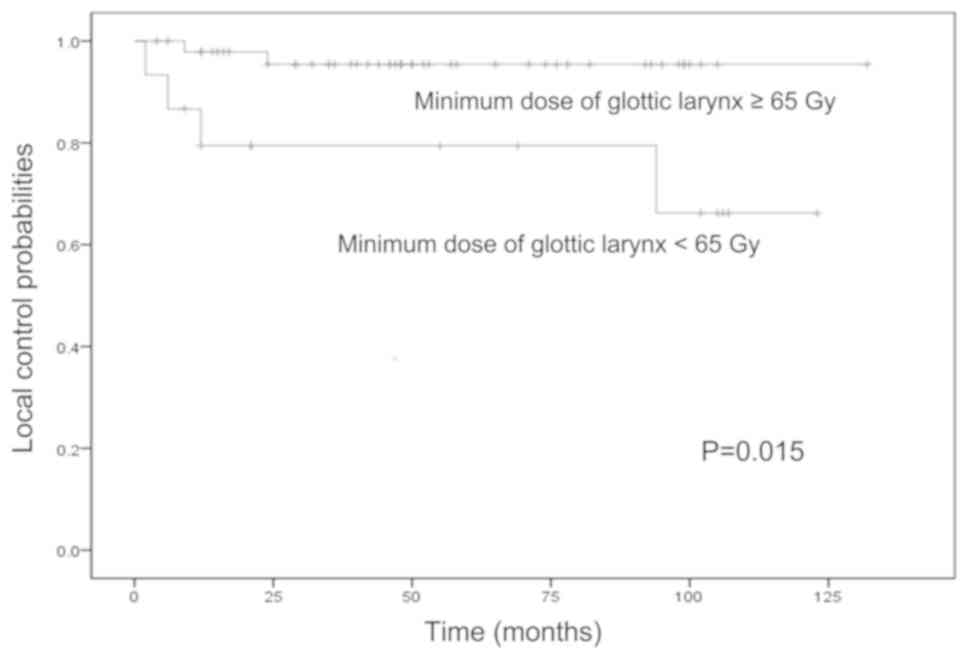
(P=0.025). The median minimum dose to the glottic larynx was ~65
Gy. The 5-year local control rates for patients with minimum doses
to the glottic larynx of <65 and ≥65 Gy were 79 and 95%,
respectively (Fig. 4). The
difference in the local control rate between patients who received
<65 and ≥65 Gy as the minimum dose to the glottic larynx,
calculated using the log-rank test, was statistically significant
(P=0.015).
 | Table IIClinical risk factors associated with
local failure. |
Table II
Clinical risk factors associated with
local failure.
| | Univariate
analysis |
|---|
| Risk factors | Local failure,
n=6 | P-value | Hazard ratio (95%
CI) |
|---|
| Sex (male vs.
female) | 11% (6/57) vs. 0%
(0/7) | >0.999 | Uncomputable |
| Age, years (<75
vs. ≥75) | 10% (4/39) vs. 8%
(2/25) | >0.999 | 0.837
(0.086-8.106) |
| PS score (0 vs.
≥1) | 10% (6/60) vs. 0%
(0/4) | >0.999 | Uncomputable |
| T stage (T1a vs.
T1b) | 12% (5/43) vs. 5%
(1/21) | 0.654 | 3.481
(0.282-42.978) |
| OTT (≤49 vs.
>49) | 11% (6/56) vs. 0%
(0/8) | >0.999 | Uncomputable |
| ACI (yes vs. no) | 9% (2/23) vs. 10%
(4/41) | >0.999 | 1.622
(0.231-11.395) |
| Smoking during/after
treatment (yes vs. no) | 11% (3/28) vs. 8%
(3/36) | >0.999 | 1.109
(0.141-8.708) |
| Histological tumor
grade (well vs. moderately/poorly differentiated) | 7% (3/45) vs. 16%
(3/19) | 0.351 | 0.567
(0.099-3.251) |
| Pretreatment
hemoglobin level, g/dl (≤14 vs. >14) | 4% (1/25) vs. 13%
(5/39) | 0.391 | 1.273
(0.107-15.196) |
 | Table IIIAssociation between dosimetric factors
and local failure. |
Table III
Association between dosimetric factors
and local failure.
| | Local failure | |
|---|
| Dose, Gy | Yes | No | P-value |
|---|
| Glottic larynx |
|
Max
dose | 69.0 (66.6-71.8) | 69.0 (66.6-71.8) | 0.613 |
|
Mean
dose | 66.7 (65.6-68.4) | 67.1 (64.9-69.9) | 0.478 |
|
Min
dose | 64.6 (64.2-65.4) | 65.6
(62.7-69.4) | 0.025 |
|
HI | 1.06
(1.02-1.09) | 1.04
(1.00-1.08) | 0.053 |
| CTV |
|
Max
dose | 68.8
(66.2-71.1) | 68.8
(66.5-71.8) | 0.920 |
|
Mean
dose | 65.7
(65.0-66.9) | 65.8
(64.6-68.9) | 0.506 |
|
Min
dose | 62.3
(61.1-62.7) | 62.1
(52.1-66.6) | 0.728 |
|
HI | 1.11
(1.06-1.14) | 1.11
(1.04-1.34) | 0.728 |
| PTV |
|
Max
dose | 69.0
(66.5-71.1) | 68.9
(66.6-71.8) | 0.991 |
|
Mean
dose | 65.4
(64.5-66.2) | 65.3
(63.8-68.0) | 0.866 |
|
Min
dose | 52.1
(22.9-57.8) | 40.4
(8.9-60.5) | 0.122 |
|
HI | 1.34
(1.15-3.09) | 1.69
(1.11-7.88) | 0.106 |
Adverse effects
The acute and late adverse effects of RT are shown
in Table IV. Of the 64 patients, 16
(25%) had grade 2 acute dermatitis and 2 (3%) had grade 3 acute
dermatitis. Although 28 patients (44%) had grade 2 acute mucositis,
none demonstrated acute adverse effects or late adverse effects of
grade ≥3.
 | Table IVAcute and late radiation-related
toxicities. |
Table IV
Acute and late radiation-related
toxicities.
| | Grade |
|---|
| Toxicities | 0 or 1 | 2 | 3 | 4 |
|---|
| Acute |
|
Dermatitis | 46 | 16 | 2 | 0 |
|
Mucositis | 36 | 28 | 0 | 0 |
| Late |
|
Laryngeal
edema | 64 | 0 | 0 | 0 |
|
Dermatitis | 64 | 0 | 0 | 0 |
|
Myelopathy | 64 | 0 | 0 | 0 |
The clinical data and dosimetric factors for all
cases are listed in Tables V and
VI.
 | Table VClinical risk factors for local
failure in all cases. |
Table V
Clinical risk factors for local
failure in all cases.
| No. | Age, years | Sex | PS score | T stage | OTT (days) | ACI (yes vs.
no) | Smoking (yes vs.
no) | Histological tumor
grade (well vs. moderate/poorly differentiated) | Pretreatment
hemoglobin (g/dl) |
|---|
| 1 | 52 | M | 0 | 1a | 45 | Yes | Yes | Well | 14.6 |
| 2 | 73 | M | 0 | 1b | 46 | Yes | Yes | Well | 15 |
| 3 | 65 | M | 0 | 1b | 50 | Yes | Yes | Well | 14.8 |
| 4 | 80 | M | 0 | 1a | 45 | Yes | No | Well | 14.4 |
| 5 | 79 | M | 0 | 1a | 47 | No | No | Well | 15.6 |
| 6 | 77 | F | 0 | 1b | 45 | Yes | No | Well | 11 |
| 7 | 57 | M | 0 | 1a | 44 | No | Yes | Moderate-poor | 15.7 |
| 8 | 83 | M | 0 | 1b | 45 | Yes | No | Well | 15.4 |
| 9 | 65 | F | 0 | 1a | 45 | No | No | Moderate-poor | 14.5 |
| 10 | 55 | F | 0 | 1a | 47 | Yes | No | Well | 13 |
| 11 | 75 | M | 0 | 1a | 44 | No | No | Well | 13.2 |
| 12 | 63 | M | 0 | 1b | 44 | Yes | No | Well | 15.5 |
| 13 | 58 | F | 0 | 1a | 39 | No | No | Well | 14.5 |
| 14 | 47 | M | 0 | 1a | 50 | No | Yes | Well | 14 |
| 15 | 71 | M | 3 | 1a | 46 | Yes | Yes | Moderate-poor | 11.3 |
| 16 | 72 | M | 0 | 1b | 49 | No | No | Well | 14.7 |
| 17 | 73 | M | 0 | 1a | 44 | No | Yes | Moderate-poor | 15.9 |
| 18 | 76 | M | 0 | 1a | 47 | No | No | Moderate-poor | 14.9 |
| 19 | 64 | M | 0 | 1a | 51 | No | Yes | Well | 15.6 |
| 20 | 71 | M | 0 | 1a | 45 | No | Yes | Moderate-poor | 14.8 |
| 21 | 73 | M | 0 | 1b | 50 | No | Yes | Well | 12.4 |
| 22 | 76 | M | 0 | 1a | 50 | Yes | No | Well | 14.5 |
| 23 | 84 | M | 0 | 1a | 45 | No | No | Well | 14.3 |
| 24 | 70 | M | 0 | 1a | 45 | No | No | Moderate-poor | 16.5 |
| 25 | 65 | M | 0 | 1b | 44 | No | Yes | Well | 13.6 |
| 26 | 70 | M | 0 | 1a | 47 | No | Yes | Well | 15.9 |
| 27 | 73 | M | 0 | 1a | 43 | No | No | Well | 10.6 |
| 28 | 70 | M | 0 | 1a | 44 | No | Yes | Moderate-poor | 15.1 |
| 29 | 82 | M | 0 | 1a | 45 | No | No | Moderate-poor | 14.3 |
| 30 | 65 | M | 0 | 1b | 46 | Yes | No | Well | 14.4 |
| 31 | 58 | M | 0 | 1a | 43 | No | Yes | Well | 14.2 |
| 32 | 64 | M | 0 | 1b | 45 | Yes | No | Moderate-poor | 14 |
| 33 | 69 | M | 0 | 1a | 46 | No | Yes | Well | 13.6 |
| 34 | 75 | M | 0 | 1a | 46 | No | No | Moderate-poor | 14.4 |
| 35 | 70 | M | 0 | 1a | 45 | Yes | Yes | Moderate-poor | 14.8 |
| 36 | 73 | M | 0 | 1b | 46 | Yes | No | Well | 18 |
| 37 | 86 | M | 1 | 1a | 49 | No | No | Well | 15 |
| 38 | 81 | M | 0 | 1a | 48 | No | No | Well | 12.4 |
| 39 | 80 | M | 0 | 1b | 44 | yes | No | Well | 13.6 |
| 40 | 86 | M | 0 | 1a | 46 | No | No | Well | 12.4 |
| 41 | 70 | M | 0 | 1b | 44 | No | No | Well | 15.9 |
| 42 | 84 | M | 0 | 1a | 50 | No | No | Moderate-poor | 13.3 |
| 43 | 63 | F | 0 | 1a | 49 | No | Yes | Well | 13.2 |
| 44 | 70 | M | 0 | 1a | 44 | No | No | Well | 15 |
| 45 | 77 | M | 0 | 1a | 48 | No | No | Well | 14 |
| 46 | 79 | M | 0 | 1a | 49 | No | Yes | Well | 12.6 |
| 47 | 64 | F | 0 | 1a | 52 | No | No | Well | 13.6 |
| 48 | 66 | M | 0 | 1a | 51 | Yes | Yes | Well | 15.5 |
| 49 | 84 | M | 0 | 1a | 44 | Yes | No | Well | 15.2 |
| 50 | 84 | M | 1 | 1a | 44 | No | No | Moderate-poor | 11 |
| 51 | 85 | M | 0 | 1b | 45 | Yes | No | Well | 13.6 |
| 52 | 72 | M | 0 | 1b | 48 | Yes | Yes | Moderate-poor | 14.9 |
| 53 | 72 | M | 0 | 1b | 45 | No | Yes | Well | 12.5 |
| 54 | 66 | M | 0 | 1b | 44 | No | Yes | Moderate-poor | 15.4 |
| 55 | 80 | F | 0 | 1a | 45 | No | No | Well | 13.8 |
| 56 | 83 | M | 0 | 1b | 44 | No | No | Well | 12.1 |
| 57 | 67 | M | 0 | 1a | 44 | Yes | No | Moderate-poor | 16.6 |
| 58 | 73 | M | 0 | 1a | 45 | No | Yes | Well | 15.8 |
| 59 | 84 | M | 0 | 1b | 49 | Yes | No | Moderate-poor | 13.4 |
| 60 | 71 | M | 0 | 1a | 44 | No | Yes | Well | 16.2 |
| 61 | 84 | M | 1 | 1a | 45 | No | Yes | Moderate-poor | 14.8 |
| 62 | 70 | M | 0 | 1b | 45 | Yes | Yes | Well | 14.2 |
| 63 | 76 | M | 0 | 1a | 48 | No | Yes | Well | 14.3 |
| 64 | 68 | M | 0 | 1b | 48 | Yes | Yes | Well | 13.6 |
 | Table VIDosimetric risk factors for local
failure in all cases. |
Table VI
Dosimetric risk factors for local
failure in all cases.
| | Dose to glottic
larynx (Gy) | Dose to CTV
(Gy) | Dose to PTV
(Gy) | | |
|---|
| No. | Max | Mean | Min | HI | Max | Mean | Min | HI | Max | Mean | Min | HI | Local control | Local control
duration (months) |
|---|
| 1 | 68.5 | 67.0 | 65.4 | 1.047 | 68.6 | 65.7 | 62.2 | 1.103 | 68.6 | 65.2 | 36.7 | 2.024 | Control | 65 |
| 2 | 66.9 | 66.1 | 65.3 | 1.025 | 67.0 | 65.4 | 63.2 | 1.060 | 67.2 | 65.1 | 25.0 | 1.111 | Control | 132 |
| 3 | 66.5 | 65.9 | 64.8 | 1.026 | 66.6 | 65.2 | 61.0 | 1.092 | 66.9 | 64.5 | 51.3 | 1.823 | Control | 21 |
| 4 | 67.7 | 65.5 | 63.8 | 1.061 | 67.8 | 64.6 | 59.4 | 1.141 | 67.9 | 64.0 | 41.1 | 2.716 | Control | 123 |
| 5 | 68.4 | 66.3 | 64.5 | 1.060 | 68.1 | 65.5 | 61.1 | 1.115 | 68.4 | 65.1 | 49.6 | 1.333 | Failure | 2 |
| 6 | 66.6 | 66.4 | 66.0 | 1.009 | 66.7 | 65.8 | 63.8 | 1.045 | 66.7 | 64.8 | 35.9 | 1.623 | Control | 82 |
| 7 | 67.9 | 66.6 | 65.0 | 1.045 | 68.1 | 64.7 | 60.2 | 1.131 | 68.4 | 64.1 | 38.0 | 1.379 | Control | 102 |
| 8 | 67.9 | 67.3 | 66.1 | 1.027 | 67.9 | 65.9 | 62.1 | 1.093 | 67.9 | 65.1 | 52.4 | 1.891 | Control | 4 |
| 9 | 66.6 | 66.3 | 65.6 | 1.015 | 66.8 | 65.7 | 63.9 | 1.045 | 66.9 | 65.1 | 47.8 | 1.761 | Control | 105 |
| 10 | 66.5 | 66.0 | 65.5 | 1.015 | 66.5 | 65.1 | 62.8 | 1.059 | 66.6 | 64.6 | 42.2 | 1.271 | Control | 76 |
| 11 | 67.0 | 66.6 | 66.0 | 1.015 | 67.3 | 66.1 | 64.1 | 1.050 | 67.6 | 65.6 | 26.0 | 1.414 | Control | 98 |
| 12 | 66.9 | 66.0 | 64.2 | 1.042 | 67.0 | 65.0 | 61.7 | 1.086 | 67.0 | 64.5 | 19.0 | 1.588 | Failure | 94 |
| 13 | 67.5 | 66.4 | 65.0 | 1.038 | 67.4 | 64.9 | 62.4 | 1.080 | 67.6 | 64.3 | 24.5 | 2.600 | Control | 44 |
| 14 | 66.8 | 66.2 | 63.3 | 1.055 | 67.2 | 65.9 | 53.5 | 1.256 | 67.5 | 64.4 | 28.5 | 3.553 | Control | 107 |
| 15 | 66.2 | 65.8 | 65.1 | 1.017 | 66.6 | 64.9 | 62.7 | 1.062 | 66.7 | 64.3 | 57.8 | 2.722 | Control | 14 |
| 16 | 68.2 | 67.8 | 66.3 | 1.029 | 68.4 | 66.6 | 62.4 | 1.096 | 68.7 | 65.8 | 55.7 | 2.411 | Control | 95 |
| 17 | 66.2 | 65.6 | 64.4 | 1.028 | 66.2 | 65.0 | 62.5 | 1.059 | 66.5 | 64.7 | 35.3 | 1.151 | Failure | 6 |
| 18 | 66.6 | 66.2 | 65.6 | 1.015 | 66.7 | 65.4 | 63.0 | 1.059 | 67.1 | 65.0 | 39.7 | 1.205 | Control | 100 |
| 19 | 67.6 | 66.5 | 64.6 | 1.046 | 67.7 | 64.8 | 60.8 | 1.113 | 67.7 | 64.3 | 59.3 | 1.918 | Control | 106 |
| 20 | 67.3 | 66.1 | 64.7 | 1.040 | 68.4 | 65.3 | 59.4 | 1.152 | 68.4 | 64.7 | 26.9 | 1.723 | Control | 105 |
| 21 | 67.1 | 66.3 | 65.2 | 1.029 | 67.3 | 65.5 | 63.0 | 1.068 | 67.5 | 65.5 | 42.6 | 1.138 | Control | 71 |
| 22 | 67.8 | 66.9 | 64.3 | 1.054 | 69.0 | 65.2 | 58.7 | 1.175 | 69.0 | 63.9 | 35.1 | 2.565 | Control | 102 |
| 23 | 68.3 | 67.4 | 66.0 | 1.035 | 68.7 | 65.7 | 60.5 | 1.136 | 68.8 | 64.9 | 47.6 | 1.615 | Control | 93 |
| 24 | 70.2 | 67.9 | 65.6 | 1.070 | 70.3 | 65.9 | 60.7 | 1.158 | 70.3 | 65.5 | 46.3 | 2.003 | Control | 42 |
| 25 | 66.5 | 66.3 | 66.0 | 1.008 | 66.7 | 65.6 | 63.3 | 1.054 | 67.0 | 65.1 | 53.0 | 1.408 | Control | 99 |
| 26 | 69.3 | 67.4 | 65.2 | 1.063 | 69.3 | 65.3 | 60.2 | 1.151 | 69.3 | 64.9 | 47.4 | 1.497 | Control | 99 |
| 27 | 69.1 | 66.9 | 65.0 | 1.063 | 70.8 | 65.7 | 60.5 | 1.170 | 70.8 | 65.4 | 52.8 | 1.336 | Control | 58 |
| 28 | 70.4 | 67.7 | 65.2 | 1.080 | 70.4 | 65.6 | 54.9 | 1.282 | 70.5 | 65.0 | 28.9 | 1.487 | Control | 92 |
| 29 | 71.1 | 68.0 | 65.3 | 1.089 | 71.1 | 66.3 | 62.4 | 1.139 | 71.1 | 66.2 | 8.9 | 1.347 | Failure | 24 |
| 30 | 70.3 | 67.0 | 65.0 | 1.082 | 71.8 | 65.8 | 60.0 | 1.197 | 71.8 | 65.5 | 46.1 | 2.484 | Control | 17 |
| 31 | 69.6 | 67.9 | 66.3 | 1.050 | 70.1 | 66.0 | 60.3 | 1.163 | 70.1 | 65.1 | 57.8 | 7.876 | Control | 74 |
| 32 | 67.8 | 67.0 | 65.7 | 1.032 | 68.0 | 65.8 | 62.1 | 1.095 | 68.0 | 65.3 | 44.9 | 1.475 | Control | 78 |
| 33 | 69.6 | 67.1 | 64.8 | 1.074 | 69.5 | 65.9 | 62.7 | 1.108 | 69.6 | 65.7 | 22.9 | 1.204 | Failure | 12 |
| 34 | 67.9 | 66.1 | 64.0 | 1.061 | 68.5 | 64.7 | 57.9 | 1.183 | 68.9 | 63.8 | 13.6 | 1.535 | Control | 55 |
| 35 | 70.3 | 68.5 | 65.4 | 1.075 | 70.8 | 66.9 | 62.2 | 1.138 | 70.8 | 65.9 | 45.8 | 3.092 | Failure | 9 |
| 36 | 67.7 | 66.9 | 64.2 | 1.055 | 67.7 | 65.7 | 59.4 | 1.140 | 67.7 | 64.1 | 12.6 | 4.978 | Control | 69 |
| 37 | 66.6 | 66.2 | 65.7 | 1.014 | 66.6 | 65.8 | 62.8 | 1.061 | 66.6 | 65.3 | 39.1 | 1.454 | Control | 12 |
| 38 | 68.7 | 68.0 | 66.3 | 1.036 | 69.2 | 67.3 | 63.4 | 1.091 | 69.5 | 66.4 | 8.9 | 5.516 | Control | 53 |
| 39 | 67.4 | 66.7 | 65.8 | 1.024 | 67.8 | 66.2 | 63.8 | 1.063 | 68.9 | 65.7 | 19.1 | 1.762 | Control | 52 |
| 40 | 68.5 | 68.1 | 66.5 | 1.030 | 69.0 | 67.0 | 60.6 | 1.139 | 69.2 | 65.2 | 34.5 | 7.775 | Control | 50 |
| 41 | 70.3 | 69.9 | 68.7 | 1.023 | 70.3 | 68.7 | 65.2 | 1.078 | 70.3 | 67.6 | 41.7 | 3.681 | Control | 57 |
| 42 | 69.0 | 67.8 | 66.2 | 1.042 | 69.6 | 67.1 | 63.3 | 1.100 | 69.6 | 66.2 | 23.7 | 2.017 | Control | 50 |
| 43 | 69.0 | 68.3 | 67.1 | 1.028 | 69.0 | 67.5 | 65.2 | 1.058 | 69.0 | 66.6 | 47.3 | 1.655 | Control | 48 |
| 44 | 70.3 | 68.9 | 67.2 | 1.046 | 70.3 | 67.5 | 62.1 | 1.132 | 70.3 | 66.6 | 45.9 | 2.966 | Control | 48 |
| 45 | 68.9 | 68.4 | 67.3 | 1.024 | 68.9 | 67.5 | 63.7 | 1.082 | 68.9 | 66.9 | 55.3 | 1.457 | Control | 48 |
| 46 | 68.2 | 66.9 | 66.0 | 1.033 | 68.7 | 66.0 | 59.1 | 1.162 | 68.7 | 65.2 | 25.8 | 1.497 | Control | 46 |
| 47 | 69.9 | 69.7 | 69.4 | 1.007 | 69.9 | 68.9 | 66.6 | 1.050 | 70.0 | 68.0 | 52.8 | 1.266 | Control | 47 |
| 48 | 67.7 | 67.5 | 66.7 | 1.015 | 68.6 | 66.7 | 63.9 | 1.074 | 68.8 | 65.8 | 31.9 | 2.667 | Control | 46 |
| 49 | 69.3 | 68.0 | 65.3 | 1.061 | 69.6 | 66.3 | 56.5 | 1.232 | 69.6 | 65.7 | 51.1 | 1.318 | Control | 40 |
| 50 | 69.5 | 68.6 | 66.9 | 1.039 | 69.5 | 67.3 | 62.3 | 1.116 | 69.5 | 66.8 | 37.7 | 2.179 | Control | 39 |
| 51 | 68.8 | 67.4 | 65.1 | 1.057 | 69.6 | 65.6 | 55.7 | 1.250 | 70.1 | 65.3 | 48.0 | 1.372 | Control | 24 |
| 52 | 69.2 | 68.2 | 66.5 | 1.041 | 69.2 | 66.3 | 61.1 | 1.133 | 69.2 | 66.0 | 59.8 | 1.836 | Control | 36 |
| 53 | 69.1 | 68.2 | 66.1 | 1.045 | 69.3 | 67.0 | 62.8 | 1.104 | 69.4 | 66.9 | 30.2 | 1.446 | Control | 35 |
| 54 | 70.3 | 69.3 | 66.8 | 1.052 | 70.5 | 67.7 | 62.3 | 1.132 | 71.5 | 67.7 | 22.0 | 1.196 | Control | 35 |
| 55 | 68.3 | 67.6 | 66.0 | 1.035 | 68.3 | 66.6 | 64.3 | 1.062 | 68.3 | 66.3 | 60.5 | 2.262 | Control | 32 |
| 56 | 70.3 | 69.7 | 68.2 | 1.031 | 70.4 | 67.8 | 64.8 | 1.086 | 70.5 | 67.5 | 35.3 | 3.205 | Control | 6 |
| 57 | 70.9 | 69.5 | 67.0 | 1.058 | 70.9 | 67.6 | 62.6 | 1.133 | 71.2 | 67.9 | 37.2 | 1.177 | Control | 29 |
| 58 | 69.3 | 67.6 | 65.6 | 1.056 | 70.4 | 66.7 | 61.0 | 1.154 | 70.6 | 66.6 | 59.4 | 2.000 | Control | 29 |
| 59 | 69.0 | 66.7 | 63.7 | 1.083 | 70.3 | 65.8 | 57.8 | 1.216 | 70.6 | 65.5 | 49.2 | 1.898 | Control | 21 |
| 60 | 68.5 | 67.4 | 65.4 | 1.047 | 68.5 | 65.4 | 61.0 | 1.123 | 68.7 | 65.7 | 49.8 | 1.157 | Control | 15 |
| 61 | 68.9 | 66.8 | 63.5 | 1.085 | 69.3 | 65.0 | 56.2 | 1.233 | 70.0 | 64.8 | 45.2 | 1.423 | Control | 9 |
| 62 | 69.9 | 68.9 | 66.8 | 1.046 | 70.1 | 67.5 | 63.9 | 1.097 | 70.3 | 67.6 | 49.2 | 1.412 | Control | 16 |
| 63 | 67.3 | 64.9 | 62.7 | 1.073 | 69.8 | 65.4 | 52.1 | 1.340 | 70.1 | 64.7 | 36.7 | 1.551 | Control | 12 |
| 64 | 69.9 | 67.9 | 65.3 | 1.070 | 70.7 | 66.2 | 58.8 | 1.202 | 70.7 | 65.8 | 25.0 | 1.437 | Control | 12 |
Discussion
In the present study, the 5-year local control rates
for T1 glottic carcinomas treated with minimum doses of <65 and
≥65 Gy to the glottic larynx were 79 and 95%, respectively. The
difference in the local control rate between patients treated with
minimum doses of <65 and ≥65 Gy to the glottic larynx was
statistically significant (P=0.015).
Several previous studies have reported on the risk
factors for local failure in patients with T1 glottic carcinoma.
The local control rate for Tl tumors with an overall treatment time
of 42-49 days was previously reported to be significantly higher
compared with that of tumors with corresponding treatment times of
>49 days (P<0.02) (11). In
addition, previous studies have demonstrated an association between
low hemoglobin levels and poor local control, i.e., pre-treatment
anemia was an adverse factor for survival in patients with
early-stage glottic carcinoma (16,17);
this was not observed in the present study. There was a significant
decrease in the 10-year overall survival rate in patients with
pre-RT anemia compared with those without pre-RT anemia (52 vs.
68%, respectively) (18).
Furthermore, a recent systematic review and meta-analysis was
performed to determine the risk factors for RT failure in
early-stage glottic carcinoma (19).
There was a higher risk of RT failure in male patients [relative
risk (RR)=0.927, P<0.001], patients with low hemoglobin levels
(RR=0.891, P<0.001), tumors with ACI (RR=0.904, P<0.001),
tobacco use during/after therapy (RR=0.824, P<0.001), and
‘bulky’ tumors (RR=1.270, P<0.001) or large tumors (RR=1.332,
P<0.001). In most previous studies, sex, age, comorbidities,
tobacco use during/after RT, alcohol consumption, hemoglobin level,
tumor stage, ACI, tumor size/volume, subglottic extension and
grade, among others, were predictive factors for the survival of
patients with early glottic squamous cell carcinomas following
definitive RT. By contrast, in the present study, none of these
clinical factors were indicative of RT failure in early-stage
glottic carcinoma.
To the best of our knowledge, only a few studies
have evaluated the dosimetric risk factors for local failure.
Several studies investigated the association between total dose and
local failure in early glottic carcinomas (18,20-26).
The majority of those studies compared the total dose between ≤66
and >66 Gy with regard to local failure, which was not
significantly different. The present study was the first to
investigate the dosimetric factors of local failure for early-stage
glottic carcinoma that was definitively irradiated to a
prescription dose of 66 Gy. Furthermore, in the present study, the
HI for glottic larynx did not reach the required levels of
significance to be considered as a confounding factor. However, the
P-value was reasonably low, confirming its importance. This finding
indicates that techniques using RT for uniform dose distribution to
the target volume, such as intensity-modulated RT (IMRT), may
improve the local control rate for early-stage glottic carcinoma
treated with definitive RT. Only a limited number of studies have
evaluated the treatment outcomes of IMRT for early-stage squamous
cell carcinoma of the glottis (27,28). In
these studies, the local control rate did not differ significantly
between patients treated with IMRT and those treated with RT.
However, the prescription dose for patients treated with IMRT was
63 Gy/28 fractions. Therefore, there is potential for improving the
local control rate in patients treated with IMRT by setting the
prescription dose to 66 Gy/33 fractions, and the minimum dose of
the glottic larynx to ≥65 Gy.
The main limitation of the present study was the
possible selection bias for the predictive factors owing to the
retrospective nature of the study. Therefore, prospective studies
are required in the future to confirm our findings.
In conclusion, the minimum dose to the glottic
larynx was the only factor found to be significantly associated
with the occurrence of local failure. Setting the minimum dose to
the glottic larynx at ≥65 Gy may improve the local control rate for
early-stage glottic carcinomas irradiated to a prescription dose of
66 Gy.
Acknowledgements
The authors wish to thank the radiographer Mr
Hideaki Tiba, and Dr Yu Tajima, Department of Radiology, Tokyo
Medical University Hospital, for their professional assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MO, TI, TS and ShS conceived the study, and wrote
and revised the manuscript. RM, AS and SaS reviewed, collected and
analyzed the data. JP, KT and KS designed the study and acquired
the data. All authors contributed to the writing of the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Tokyo Medical University Hachioji Medical Center
(Tokyo, Japan) and patient written informed consent was waived due
to the retrospective design.
Patient consent for publication
Patient consent for publication was waived due to
retrospective design.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mendenhall WM, Werning JW, Hinerman RW,
Amdur RJ and Villaret DB: Management of T1-T2 glottic carcinomas.
Cancer. 100:1786–1792. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Beitler JJ and Johnson JT: Transoral laser
excision for early glottic cancer. Int J Radiat Oncol Biol Phys.
56:1063–1036. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Back G and Sood S: The management of early
laryngeal cancer: Options for patients and therapists. Curr Opin
Otolaryngol Head Neck Surg. 13:85–91. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Akine Y, Tokita N, Ogino T, Tsukiyama I,
Egawa S, Saikawa M, Ohyama W, Yoshizumi T and Ebihara S:
Radiotherapy of T1 glottic cancer with 6 MeV X rays. Int J Radiat
Oncol Biol Phys. 20:1215–1218. 1991.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fein DA, Lee WR, Hanlon AL, Ridge JA,
Curran WJ and Coia LR: Do overall treatment time, field size, and
treatment energy influence local control of T1-T2 squamous cell
carcinomas of the glottic larynx? Int J Radiat Oncol Biol Phys.
34:823–831. 1996.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mendenhall WM, Parsons JT, Stringer SP,
Cassisi NJ and Million RR: T1-T2 vocal cord carcinoma: A basis for
comparing the results of radiotherapy and surgery. Head Neck Surg.
10:373–377. 1988.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rudoltz MS, Benammar A and Mohiuddin M:
Prognostic factors for local control and survival in T1 squamous
cell carcinoma of the glottis. Int J Radiat Oncol Biol Phys.
26:767–772. 1993.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Small W Jr, Mittal BB, Brand WN, Shetty
RM, Rademaker AW, Beck GG and Hoover SV: Results of radiation
therapy in early glottic carcinoma: Multivariate analysis of
prognostic and radiation therapy variables. Radiology. 183:789–794.
1992.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Reddy SP, Hong RL, Nagda S and Emami B:
Effect of tumor bulk on local control and survival of patients with
T1 glottic cancer: A 30-year experience. Int J Radiat Oncol Biol
Phys. 69:1389–1394. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sakata K, Aoki Y, Karasawa K, Hasezawa K,
Muta N, Nakagawa K, Terahara A, Onogi Y, Sasaki Y and Akanuma A:
Radiation therapy in early glottic carcinoma: Uni- and multivariate
analysis of prognostic factors affecting local control. Int J
Radiat Oncol Biol Phys. 30:1059–1064. 1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nishimura Y, Nagata Y, Okajima K,
Mitsumori M, Hiraoka M, Masunaga S, Ono K, Shoji K and Kojima H:
Radiation therapy for T1,2 glottic carcinoma: Impact of overall
treatment time on local control. Radiother Oncol. 40:225–232.
1996.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mendenhall WM, Parsons JT, Million RR and
Fletcher GH: T1-T2 squamous cell carcinoma of the glottic larynx
treated with radiation therapy: Relationship of dose-fractionation
factors to local control and complications. Int J Radiat Oncol Biol
Phys. 15:1267–1273. 1988.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: (eds): AJCC Cancer Staging Manual. 8th edition.
New York, Springer International Publishing, 2017.
|
|
14
|
National Cancer Institute. Common
Terminology Criteria for Adverse Events (CTCAE) [Internet], 2018
(cited 2019 Aug 5). Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50.
|
|
15
|
Kataria T, Sharma K, Subramani V,
Karrthick KP and Bisht SS: Homogeneity index: An objective tool for
assessment of conformal radiation treatments. J Med Phys.
37:207–213. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao KL, Liu G, Jiang GL, Wang Y, Zhong
LJ, Wang Y, Yao WQ, Guo XM, Wu GD, Zhu LX, et al: Association of
haemoglobin level with morbidity and mortality of patients with
locally advanced oesophageal carcinoma undergoing radiotherapy-a
secondary analysis of three consecutive clinical phase III trials.
Clin Oncol (R Coll Radiol). 18:621–627. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shin NR, Lee YY, Kim SH, Choi CH, Kim TJ,
Lee JW, Bae DS and Kim BG: Prognostic value of pretreatment
hemoglobin level in patients with early cervical cancer. Obstet
Gynecol Sci. 57:28–36. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Al-Mamgani A, van Rooij PH, Woutersen DP,
Mehilal R, Tans L, Monserez D and aatenburg de Jong RJ:
Radiotherapy for T1-2N0 glottic cancer: A multivariate analysis of
predictive factors for the long-term outcome in 1050 patients and a
prospective assessment of quality of life and voice handicap index
in a subset of 233 patients. Clin Otolaryngol. 38:306–312.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Eskiizmir G, Baskın Y, Yalçın F, Ellidokuz
H and Ferris RL: Risk factors for radiation failure in early-stage
glottic carcinoma: A systematic review and meta-analysis. Oral
Oncol. 62:90–100. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Franchin G, Minatel E, Gobitti C, Talamini
R, Sartor G, Caruso G, Grando G, Politi D, Gigante M, Toffoli G, et
al: Radiation treatment of glottic squamous cell carcinoma, stage I
and II: Analysis of factors affecting prognosis. Int J Radiat Oncol
Biol Phys. 40:541–548. 1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Marshak G, Brenner B, Shvero J, Shapira J,
Ophir D, Hochman I, Marshak G, Sulkes A and Rakowsky E: Prognostic
factors for local control of early glottic cancer: The Rabin
Medical Center retrospective study on 207 patients. Int J Radiat
Oncol Biol Phys. 43:1009–1013. 1999.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Narayana A, Vaughan AT, Kathuria S, Fisher
SG, Walter SA and Reddy SP: P53 overexpression is associated with
bulky tumor and poor local control in T1 glottic cancer. Int J
Radiat Oncol Biol Phys. 46:21–26. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cellai E, Frata P, Magrini SM, Paiar F,
Barca R, Fondelli S, Polli C, Livi L, Bonetti B, Vitali E, et al:
Radical radiotherapy for early glottic cancer: Results in a series
of 1087 patients from two Italian radiation oncology centers. I.
The case of T1N0 disease. Int J Radiat Oncol Biol Phys.
63:1378–1386. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nur DA, Oguz C, Kemal ET, Ferhat E, Sülen
S, Emel A, Münir K, Ann CS and Mehmet S: Prognostic factors in
early glottic carcinoma implications for treatment. Tumori.
91:182–187. 2005.PubMed/NCBI
|
|
25
|
Murakami R, Nishimura R, Baba Y, Furusawa
M, Ogata N, Yumoto E and Yamashita Y: Prognostic factors of glottic
carcinomas treated with radiation therapy: Value of the adjacent
sign on radiological examinations in the sixth edition of the UICC
TNM staging system. Int J Radiat Oncol Biol Phys. 61:471–475.
2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tong CC, Au KH, Ngan RK, Cheung FY, Chow
SM, Fu YT, Au JS and Law SC: Definitive radiotherapy for early
stage glottic cancer by 6 MV photons. Head Neck Oncol.
4(23)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Berwouts D, Swimberghe M, Duprez F,
Boterberg T, Bonte K, Deron P, De Gersem W, De Neve W and Madani I:
Intensity-modulated radiotherapy for early-stage glottic cancer.
Head Neck. 38 (Suppl 1):E179–E184. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zumsteg ZS, Riaz N, Jaffery S, Hu M,
Gelblum D, Zhou Y, Mychalczak B, Zelefsky MJ, Wolden S, Rao S and
Lee NY: Carotid sparing intensity-modulated radiation therapy
achieves comparable locoregional control to conventional
radiotherapy in T1-2N0 laryngeal carcinoma. Oral Oncol. 51:716–723.
2015.PubMed/NCBI View Article : Google Scholar
|