Introduction
Programmed death-1 (PD-1) inhibitors, including
pembrolizumab and nivolumab, can activate T cells to kill tumor
cells by blocking the binding of the PD-1 receptor and programmed
death ligand 1 and 2 (PD-L1 and PD-L2) (1). It is important to note that the
dramatic efficacies of PD-1 inhibitors commonly accompanied by
fatal side effects such as cardiotoxicity, pneumonitis and
neurological toxicities (2).
Guillain-Barre syndrome (GBS) is a rare but fatal
autoimmune-mediated peripheral neuropathy characterized by muscular
weakness in the extremities and peripheral paresthesia. The
pathogenesis of GBS is the autoimmune response caused by molecular
mimicry between microbial and nerve antigens (3). The present study reports on the case of
a patient with advanced renal cell carcinoma who received
pembrolizumab combined with sunitinib and presented with GBS. To
date, no similar case has been reported. We reviewed some
literatures to analyze the underlying mechanism of this rare
disease and we aim to highlight that early recognition and suitable
management is crucial to improve patient outcomes.
Case report
A 55-year-old male presented with a right renal mass
on CT scan. He was diagnosed with renal clear cell carcinoma with
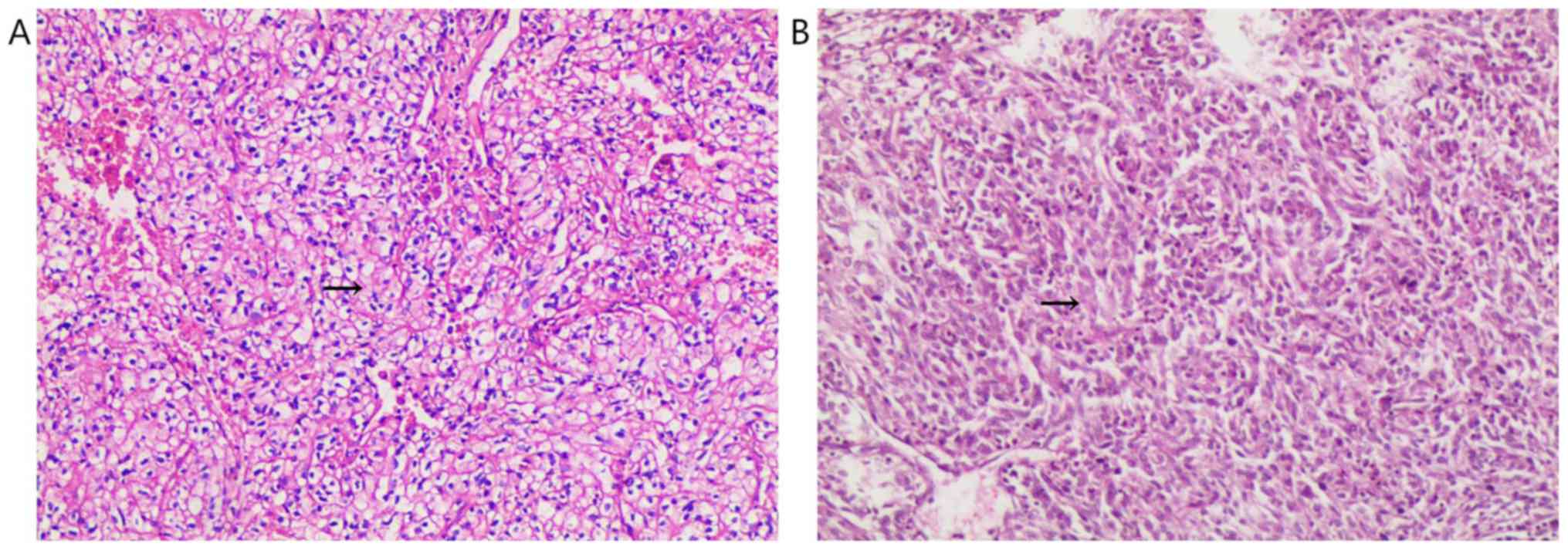
sarcomatoid differentiation after robot-assisted laparoscopic
resection of the local mass (Fig.
1). Adjuvant targeted therapy with sunitinib (50 mg/day; 2
weeks on, 1 week off) was performed for six cycles. On March 21st
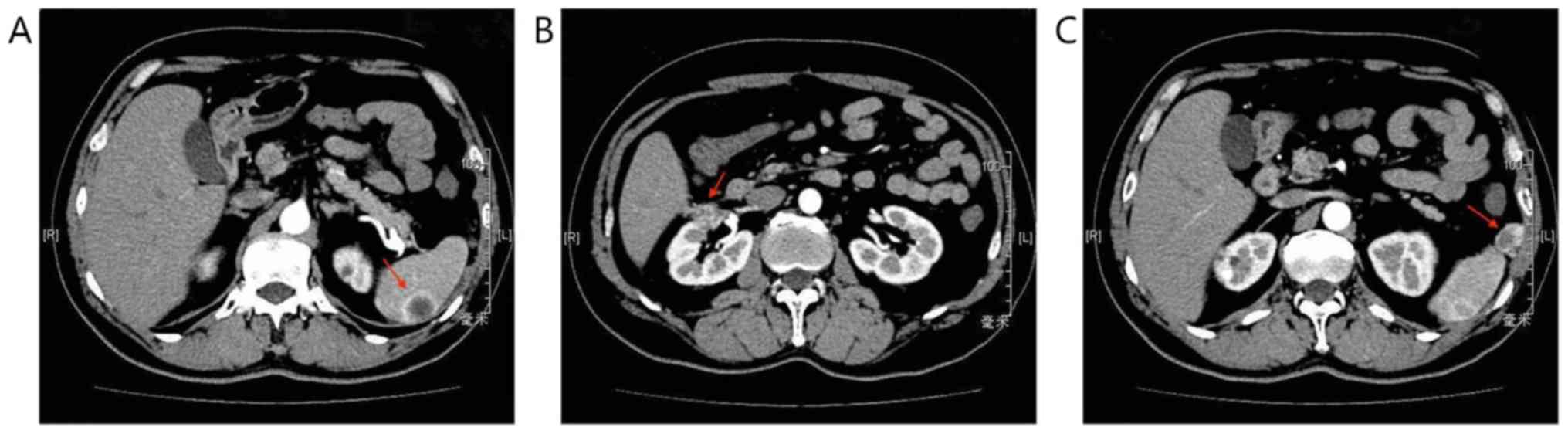
2018, a CT scan revealed local recurrence, and spleen and abdominal
cavity metastasis (Fig. 2). As a
result of this, the patient was started on pembrolizumab (2 mg/kg
once every 3 weeks) combined with sunitinib (37.5 mg/day; 2 weeks
on, 1 week off) on March 27th 2018. After 4 months, the patient
developed limb weakness and numbness of the extremities.
Neurological examination showed paresthesia of the four
extremities, absence of tendon reflex of the limbs, and a mild
decrease in muscle strength of the limbs with a grade of 4/5, but
no pathological reflex was observed. This patient had no history of
GBS, and laboratory examination results showed no evidence of
infection. Procalcitonin, c-reactive protein, influenza virus
antibody, cytomegalovirus antibody, rubella virus antibody, herpes
simplex virus antibody, Epstein-Barr virus antibody, detection of
mycoplasma and chlamydia DNA, and hepatitis virus tests were all
negative. Rheumatic immune disease was also ruled out. Magnetic
resonance imaging of the brain and spinal cord was normal.
Cerebrospinal fluid (CSF) analysis showed albuminocytologic
dissociation (protein, 583.30 mg/l; cell count, 8,000/µl), and
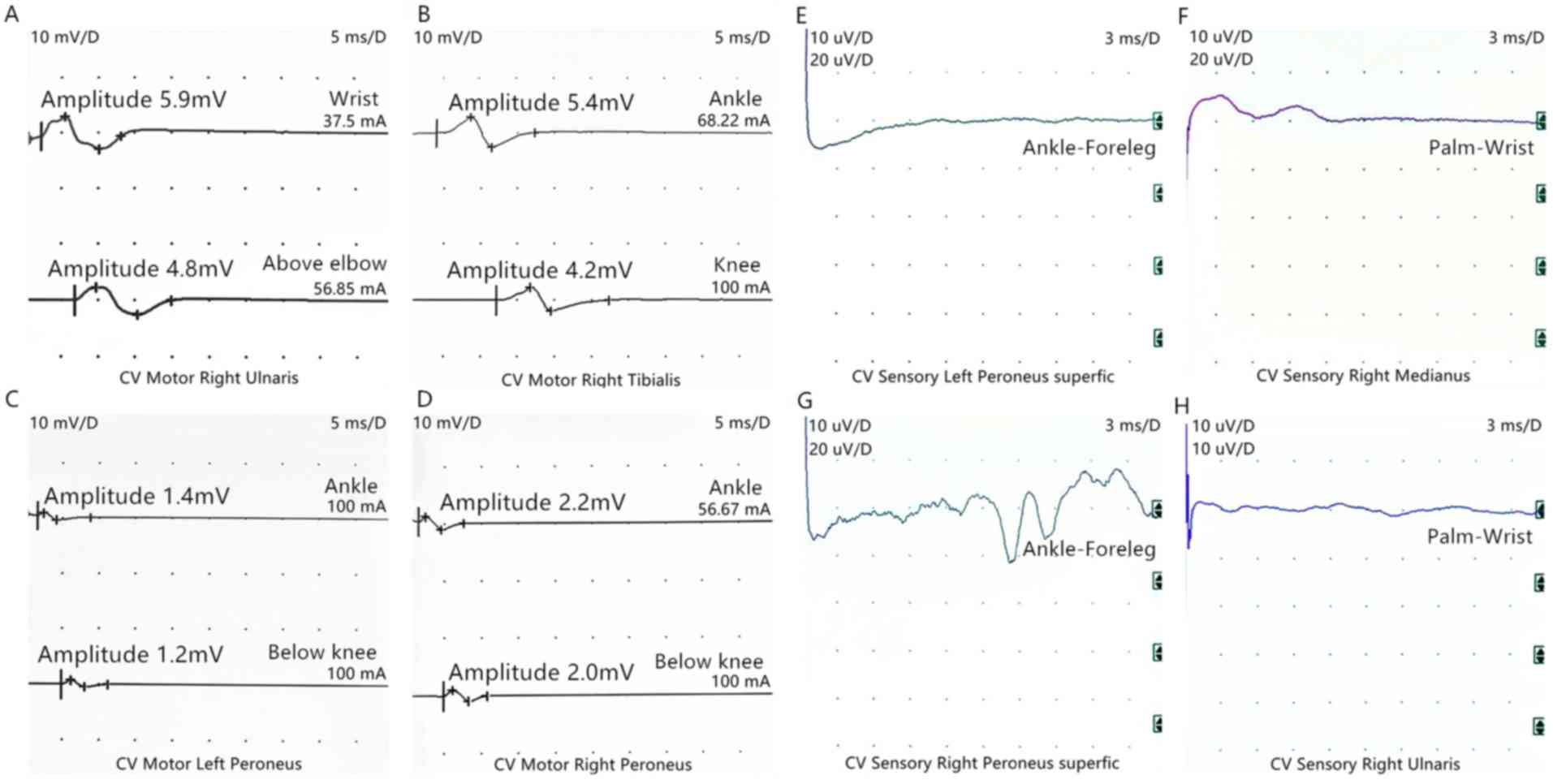
Pandy's test was positive. Electroneuromyography revealed acute
motor and sensory axonal neuropathy (Fig. 3). Based on these results, the patient
was diagnosed with GBS, which can be induced by pembrolizumab.
Pembrolizumab was permanently discontinued due to this severe
immune-related adverse event (irAE). The symptoms were gradually
aggravated after oral treatment with dexamethasone (10 mg/day). The
patient was then switched to intravenous immunoglobulin (IVIG; 0.4
g/kg for 5 days) along with prednisone (1 mg/kg per day). The
patient's symptoms improved during 1 week of treatment. After 1
month, although he experienced difficulty with fine movements, he
was able to walk and stand normally, and the doctors began to taper
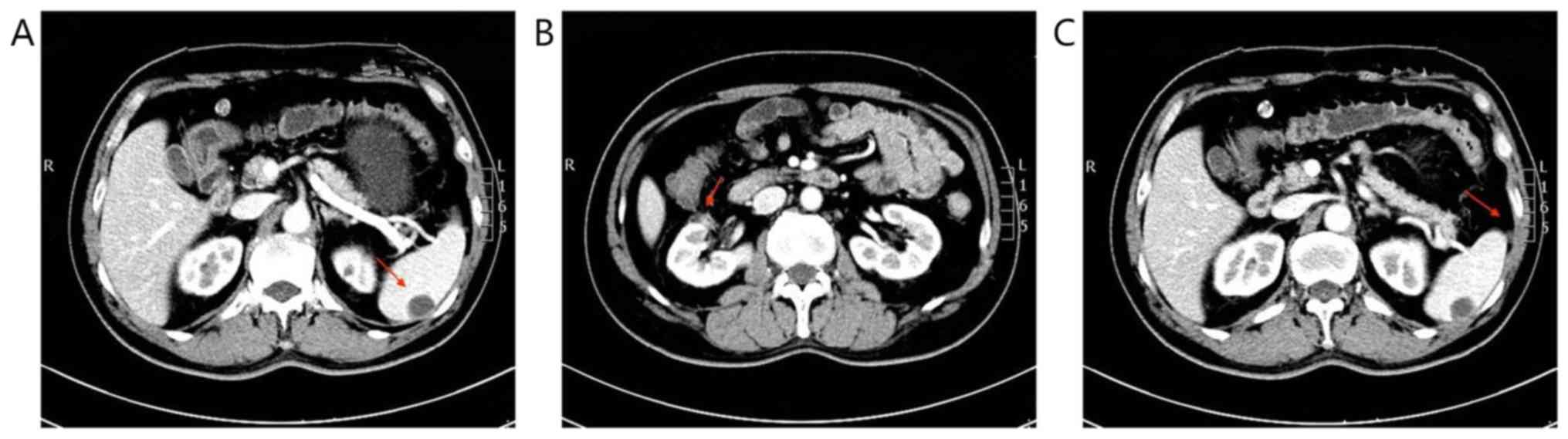
off the prednisone dosage. CT scan reexamination showed that the
masses in the right kidney and abdominal cavity had disappeared,
and the enhancement degree of spleen metastases was reduced
(Fig. 4). No recurrence was observed
during the 10 months of follow-up.
Discussion
Pembrolizumab is a potent and highly selective fully
human IgG4 PD-1 immune-checkpoint inhibitor. Studies have confirmed
its efficacy in several types of advanced cancer, such as melanoma,
non-small cell lung cancer, head and neck carcinoma and renal
cancer (3-6).
Immunotherapy can cause a series of irAEs, including skin
reactions, colitis, hepatitis, endocrinopathies, pneumonitis and,
rarely, neurological toxicity (7,8).
GBS is an autoimmune-mediated disease in which most
patients have prodromal infection. Common infectious pathogens
include cytomegalovirus, Epstein-Barr virus, influenza virus, human
immunodeficiency virus, mycoplasma, Haemophilus and
Campylobacter jejuni (1). It
is now believed that the primary pathogenesis is the autoimmune
response caused by molecular mimicry between microbial and nerve
antigens. Both cellular and humoral immunity are abnormally
activated. T cells invade the peripheral nerves, and peripheral
demyelination is mediated by macrophages. Immunoglobulin and
complement deposits appear on the myelin sheath and Schwann cells
(9). Regulatory T cells (Treg cells)
can downregulate the immune response, maintain autoimmunological
tolerance, and prevent autoimmune diseases (10). The binding of PD-1 and its ligands
PD-L1/PD-L2 causes CD25+Foxp3+ Treg cells to
suppress anti-tumor immunity (11),
and an increase in the number of CD25+Foxp3+
Treg cells has been reported in many malignant tumors (12-15),
which is often associated with poor prognosis (16). The effect of PD-1 inhibitors is not
limited to tumor-specific T cells, and blocking PD-1/PD-L1 and
PD-L2 signals not only promotes anti-tumor immunity, but it also
inhibits the generation of Treg cells in normal tissues, causing
autoimmune adverse events (11). The
patient in the present study had no symptoms of preexisting
infection, and influenza virus, Epstein-Barr virus, human
immunodeficiency virus, mycoplasma, hepatitis virus and
cytomegalovirus infection were excluded by laboratory tests. It has
been suggested that pembrolizumab may cause immune hyperfunction by
increasing T cell activity, promoting T cell proliferation and
inhibiting Treg cell function, thus disrupting immune homeostasis
and inducing GBS.
Sunitinib is a multi-target tyrosine kinase receptor
inhibitor targeting vascular endothelial growth factor receptor
(VEGFR)-1, VEGFR-2, VEGFR-3, platelet-derived growth factor-α
receptor (PDGFR-α), PDGFR-β, stem cell receptor and
colony-stimulating factor 1 receptor, which was approved by the US
Food and Drug Administration for the first-line treatment of
metastatic renal cell carcinoma, and postoperative adjuvant
treatment of renal cell carcinoma with a high risk of recurrence
(17,18). In the KEYNOTE-426 study,
pembrolizumab in combination with acitinib for advanced renal cell
carcinoma significantly improved median progression-free survival
(15.1 months vs. 11.1 months) over sunitinib alone (6), suggesting that pembrolizumab combined
with a multi-target tyrosine kinase receptor inhibitor may be a
promising treatment option. The most common side effects of
sunitinib include fatigue, anorexia, hypertension,
myelosuppression, diarrhea, mucositis, rashes and hand-foot
syndrome (19). GBS developed in the
patient in the present study, who had favorable responses to
sunitinib in combination with pembrolizumab. Currently, two cases
of GBS induced by sunitinib have been reported (20,21), to
the best of our knowledge, and the mechanism may be related to the
sunitinib-mediated inhibition of VEGFRs causing a corresponding
increase in VEGF levels, which increases the numbers of B
lymphocytes and immature myeloid cells (22). Elevated VEGF levels may also disrupt
the blood-nerve barrier by altering microvascular permeability
(22). Considering that both T cells
and B cells are important in the pathogenesis of GBS, it is
speculated that combination therapy may have a synergistic
pathogenicity. However, further studies are warranted to confirm
this hypothesis.
Management of irAEs must never be disregarded. In
the PubMed database, eight cases of nivolumab and four cases of
pembrolizumab causing GBS have been reported (23-33)
(Table I). Many of these cases were
treated with nivolumab or pembrolizumab monotherapy (10/13); two
patients were treated with ipilimumab and nivolumab in combination,
and one was treated with pembrolizumab followed by sequential
dabrafenib and trametinib. Most cases were male (9/12), and the
neurological symptoms were mostly sensory and movement disorders,
and reduced or absent deep tendon reflexes and only one patient had
a precursor infection. CSF tests showed that a high proportion of
patients had albuminocytologic dissociation (9/12), and the
etiological examinations were all negative. Most patients recovered
with corticosteroids, IVIG and plasmapheresis, but two died of
respiratory failure. The present patient did not improve with
corticosteroids alone, but IVIG was effective, suggesting that IVIG
may be suitable for checkpoint inhibitor-induced peripheral
neuropathy, although corticosteroids are recommended for the
management of irAEs. To the best of our knowledge, this is the
first case of GBS during treatment with pembrolizumab in
combination with sunitinib in advanced renal cell carcinoma,
highlighting the importance of correctly identifying immune-related
neurotoxicity and accumulating experience in the management of GBS
induced by pembrolizumab and sunitinib.
 | Table ILiterature regarding GBS induced by
PD-1 inhibitors. |
Table I
Literature regarding GBS induced by
PD-1 inhibitors.
| Study | Disease type | Checkpoint
inhibitors | GBS treatment | Outcome | CSF | (Refs.) |
|---|
| Fukumoto et
al, 2018 | NSCLC | Nivolumab | IVIG, steroids | Alive | Albuminocytologic
dissociation | (23) |
| Gu et al,
2017 | Melanoma | Nivolumab+
ipilimumab | IVIG, steroids,
plasma exchange, mycophenolate | Alive | Albuminocytologic
dissociation | (24) |
| Jacob et al,
2016 | NSCLC | Nivolumab | IVIG, plasma
exchange | Dead | Albuminocytologic
dissociation | (25) |
| Kyriazoglou et
al, 2019 | Bladder cancer | Nivolumab | IVIG, steroids | Alive | Albuminocytologic
dissociation | (26) |
| Nukui et al,
2018 | Nasal cancer | Nivolumab | IVIG, steroids | Alive | Albuminocytologic
dissociation | (27) |
| Schneiderbauer et
al, 2017 | Melanoma | Nivolumab | IVIG, steroids | Alive | Albuminocytologic
dissociation | (28) |
| Supakornnumporn et
al, 2017 | Melanoma | Nivolumab+
ipilimumab | IVIG, steroids | Alive | Albuminocytologic
dissociation | (29) |
| Thapa et al,
2018 | NSCLC | Nivolumab | IVIG, steroids | Alive | Normal | (30) |
| Manam et al,
2018 | NSCLC | Pembrolizumab | IVIG, steroids,
plasma exchange | Alive | Albuminocytologic
dissociation | (32) |
| Manam et al,
2018 | Melanoma |
Pembrolizumab-dabrafenib and
trametinib | IVIG, steroids,
plasma exchange | Dead | Albuminocytologic
dissociation | (32) |
| de Maleissye et
al, 2016 | Melanoma | Pembrolizumab | IVIG, steroids | Alive | Albuminocytologic
dissociation | (31) |
| Ong et al,
2018 | NSCLC | Pembrolizumab | IVIG, steroids | Alive | Unknown | (33) |
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CHa designed the experiments and wrote the initial
draft of the manuscript. JM, YZ, YJ, CHu and YW performed the
experiments and assisted in the preparation of the manuscript. JM
and YZ analyzed the data. CHa, CHu and YW revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The reported case has been approved by the patient
for academic exchange only.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wijdicks EF and Klein CJ: Guillain-barré
syndrome. Mayo Clin Proc. 92:467–479. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Barbee MS, Ogunniyi A, Horvat TZ and Dang
TO: Current status and future directions of the immune checkpoint
inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology.
Ann Pharmacother. 49:907–937. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Robert C, Schachter J, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med.
372:2521–2532. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Seiwert TY, Burtness B, Mehra R, Weiss J,
Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et
al: Safety and clinical activity of pembrolizumab for treatment of
recurrent or metastatic squamous cell carcinoma of the head and
neck (KEYNOTE-012): An open-label, multicentre, phase 1B trial.
Lancet Oncol. 17:956–965. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: Pembrolizumab plus axitinib versus sunitinib for advanced
renal-cell carcinoma. N Engl J Med. 380:1116–1127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S, Larkin J and Jordan K: ESMO Guidelines Committee:
Management of toxicities from immunotherapy: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28
(suppl 4):iv119–iv142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
van den Berg B, Walgaard C, Drenthen J,
Fokke C, Jacobs BC and van Doorn PA: Guillain-Barre syndrome:
Pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol.
10:469–482. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Smigiel KS, Srivastava S, Stolley JM and
Campbell DJ: Regulatory T-cell homeostasis: Steady-state
maintenance and modulation during inflammation. Immunol Rev.
259:40–59. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kumar P, Bhattacharya P and Prabhakar BS:
A comprehensive review on the role of co-signaling receptors and
Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun.
95:77–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fujii H, Josse J, Tanioka M, Miyachi Y,
Husson F and Ono M: Regulatory T cells in melanoma revisited by a
computational clustering of FOXP3+ T cell subpopulations. J
Immunol. 196:2885–2892. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ormandy LA, Hillemann T, Wedemeyer H,
Manns MP, Greten TF and Korangy F: Increased populations of
regulatory T cells in peripheral blood of patients with
hepatocellular carcinoma. Cancer Res. 65:2457–2464. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tao H, Mimura Y, Aoe K, Kobayashi S,
Yamamoto H, Matsuda E, Okabe K, Matsumoto T, Sugi K and Ueoka H:
Prognostic potential of FOXP3 expression in non-small cell lung
cancer cells combined with tumor-infiltrating regulatory T cells.
Lung Cancer. 75:95–101. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Syed Khaja AS, Toor SM, El Salhat H, Faour
I, Ul Haq N, Ali BR and Elkord E: Preferential accumulation of
regulatory T cells with highly immunosuppressive characteristics in
breast tumor microenvironment. Oncotarget. 8:33159–33171.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Takeuchi Y and Nishikawa H: Roles of
regulatory T cells in cancer immunity. Int Immunol. 28:401–409.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J. 356:115–124. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ravaud A, Motzer RJ, Pandha HS, George DJ,
Pantuck AJ, Patel A, Chang YH, Escudier B, Donskov F, Magheli A, et
al: Adjuvant sunitinib in high-risk renal-cell carcinoma after
nephrectomy. N Engl J Med. 375:2246–2254. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Randrup Hansen C, Grimm D, Bauer J,
Wehland M and Magnusson NE: Effects and side effects of using
sorafenib and sunitinib in the treatment of metastatic renal cell
carcinoma. Int J Mol Sci. 18(pii: E461)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mulherin B, Loconte NK and Holen KD:
Guillain-Barre syndrome after treatment with sunitinib malate?
Oncology (Williston Park). 22:66–67. 2008.PubMed/NCBI
|
|
21
|
Kanaan Z, Kulairi Z, Titianu M, Saha S and
Kumar S: Guillain-barre syndrome following treatment with sunitinib
malate. Case Rep Oncol Med. 2014(712040)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aparicio-Gallego G, Blanco M, Figueroa A,
Garcia-Campelo R, Valladares-Ayerbes M, Grande-Pulido E and
Anton-Aparicio L: New insights into molecular mechanisms of
sunitinib-associated side effects. Mol Cancer Ther. 10:2215–2223.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fukumoto Y, Kuwahara M, Kawai S, Nakahama
K and Kusunoki S: Acute demyelinating polyneuropathy induced by
nivolumab. J Neurol Neurosurg Psychiatry. 89:435–437.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gu Y, Menzies AM, Long GV, Fernando SL and
Herkes G: Immune mediated neuropathy following checkpoint
immunotherapy. J Clin Neurosci. 45:14–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jacob A, Unnikrishnan DC, Mathew A,
Thyagarajan B and Patel S: A case of fatal Guillain-Barre syndrome
from anti-PD1 monoclonal antibody use. J Cancer Res Clin.
142:1869–1870. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kyriazoglou A, Liontos M, Papadopoulos C,
Bilali A, Kostouros E, Pagoni S, Doumas K, Dimopoulos MA and Bamias
A: Guillain-barre syndrome related to nivolumab: Case report of a
patient with urothelial cancer and review of the literature. Clin
Genitourin Cancer. 17:e360–e364. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nukui T, Nakayama Y, Yamamoto M, Taguchi
Y, Dougu N, Konishi H, Hayashi T and Nakatsuji Y: Nivolumab-induced
acute demyelinating polyradiculoneuropathy mimicking Guillain-Barre
syndrome. J Neurol Sci. 390:115–116. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schneiderbauer R, Schneiderbauer M, Wick
W, Enk AH, Haenssle HA and Hassel JC: PD-1 antibody-induced
guillain-barre syndrome in a patient with metastatic melanoma. Acta
Derm Venereol. 97:395–396. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Supakornnumporn S and Katirji B:
Guillain-barre syndrome triggered by immune checkpoint inhibitors:
A case report and literature review. J Clin Neuromuscul. 19:80–83.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Thapa B, Khalid S, Vakili R, Ui J and
Misbah S: Nivolumab-associated Guillain-barre syndrome in a patient
with non-small-cell lung cancer. Am J Ther. 25:e761–e763.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
de Maleissye MF, Nicolas G and Saiag P:
Pembrolizumab-induced demyelinating polyradiculoneuropathy. N Engl
J Med. 375:296–297. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Manam R, Martin JL, Gross JA, Chaudhary D,
Chowdhary S, Espinosa PS and Santos ES: Case reports of
pembrolizumab-induced acute inflammatory demyelinating
polyneuropathy. Cureus. 10(e3371)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ong S, Chapman J, Young G and Mansy T:
Guillain-Barre-like syndrome during pembrolizumab treatment. Muscle
Nerve. 58:e8–e10. 2018.PubMed/NCBI View Article : Google Scholar
|