Introduction
Mammary myofibroblastoma (MFB) is a rare benign
mesenchymal tumor originating from mammary stromal that was
described for the first time in 1981(1) and first named by Wargotz et al
in 1987(2). Some years later, in
2001, McMenamin and Fletcher described the first case of extra
mammary myofibroblastoma (MTM) (3).
Both of these entities are histologically and immune-phenotypically
identical. Traditionally, mammary myofibroblastoma mainly affects
older men (between 60 and 70 years), although some cases were also
described in postmenopausal women (4). In women, thanks to mammary screening,
this entity can be detected at a smaller size and, in the last few
years, the incidence has been on the increase. Conversely, in men
it is not uncommon to identify at diagnosis a painless and palpable
mass.
MFB was described in multiple races with no
predilection for ethnicity. Owing to its rarity, this tumor can be
confused, both clinically and radiologically, with other types of
benign or malignant breast cancers. Differential diagnosis is
fundamental to avoid excessive treatment in a condition where the
correct approach is the excision of the lesion.
In the present study, we report two cases of male
mammary myofibroblastoma treated in the Senology Unit of the
University Hospital of Modena (Modena, Italy) between September
2010 and December 2018, with a short literature review.
Case reports
Case 1
In 2010, a 65-year-old man presented at the Division
of Breast Surgery of the University Hospital of Modena (Modena,
Italy) with a palpable mass in his left breast. He had no family
history of breast cancer, ovarian cancer or other types of
malignancies. At the clinical examination there were no skin
changes, no nipple changes or retraction and there were not
lymphadenopathies at the supraclavicular or axillary sites. No
gynecomastia was identified.
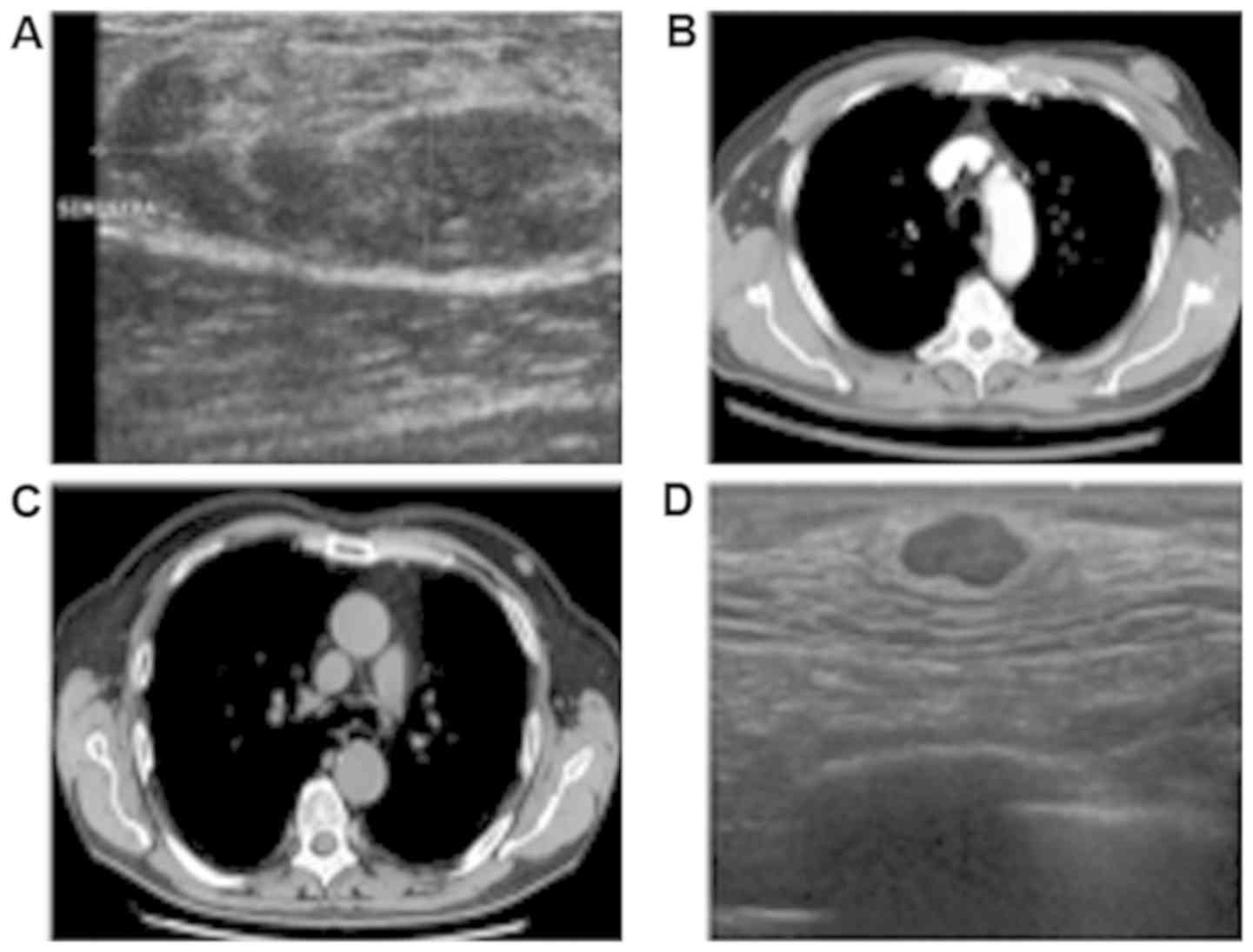
A diagnostic bilateral mammography and
ultrasonography (Fig. 1A) were
performed demonstrating a nodular, well-circumscribed lesion with
no microcalcifications and an iso-hypoechoic, oval, solid mass,
respectively. The lesion occupied the upper outer quadrant of the
left breast and measured 41x18 mm. The right mammography and
ultrasonography were normal. Fine needle aspiration cytology (FNAC)
resulted in a suspicious specimen of malignant neoplasm (Category
4-C4). Pre-operatory abdomen and chest contrast-enhanced computed
tomography (CT) scan (Fig. 1B) were
negative and the patient underwent a radical left mastectomy plus
axillary dissection.
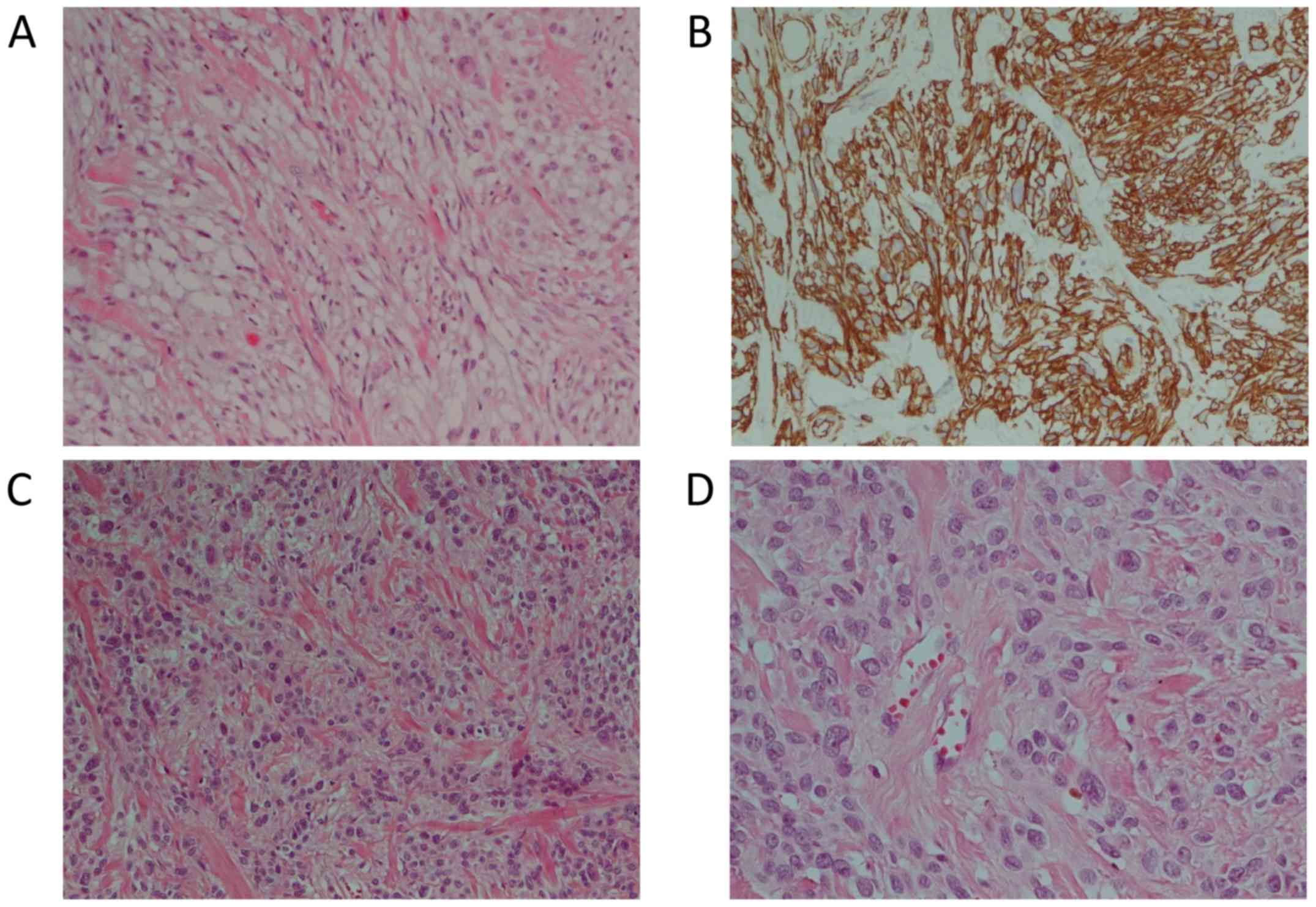
The excised mass measured 31 mm in the major axis
and was mainly composed of epithelioid cells with some spindle
cells, adipocytes and hyalinized collagen fibers in hematoxylin and
eosin (H&E) stain (Fig. 2A).
Some nuclear atypia were observed in epithelioid cells.
Histopathologic examination also showed immunoreactivity for CD34
(Fig. 2B), desmin, alpha smooth
muscle actin (α-SMA) and myosin. Tumor cells were negative for
S100, p63 and cytokeratins (Table
I). The Ki67 proliferative index was <5%. No pathological
lymph node was found. The immunohistochemical pattern supported a
mammary stromal origin and the diagnosis of epithelioid mammary
myofibroblastoma was performed with the support of Professor C.D.M.
Fletcher at Harvard Medical School, Brigham and Women's Hospital,
Boston, USA.
 | Table ISummary of the immunohistochemical
findings in our cases. |
Table I
Summary of the immunohistochemical
findings in our cases.
| Variables | Case 1 | Case 2 |
|---|
| Vimentin | / | / |
| CD34 | + | + |
| Desmin | + | + |
| Bcl-2 | / | + |
| SMA | + | +/- |
| Myosin | + | / |
| ER | / | + (80%) |
| PgR | / | + (80%) |
| AR | / | + (98%) |
| CD99 | / | - |
| S100 | - | - |
| Cytokeratins | - | - |
| Melan A | / | - |
| p63 | - | - |
Case 2
In 2017, a 76-year-old man came to our attention
with a 13 mm oval mass in his left breast found during a chest CT
scan (Fig. 1C). He was a strong
smoker and he had a severe cough for a long period. This patient
also had no family history of breast cancer, ovarian cancer or
other type of malignancies. At clinical inspection there were no
skin changes, no nipple changes or retraction and there were no
lymphadenopathies at the supraclavicular or axillary sites. There
were no breast masses or gynecomastia.
Bilateral mammogram and ultrasonography (Fig. 1D) showed a 15 mm oval solid mass in
the retroareolar region of the left breast (BIRADS R5 and US5). The
right mammography and ultrasonography were normal. The subsequent
ultrasound-guided needle biopsy resulted in C4.
The patient underwent a radical left mastectomy and
the removal of the sentinel lymph node. The removed mass was 12 mm
in its major axis. Histological examination in the H&E stain
showed epithelioid and mesenchymal cells with hyalinized collagen
fibers (Fig. 2C and D). There was no necrosis.
Immunohistochemistry showed positive reaction for desmin, actin,
Bcl-2 and CD34. Neoplastic cells were also positive for estrogen
(ER), androgen (AR) and progesterone (PR) receptors while S100,
p63, CD99 and cytokeratins were negative (Table I). The Ki67 proliferative index was
1%. No pathological lymph nodes were identified. All of these
findings indicated the diagnosis of myofibroblastoma.
Discussion
Myofibroblastoma is a tumor with myofibroblastic
differentiation, most frequently detected in men, and which
represents the most common type of benign spindle cell lesion
(1,5). At clinical examination, it generally
presents as a unilateral, solitary, firm, mobile and painless
breast mass with slow growth (6).
Bilaterality and unilateral multicentricity are very rare.
Otherwise, mammary-type myofibroblastoma occurs predominantly along
the embryonic milk-line such as the axillary, perianal, vulvar and
para-testicular regions (3,5,6).
Usually, the tumor mass is <40 mm but the
literature also reports larger tumors and some cases of giant
masses (around 150-160 mm) (7). Some
patients were documented with gynecomastia (8) and that evidence suggests a role of the
estrogen pathway. Interestingly, O'Bryan et al recently
published the first case of MFB occurring in a transgender
individual after 13 months of treatment with hormone replacement
therapy (9). In 1998, Morgan and
Pitha postulated that androgen receptor or its ligands could be
pathologically related to the development of MFB, but the results
did not resolutely prove a causal mechanism of hormonal
tumorigenesis (10). MFB has also
been described at the surgical scar after breast cancer excision
(11), and after wide excision and
radiation therapy for ductal carcinoma in situ (12). Several publications have also
reported MFB in the setting of prior cancers such as prostatic,
renal and pancreatic tumors (3,13).
Radiological features of MFB are non-specific and it
is often mistaken for fibroadenomas or other benign and malignant
lesions. Breast ultrasound usually reveals a well-circumscribed,
oval and dense mass, with variable echogenicity and rare
calcifications that are more common in cases of fibroadenoma
(13-15).
The mammography shows a well-circumscribed dense mass, typically
round to oval without calcifications (14). For these reasons,
immunohistochemistry and histological examination play major roles
in making the correct diagnosis.
According to the macroscopic aspect, MFB is an
unencapsulated mass, well circumscribed from the adjacent
parenchyma (4,6). Necrosis, hemorrhage and cystic
degeneration are not characteristics of MFB (6). At the microscopic level, the classical
type is composed of uniform, slender, spindle cells arranged in
clusters separated by broad bands of hyalinized collagen (13). A variable adipocyte component and
mast cells are also described. Mammary ducts and lobules are
absent. The tumor vascular component is variably represented by
small to medium-sized vessels frequently showing hyalinization and
foamy histiocytes in their walls. In most cases
immunohistochemistry is positive for CD34 and vimentin (5,6). It is
also frequently positive, with variable extension of
immunoreactivity, for SMA, desmin, CD99, Bcl-2, CD10, ER, PR and
AR, while it does not express cytokeratin, EMA, c-Kit (CD117), p63
and S-100 protein (5,6). Proliferative activity is low with two
or fewer mitoses per 10 high-power fields (HPF) (5,6). In both
our cases, immunohistochemical analysis was in line with this
evidence, but for the first patient there was no information with
regard to ER and PR status. In addition, there was no information
regarding the expression of vimentin, which is typically positive
in MFB.
Some case series described nuclear atypia without
clinical consequences and, according to Howitt and Fletcher
(4), atypical cells were present in
approximately 10% of 141 MFB cases reviewed. According to their
histological composition, several patterns of MFB have been
identified in addition to the classical type: Collagenized/fibrous,
cellular, lipomatous, infiltrative, myxoid, epitheliod and
deciduoid-like variant (5,6). Two different morphological patterns may
potentially albeit rarely coexist in the same MFB.
Cytogenetic studies have shown that MFB exhibits
chromosome 13 rearrangements. In particular, in most cases it was
associated with the 13q14 deletion that includes the loss of RB1
and/or FOXO1 loci (16). These
deletions have been confirmed by FISH analyses and were also
described in spindle cell lipoma and in cellular angiomiofibroma,
suggesting a close relationship among these types of lesions
(16).
The principal differential diagnosis includes tumors
that can arise primarily in the breast parenchyma such as
leiomyoma, spindle cell lipoma, solitary fibrous tumor, spindle
cell sarcoma, nodular fasciitis, desmoid-type fibromatosis,
angiomyolipomas, pseudoangiomatous stroma hyperplasia and spindle
cell carcinoma (5). In particular,
the epithelioid variant can be confused with invasive lobular
carcinoma due to the pseudo-infiltrative growth pattern and the
expression of ER and PR. As the name implies, the epitheliod
variant is composed, exclusively or predominantly, by cells with
epitheliod morphology (at least 50% of the entire tumor) (5) and for this reason, it is a rare
subtype. The cases presented in this study emphasize that the
correct diagnosis of MFB is fundamental to avoid its overtreatment.
Generally, the absences of cytologic atypia and necrosis as well as
the lack of high mitotic activity and atypical mitoses at the
diagnostic biopsy are useful in the exclusion of malignancy. No
therapies are necessary after surgical removal, since recurrence is
unlikely following excision with clear resection margins (R0) and
no distant metastasis has been described after a follow-up period
of 15 years (17).
Non-specific imaging of this type of tumor
necessitates the support of histopathological analysis for correct
diagnosis. The careful analysis of cellular composition, growth
pattern and immunoreaction should help to differentiate MFB from
other benign or malignant tumors of the breast. In both our cases,
patients underwent radical surgery after the only execution of fine
needle aspiration cytology. No core biopsy was performed to help
clinicians in differential diagnosis and to avoid overtreatment, in
particular axillary dissection. For these reasons, a
multidisciplinary approach is critical to establish the appropriate
management. The long-term prognosis of MFB is excellent and the
complete surgical excision is considered curative, no additional
therapies, such as radiation or hormonal therapies are
necessary.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MV, GT, SC and LM conceived and designed this case
report. MV and AT contributed to the writing of the manuscript. MV,
LC and AT acquired the data in the diagnostic imaging and archives
of pathology. AG, AA and GT surgically treated the two patients.
LC, AT and LM made strict changes to the language of the manuscript
and made suggestions. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The local 'Area Vasta Emilia Nord (AVEN)' Ethical
Committee does not require official approval for the publication of
single case reports. Nevertheless, written informed consent was
obtained from both participants included in the pubblication.
Patient consent for publication
We obtained written informed consent for publication
from the two patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Toker C, Tang CK, Whitely JF, Berkheiser
SW and Rachman R: Benign spindle cell breast tumor. Cancer.
48:1615–1622. 1981.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wargotz ES, Weiss SW and Norris HJ:
Myofibroblastoma of the breast. Sixteen cases of a distinctive
benign mesenchymal tumor. Am J Surg Pathol. 11:493–502.
1987.PubMed/NCBI View Article : Google Scholar
|
|
3
|
McMenamin ME and Fletcher CD: Mammary-type
myofibroblastoma of soft tissue: A tumor closely related to spindle
cell lipoma. Am Surg Pathol. 25:1022–1029. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Howitt BE and Fletcher CD: Mammary-type
myofibroblastoma: Clinicopathologic characterization in a series of
143 cases. Am J Surg Pathol. 40:361–367. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Magro G: Mammary myofibroblastoma: An
update with emphasis on the most diagnostically challenging
variants. Histol Histopathol. 31:1–23. 2016.PubMed/NCBI
|
|
6
|
Magro G: Mammary myofibroblastoma: A tumor
with a wide morphologic spectrum. Arch Pathol Lab Med.
132:1813–1820. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kataria K, Srivastava A, Singh L, Suri V
and Yadav R: Giant myofibroblastoma of the male breast: a case
report and literature review. Malays J Med Sci. 19:74–76.
2012.PubMed/NCBI
|
|
8
|
Reis-Filho JS, Faoro LN, Gasparetto EL,
Totsugui JT and Schmitt FC: Mammary epithelioid myofibroblastoma
arising in bilateral gynecomastia: Case report with
immunohistochemical profile. Int J Surg Pathol. 9:331–334.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
O'Bryan J, Wolf-Gould C and Matsuo Y:
Mammary myofibroblastoma in a transgender patient on feminizing
hormones: Literature review and case report. Transgend Health.
3:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Morgan MB and Pitha JV: Myofibroblastoma
of the breast revisited: An etiologic association with androgens?
Hum Pathol. 29:347–351. 1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gocht A, Bösmüller HC, Bässler R,
Tavassoli FA, Moinfar F, Katenkamp D, Schirrmacher K, Lüders P and
Saeger W: Breast tumors with myofibroblastic differentiation:
Clinico-pathological observations in myofibroblastoma and
myofibrosarcoma. Pathol Res Pract. 195:1–10. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yagmur Y, Prasad ML and Osborne MP:
Myofibroblastoma in the irradiated breast. Breast J. 5:136–140.
1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Comer JD, Cui X, Eisen CS, Abbey G and
Arleo EK: Myofibroblastoma of the male breast: A rare entity with
radiologic-pathologic correlation. Clin Imaging. 42:109–112.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Greenberg JS, Kaplan SS and Grady C:
Myofibroblastoma of the breast in women: Imaging appearances. AJR
Am J Roentgenol. 171:71–72. 1998.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dockery WD, Singh HR and Wilentz RE:
Myofibroblastoma of the male breast: Imaging appearance and
ultrasound-guided core biopsy diagnosis. Breast J. 7:192–194.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Maggiani F, Debiec-Rychter M, Vanbockrijck
M and Sciot R: Cellular angiofibroma: Another mesenchymal tumour
with 13q14 involvement, suggesting a link with spindle cell lipoma
and (extra)-mammary myofibroblastoma. Histopathology. 51:410–412.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Magro G, Bisceglia M, Michal M and Eusebi
V: Spindle cell lipoma-like tumor, solitary fibrous tumor and
myofibroblastoma of the breast: A clinico-pathological analysis of
13 cases in favor of a unifying histogenetic concept. Virchows
Arch. 440:249–260. 2002.PubMed/NCBI View Article : Google Scholar
|