Introduction
Neuroendocrine tumours rarely occur in the genital
tract, and endometrial neuroendocrine tumours in particular are
extremely rare. Neuroendocrine tumours are classified as low-grade
neuroendocrine tumour or high-grade neuroendocrine carcinoma (NEC),
with the latter further subclassified as small-and large-cell NEC
(1). Approximately 100 cases of
small-cell NEC of the endometrium have been reported in the English
language literature (2,3), and this type of tumour shows an
aggressive clinical course.
Sex-determining region Y-box 2 (SOX2) is a member of
the SOX family and is a master transcription factor of the
self-renewal, maintenance of stem cell properties, and pluripotency
of embryonic stem cells (4). Its
expression is tightly regulated during embryonic development. It
has been reported that SOX2 also plays important roles in cell
survival and cancer growth and progression in several types of
carcinomas, such as lung, breast, gastric and pancreatic cancers,
as well as malignant melanoma (5-9).
A limited number of studies have addressed the role
of SOX2 expression in endometrial cancer (10-12).
A recent study detected SOX2 expression in 17% of patients with
endometrial carcinoma, which was significantly correlated with high
histological grade and poor prognosis (10). Moreover, a high frequency of SOX2
expression has been reported in small- and large-cell NECs of the
lung (5) and small-cell NEC of the
oesophagus (13). However, its
expression and significance in small-cell NEC of the endometrium
have not been examined. The aim of the present study was to analyse
the expression profiles of SOX2 in small-cell NEC of the
endometrium, which were compared with those of p16 and paired-box
gene (PAX) 8, a useful Müllerian marker.
Patients and methods
Patients and samples
The pathology archives of Kansai Medical University
Hospital between January 2006 and December 2017 were reviewed.
Patients with a histopathological diagnosis of endometrial
small-cell NEC were enrolled in the present study. The diagnosis of
small-cell NEC was independently confirmed by two diagnostic
pathologists. The majority of the patients evaluated in the present
study were also included in our previous study on the cytological
characteristics of endometrial small-cell NEC (14). Patients who had been administered
preoperative chemotherapy were excluded.
The present study was conducted in accordance with
the principles outlined in the Declaration of Helsinki, and the
study protocol was approved by the Institutional Review Board of
Kansai Medical University Hospital (approval no. 2016646). The need
for informed consent was waived due to the retrospective design of
the study.
Immunohistochemistry
Formalin-fixed and paraffin-embedded blocks of
resected specimens of small-cell NEC of the endometrium were cut
into 4-µm sections, deparaffinized and rehydrated.
Immunohistochemical analyses were performed using autostainers
(Discovery ULTRA System, Link 48; Roche Diagnostics, Dako
Cytomation) according to the manufacturer's instructions. The
primary antibodies used were mouse monoclonal antibody against
chromogranin A (clone LK2H10, Cell Marque, dilution 1:500), mouse
monoclonal antibody against p16 (clone. E6H4, Roche Diagnostics,
prediluted), mouse monoclonal antibody against PAX8 (clone. PAX8R1,
Abcam, dilution 1:100), rabbit polyclonal antibody against SOX2
(cat. no. PM056, Medial and Biological Laboratories Co., Ltd.,
dilution 1:500), and mouse monoclonal antibody against
synaptophysin (clone 27G12, Nichirei Bioscience, prediluted).
Immunohistochemical staining of p16, PAX8 and SOX2 was
quantitatively analysed as the proportion of positive neoplastic
cells. p16 expression was evaluated as nuclear and/or cytoplasmic
stainings, and PAX8 and SOX2 were evaluated as nuclear staining.
The expression status of chromogranin A and synaptophysin was
evaluated as the presence of positive neoplastic cells (more than
1%). Immunohistochemical staining results were independently
evaluated by at least two researchers.
Results
Clinicopathological
characteristics
The present study included 4 patients with
small-cell NEC of the endometrium. The clinicopathological
characteristics of the present series are summarized in Table I. The median age of the patients was
70 years (range, 61-73 years). All the patients were
post-menopausal.
 | Table IClinicopathological and
immunohistochemical characteristics of small-cell neuroendocrine
carcinoma of the endometrium. |
Table I
Clinicopathological and
immunohistochemical characteristics of small-cell neuroendocrine
carcinoma of the endometrium.
| | | | Immunohistochemical
characteristics | | | |
|---|
| Case no. | Age (years) | Endometrioid
carcinoma component | SOX2 (%) | p16 (%) | PAX8 (%) | Chromogranin A | Synaptophysin |
|---|
| 1 | 73 | - | 70 | 100 | 0 | + | + |
| 2 | 72 | - | 30 | 100 | 0 | + | - |
| 3 | 61 | - | 80 | 100 | 0 | + | + |
| 4 | 68 | + | 0 | 100 | 0 | + | + |
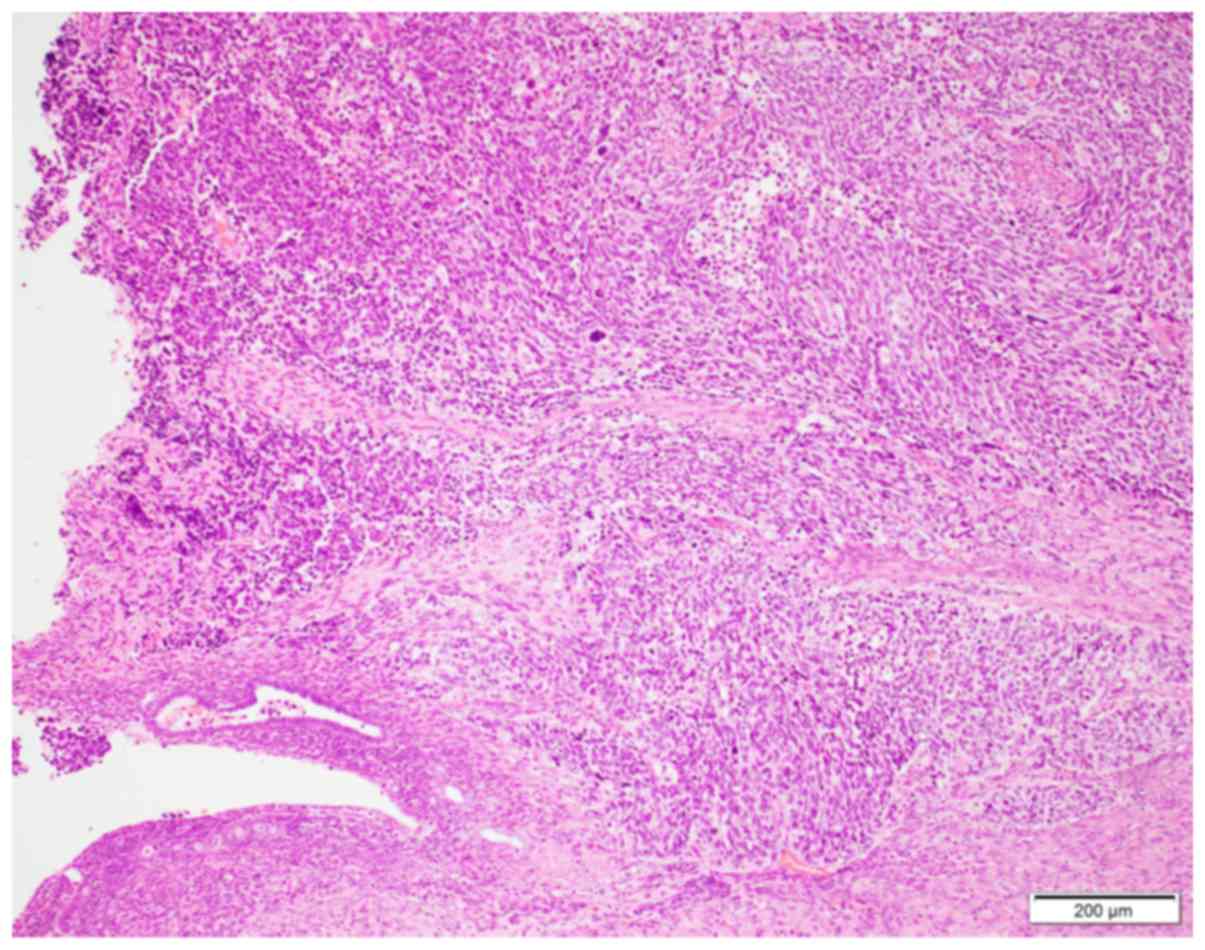
The characteristic histopathological features of
small-cell NEC are shown in Fig. 1.
Diffuse proliferation of neoplastic cells with round to oval nuclei
showing a salt and pepper chromatin pattern and high
nuclear/cytoplasmic ratio were observed. Mitotic figures (more than
10 mitotic figures/10 high-power fields) and apoptotic bodies were
frequently observed. A conventional endometrioid carcinoma
component was detected in 1 patient (Case 4).
Immunohistochemical
characteristics
The immunohistochemical results of the present study
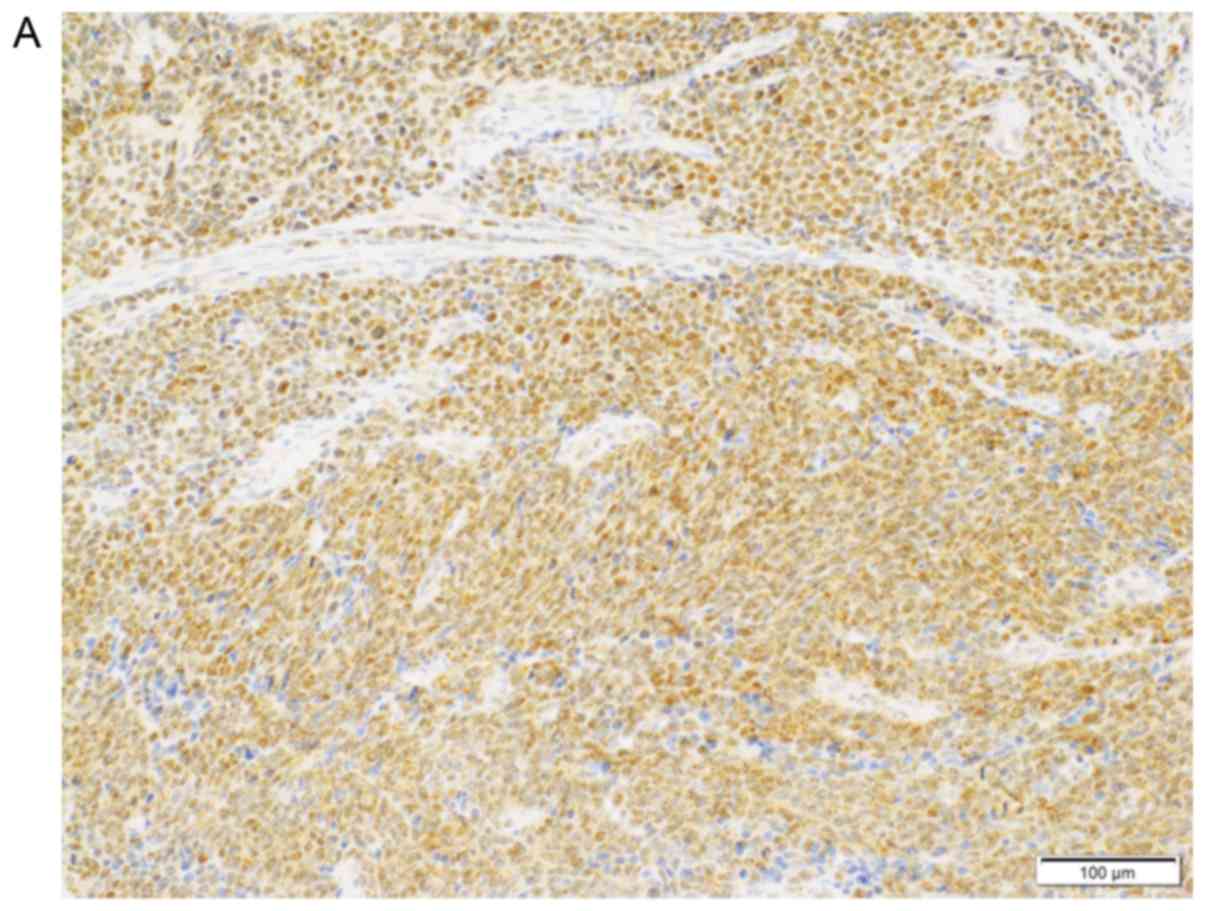
are summarized in Table I. SOX2 was
expressed in 3 of the 4 patients, and the median percentage of
positive neoplastic cells in positive patients was 70% (range,
30-80%; Fig. 2A). p16 was expressed
in all cases (100% of neoplastic cells in all cases; Fig. 2B). None of the cases exhibited
positive immunoreactivity for PAX8 in the small-cell NEC component,
although the conventional endometrioid carcinoma component in case
4 showed positive immunoreactivity for this marker.
Chromogranin A and synaptophysin expression was
noted in 4 and 3 cases, respectively.
Discussion
The present study demonstrated that SOX2 was
expressed in 3 of 4 patients with small-cell NEC of the endometrium
(the median proportion of positive neoplastic cells was 70% in
positive patients), p16 was diffusely expressed in all cases, and
none of the cases showed positive immunoreactivity for PAX8.
SOX2 is a transcription factor that plays an
important role in the growth and progression of several types of
carcinomas (5-9).
The role of SOX2 expression in small-cell NEC of some organs has
been previously analysed (13,15). A
recent study revealed high SOX2 expression in small-cell NEC of the
oesophagus and the lung, indicating that SOX2 plays a pivotal role
in the development of small-cell NEC in these locations (13). In the present study, 3 of 4 patients
with endometrial small-cell NEC exhibited positive immunoreactivity
for SOX2. Accordingly, SOX2 may play an important role in the
pathogenesis of small-cell NEC of the endometrium, lung and
oesophagus, as only 17% of patients with conventional endometrial
carcinoma, particularly those with high histological grade, express
this marker (10).
p16 plays important role in cell cycle regulation,
and its expression is observed in most cases of human
papillomavirus-related cervical carcinoma (16). It is well-known that p16 is expressed
in high-grade endometrial carcinomas, including serous carcinoma
(16). The largest case series of
small-cell NEC of the endometrium revealed p16 expression in 5/5
cases (2), which was consistent with
the results obtained in the present study (4/4 cases); therefore,
p16 expression appears to be a common finding in high-grade
endometrial carcinomas, including small-cell NEC. Moreover, p16
expression has been reported in small-cell NEC of cervical origin
(17); therefore, positive
immunoreactivity for this marker in small-cell NEC does not
indicate cervical origin.
PAX8 is a transcription marker associated with the
organogenesis of the Müllerian system, thyroid and kidney (18). Epithelial cells in the genital tract
and conventional endometrioid carcinoma cells commonly express this
marker (18). However, no samples
from small-cell NECs of the endometrium exhibited positive
immunoreactivity for PAX8 in the present study, although the
conventional endometrioid carcinoma component was positive in 1
case. This agrees with the results of a previous study, in which
PAX8 expression was detected in 2/6 cases of small-cell NEC of the
endometrium (2). These results
suggest that loss of PAX8 may be a common finding in small-cell NEC
of the endometrium, and PAX8 is not a reliable marker of
endometrial origin when diagnosing small-cell NEC of unknown
primary site (2).
In conclusion, the present study demonstrated that
SOX2 may play an important role in the development of small-cell
NEC of the endometrium, as well as that of the lung and oesophagus.
Expression of p16 or loss of PAX8 expression are common findings
associated with this type of tumour, and do not indicate the origin
of small-cell NEC. The present study included a limited number of
patients with small-cell NEC of the endometrium; therefore,
additional studies are needed to fully elucidate the expression
profiles of immunohistochemical markers and pathogenesis of
small-cell NEC of the endometrium.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during the present
study are included in this published article.
Authors' contributions
YE and MI conceived and designed the study. YE, MI,
TM, MK, HO and KT acquired and analysed the data. YE and MI drafted
the manuscript and designed the figures. The final version of the
manuscript has been read and approved by all authors.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the principles outlined in the Declaration of Helsinki, and the
study protocol was approved by the Institutional Review Board of
Kansai Medical University Hospital (approval no. 2016646).
Patient consent for publication
The need for informed consent was waived due to the
retrospective design of the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zaio R, Carinelli SG, Ellenson LH, Eng C,
Katabuchi H, Konishi I, Lax S, Matias-Guiu X, Mutter GL, Peters WA
III, et al: Epithelial tumours and precursors. In: Kurman RJ
Carcangiu ML Herrington S and Young RH (eds). WHO classification of
tumours of female reproductive organs. 4th edition. Lyon, IARC.
pp125–135. 2014.
|
|
2
|
Pocrnich CE, Ramalingam P, Euscher ED and
Malpica A: Neuroendocrine carcinoma of the endometrium: A
clinicopathologic study of 25 cases. Am J Surg Pathol. 40:577–586.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ishida M, Iwamoto N, Nakagawa T, Kaku S,
Iwai M, Kagotani A, Takahashi K, Murakami T and Okabe H: Small cell
carcinoma of the endometrium: A case report with emphasis on the
cytological features. Int J Clin Exp Pathol. 7:3332–3337.
2014.PubMed/NCBI
|
|
4
|
Adachi K, Nikaido I, Ohta H, Ohtsuka S,
Ura H, Kadota M, Wakayama T, Ueda HR and Niwa H: Context-dependent
wiring of Sox2 regulatory networks for self-renewal of embryonic
and trophoblast stem cells. Mol Cell. 52:380–392. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sholl LM, Long KB and Hornick JL: Sox2
expression in pulmonary non-small cell and neuroendocrine
carcinomas. Appl Immunohistochem Mol Morphol. 18:55–61.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shima H, Kutomi G, Satomi F, Maeda H,
Hirohashi Y, Hasegawa T, Mori M, Torigoe T and Takemasa I: SOX2 and
ALDH1 as predictors of operable breast cancer. Anticancer Res.
36:2945–2953. 2016.PubMed/NCBI
|
|
7
|
Basati G, Mohammadpour H and Emami Razavi
A: Association of high expression levels of SOX2, NANOG, and OCT4
in gastric cancer tumor tissues with progression and poor
prognosis. J Gastrointest Cancer. 51:41–47. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Herreros-Villanueva M, Bujanda L,
Billadeau DD and Zhang JS: Embryonic stem cell factors and
pancreatic cancer. World J Gastroenterol. 20:2247–2254.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Santini R, Pietrobono S, Pandolfi S,
Montagnani V, D'Amico M, Penachioni JY, Vinci MC, Borgognoni L and
Stecca B: SOX2 regulates self-renewal and tumorigenicity of human
melanoma-initiating cells. Oncogene. 33:4697–4708. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yamawaki K, Ishiguro T, Mori Y, Yoshihara
K, Suda K, Tamura R, Yamaguchi M, Sekine M, Kashima K, Higuchi M,
et al: Sox2-dependent inhibition of p21 is associated with poor
prognosis of endometrial cancer. Cancer Sci. 108:632–640.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee CJ, Sung PL, Kuo MH, Tsai MH, Wang CK,
Pan ST, Chen YJ, Wang PH, Wen KC and Chou YT: Crosstalk between
SOX2 and cytokine signaling in endometrial carcinoma. Sci Rep.
8(17550)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pityński K, Banas T, Pietrus M,
Milian-Ciesielska K, Ludwin A and Okon K: SOX-2, but not Oct4, is
highly expressed in early-stage endometrial adenocarcinoma and is
related to tumour grading. Int J Clin Exp Pathol. 8:8189–8198.
2015.PubMed/NCBI
|
|
13
|
Ishida H, Kasajima A, Kamei T, Miura T,
Oka N, Yazdani S, Ozawa Y, Fujishima F, Sakurada A, Nakamura Y, et
al: SOX2 and Rb1 in esophageal small-cell carcinoma: Their possible
involvement in pathogenesis. Mod Pathol. 30:660–671.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ebisu Y, Ishida M, Okano K, Sandoh K,
Mizokami T, Kita M, Okada H and Tsuta K: Small-cell neuroendocrine
carcinoma in directly sampled endometrial cytology: A monocentric
retrospective study of six cases. Diagn Cytopathol. 47:1297–1301.
2019.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Masai K, Tsuta K, Kawago M, Tatsumori T,
Kinno T, Taniyama T, Yoshida A, Asamura H and Tsuda H: Expression
of squamous cell carcinoma markers and adenocarcinoma markers in
primary pulmonary neuroendocrine carcinomas. Appl Immunohistochem
Mol Morphol. 21:292–297. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yemelyanova A, Ji H, Shih IeM, Wang TL, Wu
LS and Ronnett BM: Utility of p16 expression for distinction of
uterine serous carcinomas from endometrial endometrioid and
endocervical adenocarcinomas: Immunohistochemical analysis of 201
cases. Am J Surg Pathol. 33:1504–1514. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
McCluggage WG, Kennedy K and Busam KJ: An
immunohistochemical study of cervical neuroendocrine carcinomas:
Neoplasms that are commonly TTF1 positive and which may express
CK20 and P63. Am J Surg Pathol. 34:525–532. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ordóñez NG: Value of PAX 8 immunostaining
in tumor diagnosis: A review and update. Adv Anat Pathol.
19:140–151. 2012.PubMed/NCBI View Article : Google Scholar
|